3afacb92e8db3cce7f60462f3e9c4910.ppt
- Количество слайдов: 41
 Regulation and Standards Chapter 17 1
Regulation and Standards Chapter 17 1
 Extra Activities for Regulated Industries z Develop and maintain a Quality System z Product Documentation x. Design History File x. Technical File z Product submissions z Testing certifications z Extra time for: x. Submissions x. Answer questions from regulators x. Re-submissions z Audits 2
Extra Activities for Regulated Industries z Develop and maintain a Quality System z Product Documentation x. Design History File x. Technical File z Product submissions z Testing certifications z Extra time for: x. Submissions x. Answer questions from regulators x. Re-submissions z Audits 2
 The Typical Road to Market for a Non-Medical Device z. Generate a new idea for a product z. Design the product z. Test the product z. Manufacture the product z. Ship the product 3
The Typical Road to Market for a Non-Medical Device z. Generate a new idea for a product z. Design the product z. Test the product z. Manufacture the product z. Ship the product 3
 The Typical Road to Market for a Medical Device z. Generate a new idea for a product z. Design the product z. Test the product z. Submit data to the regulatory agency z. Wait z. Manufacture the product z. Ship the product 4
The Typical Road to Market for a Medical Device z. Generate a new idea for a product z. Design the product z. Test the product z. Submit data to the regulatory agency z. Wait z. Manufacture the product z. Ship the product 4
 Timing of Product Development z Establish a window of opportunity to sell the product z Determine the amount of time to manufacture the product z Determine the amount of time for regulatory approval z Determine the amount of time to test the product z Determine the amount of time to design the product z Determine the amount of time to specify the product z Start the development cycle 5
Timing of Product Development z Establish a window of opportunity to sell the product z Determine the amount of time to manufacture the product z Determine the amount of time for regulatory approval z Determine the amount of time to test the product z Determine the amount of time to design the product z Determine the amount of time to specify the product z Start the development cycle 5
 Types of Regulations z Process x. ISO 9000 family x. Audits by Notified Bodies z Product x. Food and Drug Administration (FDA) x. Medical Device Directive (MDD) x. Individual country requirements (Canada, Australia, Japan, Russia) x. City of Los Angeles x. Other standards required for certain products x. Environmental standards 6
Types of Regulations z Process x. ISO 9000 family x. Audits by Notified Bodies z Product x. Food and Drug Administration (FDA) x. Medical Device Directive (MDD) x. Individual country requirements (Canada, Australia, Japan, Russia) x. City of Los Angeles x. Other standards required for certain products x. Environmental standards 6
 Process Regulations z Basis for product regulations z Requires the company to show an experienced quality system in place z ISO 9000 family used as the gold standard z For companies with design capabilities, ISO 9001 is the foundation z For medical device companies, ISO 13485 is beginning to be accepted 7
Process Regulations z Basis for product regulations z Requires the company to show an experienced quality system in place z ISO 9000 family used as the gold standard z For companies with design capabilities, ISO 9001 is the foundation z For medical device companies, ISO 13485 is beginning to be accepted 7
 ISO 9001 z z z z z Management responsibility Quality system Contract review Design control Document and data control Purchasing Control of customer supplied product Product identification and traceability Process control Inspection and testing 8
ISO 9001 z z z z z Management responsibility Quality system Contract review Design control Document and data control Purchasing Control of customer supplied product Product identification and traceability Process control Inspection and testing 8
 ISO 9001 z Control of inspection, measuring, and test equipment Inspection and test status z Control of non-conforming product z Corrective and preventive action z Handling, storage, packaging, preservation, and delivery z Control of quality records z Internal quality audits z Training z Servicing z Statistical techniques 9
ISO 9001 z Control of inspection, measuring, and test equipment Inspection and test status z Control of non-conforming product z Corrective and preventive action z Handling, storage, packaging, preservation, and delivery z Control of quality records z Internal quality audits z Training z Servicing z Statistical techniques 9
 Design Control z Design and development planning z Organizational and technical interfaces z Design input z Design output z Design review z Verification z Validation z Design changes 10
Design Control z Design and development planning z Organizational and technical interfaces z Design input z Design output z Design review z Verification z Validation z Design changes 10
 Product Regulations z Europe x. Medical Device Directive z Other Countries x. Australia x. Canada x. Japan x. Russia z United States x. FDA 11
Product Regulations z Europe x. Medical Device Directive z Other Countries x. Australia x. Canada x. Japan x. Russia z United States x. FDA 11
 The various Medical Device Directives define a medical device as: z z "any instrument, appliance, apparatus, material or other article, whether used alone or in combination, including the software necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of: diagnosis, prevention, monitoring, treatment or alleviation of disease diagnosis, monitoring, alleviation of or compensation for an injury or handicap investigation, replacement or modification of the anatomy or of a physiological process control of conception, and which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means. " 12
The various Medical Device Directives define a medical device as: z z "any instrument, appliance, apparatus, material or other article, whether used alone or in combination, including the software necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of: diagnosis, prevention, monitoring, treatment or alleviation of disease diagnosis, monitoring, alleviation of or compensation for an injury or handicap investigation, replacement or modification of the anatomy or of a physiological process control of conception, and which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means. " 12
 Medical Device Directive z 3 divisions: AIMDD, IVMDD z. Required for selling a product in Europe z. Product must contain a CE mark z. Must have a quality system z. Product must meet a list of essential requirements z. Certificates for all testing 13
Medical Device Directive z 3 divisions: AIMDD, IVMDD z. Required for selling a product in Europe z. Product must contain a CE mark z. Must have a quality system z. Product must meet a list of essential requirements z. Certificates for all testing 13
 Medical Device Directive z. Three directives: x. Active Implantable Medical Devices Directive (AIMDD) x. Medical Devices Directive (MDD) x. In Vitro Diagnostic Medical Devices Directive (IVDMDD) 14
Medical Device Directive z. Three directives: x. Active Implantable Medical Devices Directive (AIMDD) x. Medical Devices Directive (MDD) x. In Vitro Diagnostic Medical Devices Directive (IVDMDD) 14
 Medical Device Directive Process z Analyze the device to determine which directive is applicable z Identify the applicable Essentials Requirements List (safety, risk, performance, …) z Identify any corresponding Harmonized standards z Confirm that the device meets the Essential requirements/Harmonized Standards and document the evidence z Classify the device 15
Medical Device Directive Process z Analyze the device to determine which directive is applicable z Identify the applicable Essentials Requirements List (safety, risk, performance, …) z Identify any corresponding Harmonized standards z Confirm that the device meets the Essential requirements/Harmonized Standards and document the evidence z Classify the device 15
 Medical Device Directive Process z. Decide on the appropriate conformity assessment procedure z. Identify and choose a notified body z. Obtain conformity certifications for the device z. Establish a Declaration of Conformity z. Apply for the CE mark 16
Medical Device Directive Process z. Decide on the appropriate conformity assessment procedure z. Identify and choose a notified body z. Obtain conformity certifications for the device z. Establish a Declaration of Conformity z. Apply for the CE mark 16
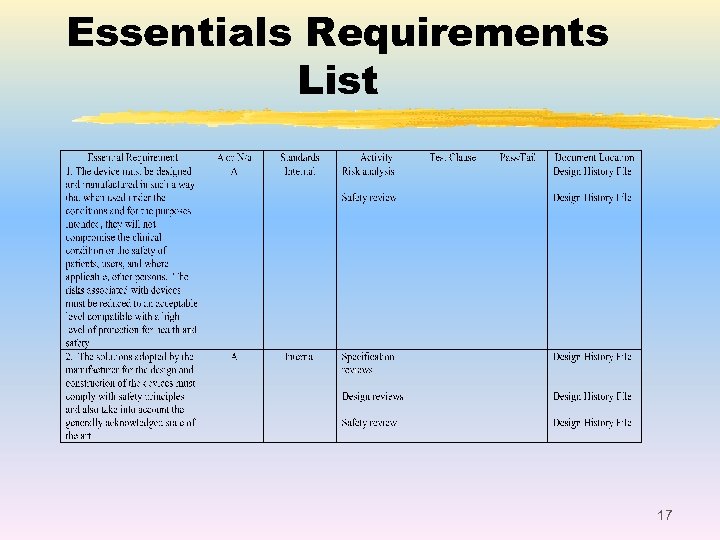 Essentials Requirements List 17
Essentials Requirements List 17
 Declaration of Conformance z Every device, other than a custom-made or clinical investigation device, must be covered by a declaration of conformity z Document that states you have met all the essential requirements for your device z Must include the serial numbers or batch numbers of the products it covers z Signed by a member of Senior Management 18
Declaration of Conformance z Every device, other than a custom-made or clinical investigation device, must be covered by a declaration of conformity z Document that states you have met all the essential requirements for your device z Must include the serial numbers or batch numbers of the products it covers z Signed by a member of Senior Management 18
 The CE Mark XXXX 19
The CE Mark XXXX 19
 Difference Between FDA and MDD z FDA: z A submission must be sent to the FDA for each product to be marketed z Must wait for approval z MDD: z A company may qualify for self-certification to MDD for their products. These are checked during scheduled audits. 20
Difference Between FDA and MDD z FDA: z A submission must be sent to the FDA for each product to be marketed z Must wait for approval z MDD: z A company may qualify for self-certification to MDD for their products. These are checked during scheduled audits. 20
 Other Product Regulations z Countries x. Japan x. Australia x. China x. Russia z Type of Device Standards x. Alarms x. Software z Environmental Standards x. EMC x. Temperature/Humidity x. Shipping 21
Other Product Regulations z Countries x. Japan x. Australia x. China x. Russia z Type of Device Standards x. Alarms x. Software z Environmental Standards x. EMC x. Temperature/Humidity x. Shipping 21
 Audits z 1 -4 people in your spaces for 3 days to several months 22
Audits z 1 -4 people in your spaces for 3 days to several months 22
 Audits z. Will cover in detail your process and products z. Auditors will “dig-in” in they find the hint of a problem z. Major discrepancies will shut you down until they are fixed z. Legal and/or punitive steps may be taken 23
Audits z. Will cover in detail your process and products z. Auditors will “dig-in” in they find the hint of a problem z. Major discrepancies will shut you down until they are fixed z. Legal and/or punitive steps may be taken 23
 Food and Drug Administration z Quality system z Testing to prove the safety and efficacy of your product z Submission material dependent on the type of product you are making z Particular attention to software z MDRs z Recalls z Audits z (see chapter 16…) 24
Food and Drug Administration z Quality system z Testing to prove the safety and efficacy of your product z Submission material dependent on the type of product you are making z Particular attention to software z MDRs z Recalls z Audits z (see chapter 16…) 24
 Food and Drug Administration z Safety and efficacy: y. Requirement verification y. Risk analysis y. Environmental testing y. Clinical testing 25
Food and Drug Administration z Safety and efficacy: y. Requirement verification y. Risk analysis y. Environmental testing y. Clinical testing 25
 Food and Drug Administration z. Submissions: y. Class I Little regulation y. Class II 510(k) y. Class III PMA 26
Food and Drug Administration z. Submissions: y. Class I Little regulation y. Class II 510(k) y. Class III PMA 26
 FDA 2004 User Fees z. Large business: z 510(k) $ 3, 480 z. PMA y 180 day supplement y. Real-time supplement $206, 811 $ 44, 464 $ 14, 890 27
FDA 2004 User Fees z. Large business: z 510(k) $ 3, 480 z. PMA y 180 day supplement y. Real-time supplement $206, 811 $ 44, 464 $ 14, 890 27
 FDA 2004 User Fees z. Small business: z 510(k) $ 2, 784 z. PMA y 180 day supplement y. Real-time supplement $ 78, 588 $ 16, 896 $ 5, 658 28
FDA 2004 User Fees z. Small business: z 510(k) $ 2, 784 z. PMA y 180 day supplement y. Real-time supplement $ 78, 588 $ 16, 896 $ 5, 658 28
 Food and Drug Administration z Software: y. Based on an bad experience in Canada y. FDA doesn’t understand it y. Therefore, they over-regulate it y. All current regulations are in draft form y. Software in a device is the same level as the device y. Excess documentation required y. Auditors free to regulate according to their own principles 29
Food and Drug Administration z Software: y. Based on an bad experience in Canada y. FDA doesn’t understand it y. Therefore, they over-regulate it y. All current regulations are in draft form y. Software in a device is the same level as the device y. Excess documentation required y. Auditors free to regulate according to their own principles 29
 Food and Drug Administration z. MDRs and Recalls: y. MDR: a report sent to the FDA detailing the circumstances of your device killing or causing serious injury to a patient x. The FDA also gets a report from the hospital or clinic where the situation occurred y. Recall: a detailed plan for making design changes in all your devices currently in the field 30
Food and Drug Administration z. MDRs and Recalls: y. MDR: a report sent to the FDA detailing the circumstances of your device killing or causing serious injury to a patient x. The FDA also gets a report from the hospital or clinic where the situation occurred y. Recall: a detailed plan for making design changes in all your devices currently in the field 30
 Food and Drug Administration z Audits: y. General y. Triggered by submissions y. Triggered by field failures y. Triggered by unsolicited information 31
Food and Drug Administration z Audits: y. General y. Triggered by submissions y. Triggered by field failures y. Triggered by unsolicited information 31
 Newest of the Regulations (US) z HIPAA z Health Insurance Portability and Accountability Act z Main components are Privacy and Security 32
Newest of the Regulations (US) z HIPAA z Health Insurance Portability and Accountability Act z Main components are Privacy and Security 32
 Protected Health Information (PHI) PHI is health Information that: 1) is created or received by a health care provider, health plan, employer, or health care clearinghouse, and 2) relates to the past, present, or future physical or mental health or condition of an individual, the provisions of health care to an individual, or the past, present, or future payment for the provision of health care to an individual, and i) that dentifies the individual or ii) with respect to which there is a reasonable basis to believe the information can be used to identify the individual. 33
Protected Health Information (PHI) PHI is health Information that: 1) is created or received by a health care provider, health plan, employer, or health care clearinghouse, and 2) relates to the past, present, or future physical or mental health or condition of an individual, the provisions of health care to an individual, or the past, present, or future payment for the provision of health care to an individual, and i) that dentifies the individual or ii) with respect to which there is a reasonable basis to believe the information can be used to identify the individual. 33
 Protected Health Information (PHI) z Any health information that can be identified to a person z It includes information about treatment and care z PHI can include: x. Name x. Dates x. Record number x. Social security number x. Full face photo x. Any other unique identifying information 34
Protected Health Information (PHI) z Any health information that can be identified to a person z It includes information about treatment and care z PHI can include: x. Name x. Dates x. Record number x. Social security number x. Full face photo x. Any other unique identifying information 34
 De-Identification z Patient information from which identifiers have been deleted, redacted, or blocked, so that remaining information cannot reasonably be used to identify a person. Identifiers to be deleted include: x. Name x. Social security number x. Address x. Telephone number x. Birth date x. Admission date x. FAX numbers x. E-mail addresses x. Medical record numbers x. Health plan beneficiary numbers x. Account numbers x. Certification/license numbers x. Full face photos. 35
De-Identification z Patient information from which identifiers have been deleted, redacted, or blocked, so that remaining information cannot reasonably be used to identify a person. Identifiers to be deleted include: x. Name x. Social security number x. Address x. Telephone number x. Birth date x. Admission date x. FAX numbers x. E-mail addresses x. Medical record numbers x. Health plan beneficiary numbers x. Account numbers x. Certification/license numbers x. Full face photos. 35
 Civil Penalties for Non. Compliance z $100 for each violation z Total of $25, 000 for all violations of an identical requirement in a calendar year 36
Civil Penalties for Non. Compliance z $100 for each violation z Total of $25, 000 for all violations of an identical requirement in a calendar year 36
 Obtainment/Disclosure of PHI z Not more than $50, 000 and/or not more than 1 year imprisonment z Not more than $100, 000 and/or not more than 5 years imprisonment if the offense is “under false pretenses” z Not more than $250, 000 and/or not more than 10 years imprisonment for the intent to sell, use for commercial advantage, personal gain, or malicious harm Protected Health Information 37
Obtainment/Disclosure of PHI z Not more than $50, 000 and/or not more than 1 year imprisonment z Not more than $100, 000 and/or not more than 5 years imprisonment if the offense is “under false pretenses” z Not more than $250, 000 and/or not more than 10 years imprisonment for the intent to sell, use for commercial advantage, personal gain, or malicious harm Protected Health Information 37
 HIPAA Philosophy What I see here, What I hear here, When I leave here, Remains here! 38
HIPAA Philosophy What I see here, What I hear here, When I leave here, Remains here! 38
 Other US Standards Groups: z AAMI z ANSI z ASQC z ASTM z IEEE z IES z IPC z NEMA z NFPA z OSHA z UL 39
Other US Standards Groups: z AAMI z ANSI z ASQC z ASTM z IEEE z IES z IPC z NEMA z NFPA z OSHA z UL 39
 Rest of World z British Standards Institute z European Committee for Normalization z European Committee for Electronic Standards z Tick. IT z International Committee on Radio Interference z Canadian Standards z IEEE z ISO (9000, 9001, 13485, 13488, 14000) z JSA 40
Rest of World z British Standards Institute z European Committee for Normalization z European Committee for Electronic Standards z Tick. IT z International Committee on Radio Interference z Canadian Standards z IEEE z ISO (9000, 9001, 13485, 13488, 14000) z JSA 40
 Trends: z. Harmonization of Regulations & Standards z. Attempts at defining Medical Informatics and the structures of medical records z. Computerization 41
Trends: z. Harmonization of Regulations & Standards z. Attempts at defining Medical Informatics and the structures of medical records z. Computerization 41


