4cacddd590690075c923e928ba9108b1.ppt
- Количество слайдов: 48
 REFRIGERANTS AND ENVIRONMENT
REFRIGERANTS AND ENVIRONMENT
 MARPOL 73/78 • Annexe VI contains Regulations for the Prevention of Air Pollution from Ships. • Divided into 3 chapters • Chapter I -General having 4 Regulations (1 -4) • Chapter II- Survey Certification of Means of Control having 7 Regulations (5 -11) • Chapter III- Requirements for Control of Emission from Ships having 8 Regulations (12 -19)
MARPOL 73/78 • Annexe VI contains Regulations for the Prevention of Air Pollution from Ships. • Divided into 3 chapters • Chapter I -General having 4 Regulations (1 -4) • Chapter II- Survey Certification of Means of Control having 7 Regulations (5 -11) • Chapter III- Requirements for Control of Emission from Ships having 8 Regulations (12 -19)
 MARPOL 73/78 Contd. CHAPTER I • Regulation 1 -Applications • Regulation 2 - Definitions • Regulation 3 - General Exceptions • Regulation 4 - Equivalents
MARPOL 73/78 Contd. CHAPTER I • Regulation 1 -Applications • Regulation 2 - Definitions • Regulation 3 - General Exceptions • Regulation 4 - Equivalents
 MARPOL 73/78 Contd. CHAPTER II • Regulation 5 -Surveys and Inspections • Regulation 6 -Issue of International Air Pollution Prevention Certificate • Regulation 7 -Issue of Certificate by another Government. • Regulation 8 -Form of Certificate
MARPOL 73/78 Contd. CHAPTER II • Regulation 5 -Surveys and Inspections • Regulation 6 -Issue of International Air Pollution Prevention Certificate • Regulation 7 -Issue of Certificate by another Government. • Regulation 8 -Form of Certificate
 MARPOL 73/78 Contd. CHAPTER II Contd. • Regulation 9 -Duration and Validity of Certificate • Regulation 10 -Port State Control on operational requirements • Regulation 11 -Detection of violations and enforcement
MARPOL 73/78 Contd. CHAPTER II Contd. • Regulation 9 -Duration and Validity of Certificate • Regulation 10 -Port State Control on operational requirements • Regulation 11 -Detection of violations and enforcement
 MARPOL 73/78 Contd. CHAPTER III • Regulation 12 - Ozone Depleting Substances • Regulation 13 - Nitrogen Oxides (NOx) • Regulation 14 - Sulphur Oxides (SOx) • Regulation 15 -Volatile Organic Compounds
MARPOL 73/78 Contd. CHAPTER III • Regulation 12 - Ozone Depleting Substances • Regulation 13 - Nitrogen Oxides (NOx) • Regulation 14 - Sulphur Oxides (SOx) • Regulation 15 -Volatile Organic Compounds
 MARPOL 73/78 Contd. CHAPTER III Contd. • Regulation 16 - Shipboard Incineration • Regulation 17 -Reception Facilities • Regulation 18 - Fuel Oil Quality • Regulation 19 - Requirements for platforms and drilling rigs
MARPOL 73/78 Contd. CHAPTER III Contd. • Regulation 16 - Shipboard Incineration • Regulation 17 -Reception Facilities • Regulation 18 - Fuel Oil Quality • Regulation 19 - Requirements for platforms and drilling rigs
 MARPOL 73/78 Contd. Regulation 12 deals with Ozone Depleting Substances • Definition: Ozone Depleting Substances means controlled substances defined in Paragraph 4 of article 1 of the Montreal Protocol on Substances that Deplete the Ozone Layer, 1987.
MARPOL 73/78 Contd. Regulation 12 deals with Ozone Depleting Substances • Definition: Ozone Depleting Substances means controlled substances defined in Paragraph 4 of article 1 of the Montreal Protocol on Substances that Deplete the Ozone Layer, 1987.
 MARPOL 73/78 Contd. Definition Contd. The following Halons are listed • Halon 1211 Bromochlorofluoromethane • Halon 1301 Bromotrifluoromethane • Halon 2402 1, 2 -Dibromo-1, 1, 2, 2 tetrafluoro ethane (also known as Halon 114 B 2)
MARPOL 73/78 Contd. Definition Contd. The following Halons are listed • Halon 1211 Bromochlorofluoromethane • Halon 1301 Bromotrifluoromethane • Halon 2402 1, 2 -Dibromo-1, 1, 2, 2 tetrafluoro ethane (also known as Halon 114 B 2)
 MARPOL 73/78 Contd. Definition Contd. The following Refrigerants are listed • CFC-11 Trichlorofluouromethane • CFC-12 Dichlorodifluouromethane • CFC-113 1, 1, 2 -Trichloro-1, 2, 2 trifluoroethane • CFC-114 1, 2 -Dichloro-1, 1, 2, 2 tetrafluoroethane • CFC-115 Chloropentafluoroethane
MARPOL 73/78 Contd. Definition Contd. The following Refrigerants are listed • CFC-11 Trichlorofluouromethane • CFC-12 Dichlorodifluouromethane • CFC-113 1, 1, 2 -Trichloro-1, 2, 2 trifluoroethane • CFC-114 1, 2 -Dichloro-1, 1, 2, 2 tetrafluoroethane • CFC-115 Chloropentafluoroethane
 MARPOL 73/78 Contd. Regulation 12 is about Ozone Depleting Substances • Subject to the provisions of Regulation 3, any deliberate emissions of ozone depleting substances shall be prohibited. • Deliberate emissions include emissions in the course of maintaining, servicing, repairing or disposing of systems or equipment except that deliberate emissions do not include minimal releases associated with the recapture or recycling
MARPOL 73/78 Contd. Regulation 12 is about Ozone Depleting Substances • Subject to the provisions of Regulation 3, any deliberate emissions of ozone depleting substances shall be prohibited. • Deliberate emissions include emissions in the course of maintaining, servicing, repairing or disposing of systems or equipment except that deliberate emissions do not include minimal releases associated with the recapture or recycling
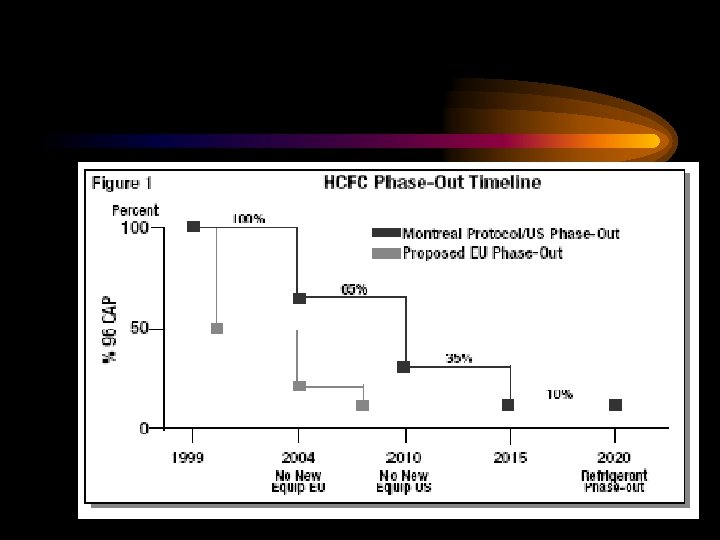
 MARPOL 73/78 Contd. Regulation 12 Contd. • Emissions arising from leakage of an ODS, whether or not the leaks are deliberate, may be regulated by Parties to the Protocol of 1997. • New installations which contain ODSs shall be prohibited on all ships, except that new installations containing hydrochlorofluoro carbons (HCFCs) are permitted until 1 January 2020.
MARPOL 73/78 Contd. Regulation 12 Contd. • Emissions arising from leakage of an ODS, whether or not the leaks are deliberate, may be regulated by Parties to the Protocol of 1997. • New installations which contain ODSs shall be prohibited on all ships, except that new installations containing hydrochlorofluoro carbons (HCFCs) are permitted until 1 January 2020.
 MARPOL 73/78 Contd. Regulation 12 Contd. • The substances referred to in this regulation and equipments containing such substances, shall be delivered to appropriate reception facilities when removed from ships.
MARPOL 73/78 Contd. Regulation 12 Contd. • The substances referred to in this regulation and equipments containing such substances, shall be delivered to appropriate reception facilities when removed from ships.
 WHAT ARE THE VARIOUS SUBSTANCES THAT CAN CAUSE OZONE DEPLETION? CFCs and HCFCs used in following applications: • Air Conditioning Fire fighting • Refrigeration Adhesives • Pesticides Aerosols • Plastic Foams Chemical feedstock etc. • Solvents
WHAT ARE THE VARIOUS SUBSTANCES THAT CAN CAUSE OZONE DEPLETION? CFCs and HCFCs used in following applications: • Air Conditioning Fire fighting • Refrigeration Adhesives • Pesticides Aerosols • Plastic Foams Chemical feedstock etc. • Solvents
 VARIOUS REFRIGERANTS • CFCs- R-12, R-113, R-114, R-115 etc. • HCFCs- R-22, R-123, R-124 etc. • HFCs- R-134 a, R-125, R-148 a, R 152 a etc.
VARIOUS REFRIGERANTS • CFCs- R-12, R-113, R-114, R-115 etc. • HCFCs- R-22, R-123, R-124 etc. • HFCs- R-134 a, R-125, R-148 a, R 152 a etc.
 VARIOUS REFRIGERANTS Contd. • Freon is Dupont’s trademark for its fluorocarbon refrigerants. • All Refrigerants have one Carbon atom as their origin is from Methane (CH 4) or Ethane C 2 H 6 where one or 2 Hydrogen atoms have been replaced with Chlorine, Fluorine or Bromine.
VARIOUS REFRIGERANTS Contd. • Freon is Dupont’s trademark for its fluorocarbon refrigerants. • All Refrigerants have one Carbon atom as their origin is from Methane (CH 4) or Ethane C 2 H 6 where one or 2 Hydrogen atoms have been replaced with Chlorine, Fluorine or Bromine.
 VARIOUS REFRIGERANTS Contd. Methane Derived Refrigerants • R No. viz. R-11, R-12, R-22 etc. First figure shows the number of Hydrogen atoms plus one. Second figure shows the number of Fluorine atoms. The total number of Hydrogen and other replacement atoms should be 4
VARIOUS REFRIGERANTS Contd. Methane Derived Refrigerants • R No. viz. R-11, R-12, R-22 etc. First figure shows the number of Hydrogen atoms plus one. Second figure shows the number of Fluorine atoms. The total number of Hydrogen and other replacement atoms should be 4
 VARIOUS REFRIGERANTS Contd. Methane Derived Refrigerants Contd. • R-11: CCl 3 F CFC • R-13: CCl. F 3 CFC • R-22: CHCl. F 2 HCFC • R-12: CCl 2 F 2 Dichlorodifluoro. Methane CFC • R 113: CCl 2 F/CCl. F 2 • R 501: Mixture 75%-R 22 and 25%-R 12 • R 502: Mixture 75%-R 12 and 25%-R 22
VARIOUS REFRIGERANTS Contd. Methane Derived Refrigerants Contd. • R-11: CCl 3 F CFC • R-13: CCl. F 3 CFC • R-22: CHCl. F 2 HCFC • R-12: CCl 2 F 2 Dichlorodifluoro. Methane CFC • R 113: CCl 2 F/CCl. F 2 • R 501: Mixture 75%-R 22 and 25%-R 12 • R 502: Mixture 75%-R 12 and 25%-R 22
 VARIOUS REFRIGERANTS Contd. Ethane Derived Refrigerants • R No: viz. R 113, R 114, R 115 etc. First Figure shows number of Carbon atoms minus 1 Second figure shows number of Hydrogen atoms plus 1 Third figure shows number of Fluorine atoms.
VARIOUS REFRIGERANTS Contd. Ethane Derived Refrigerants • R No: viz. R 113, R 114, R 115 etc. First Figure shows number of Carbon atoms minus 1 Second figure shows number of Hydrogen atoms plus 1 Third figure shows number of Fluorine atoms.
 VARIOUS REFRIGERANTS Contd. Ethane Derived Refrigerants Contd. • R-113 - 1, 1, 2 Trichloro 1, 2, 2 Trifluoro ethane • R-114 - 1, 2 Dichloro 1, 1, 2, 2 Tetrafluoroethane • R-115 - Chloropentafluoroethane • R-134 a C 2 H 2 F 4 Tetra Fluoro Ethane (a stands for isomer- same chemical composition but different atomic arrangement)
VARIOUS REFRIGERANTS Contd. Ethane Derived Refrigerants Contd. • R-113 - 1, 1, 2 Trichloro 1, 2, 2 Trifluoro ethane • R-114 - 1, 2 Dichloro 1, 1, 2, 2 Tetrafluoroethane • R-115 - Chloropentafluoroethane • R-134 a C 2 H 2 F 4 Tetra Fluoro Ethane (a stands for isomer- same chemical composition but different atomic arrangement)
 KEY REFRIGERANT CHARACTERISTICS AFFECTING ENVIRONMENT • • Ozone Depletion Potential ODP Global Warming Potential GWP Chlorine leading Potential Atmospheric Life (years)
KEY REFRIGERANT CHARACTERISTICS AFFECTING ENVIRONMENT • • Ozone Depletion Potential ODP Global Warming Potential GWP Chlorine leading Potential Atmospheric Life (years)
 MONTREAL PROTOCOL • Signed on September 16, 1987 under the auspecies of IMO. • Seeks to inhibit production, consumption and trade of ozone depleting compounds. • Also distinguishes between developed countries with higher consumption and developing countries with lower countries.
MONTREAL PROTOCOL • Signed on September 16, 1987 under the auspecies of IMO. • Seeks to inhibit production, consumption and trade of ozone depleting compounds. • Also distinguishes between developed countries with higher consumption and developing countries with lower countries.
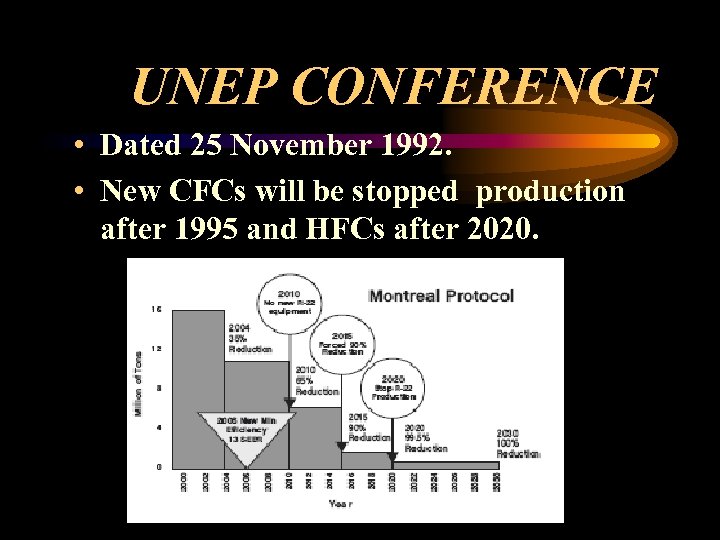 UNEP CONFERENCE • Dated 25 November 1992. • New CFCs will be stopped production after 1995 and HFCs after 2020.
UNEP CONFERENCE • Dated 25 November 1992. • New CFCs will be stopped production after 1995 and HFCs after 2020.
 ENVIRONMENT Definition The conditions and influences of the place in which an organism lives. In layman’s terms, it is known as Surroundings.
ENVIRONMENT Definition The conditions and influences of the place in which an organism lives. In layman’s terms, it is known as Surroundings.
 ECOLOGY Definition The Study of relationship between living organisms and their Environment.
ECOLOGY Definition The Study of relationship between living organisms and their Environment.
 ATMOSPHERE Definition The layer of gas surrounding a planet or a star. • Earth’s Atmosphere Composition Oxygen: 21% Nitrogen: 78% CO 2, H 2, Argon and other Gases: 1%
ATMOSPHERE Definition The layer of gas surrounding a planet or a star. • Earth’s Atmosphere Composition Oxygen: 21% Nitrogen: 78% CO 2, H 2, Argon and other Gases: 1%
 ATMOSPHERE Contd. Earth’s Atmosphere consists of the following layers TROSPHERE Closest to Earth’s surface upto 14 kms height STRATOSPHERE upto 44 km height IONOSPHERE consisting of MESOSPHERE upto 80 km height and THERMOSPHERE above 80 km height • Each of these layers is seperated by TROPOPAUSE, STRATOPAUSE, AND
ATMOSPHERE Contd. Earth’s Atmosphere consists of the following layers TROSPHERE Closest to Earth’s surface upto 14 kms height STRATOSPHERE upto 44 km height IONOSPHERE consisting of MESOSPHERE upto 80 km height and THERMOSPHERE above 80 km height • Each of these layers is seperated by TROPOPAUSE, STRATOPAUSE, AND
 OZONE • Formed by the action of Ultra Violet Light from the sun on Oxygen in the atmosphere. • Boiling Point is -112 deg C. • Chemically very active, can readily react with any other chemical atoms present closeby. • It has a great affinity to Chlorine and can engage in a catalytic reaction in which each chlorine fragment can destroy upto 100000 ozone molecules before the chemical processes remove the chlorine
OZONE • Formed by the action of Ultra Violet Light from the sun on Oxygen in the atmosphere. • Boiling Point is -112 deg C. • Chemically very active, can readily react with any other chemical atoms present closeby. • It has a great affinity to Chlorine and can engage in a catalytic reaction in which each chlorine fragment can destroy upto 100000 ozone molecules before the chemical processes remove the chlorine
 OZONE LAYER • Present in Stratosphere at heights 11 km to 50 km above Earth’s surface. Stratosphere temperature ranges from 56 deg C to -2 deg C. • Most concentrated at height of 22 km. • Highest concentration of about 10 ppm. • It accounts for less than one-millionth part of Earth’s Atmosphere. • But it is of vital importance for Earth’s life-form.
OZONE LAYER • Present in Stratosphere at heights 11 km to 50 km above Earth’s surface. Stratosphere temperature ranges from 56 deg C to -2 deg C. • Most concentrated at height of 22 km. • Highest concentration of about 10 ppm. • It accounts for less than one-millionth part of Earth’s Atmosphere. • But it is of vital importance for Earth’s life-form.
 OZONE LAYER Contd. • The ozone layer is not constantly thick all over the earth’s surface. • Ozone Molecules are formed over the tropics and are delivered along with the chlorine to the Arctic and Antarctic with atmospheric Motion. • A circulation pattern called Antarctic and Acrtic Polar Vortex traps the ozone near the poles for several months.
OZONE LAYER Contd. • The ozone layer is not constantly thick all over the earth’s surface. • Ozone Molecules are formed over the tropics and are delivered along with the chlorine to the Arctic and Antarctic with atmospheric Motion. • A circulation pattern called Antarctic and Acrtic Polar Vortex traps the ozone near the poles for several months.
 OZONE LAYER Contd. IMPORTANCE OF OZONE LAYER • It absorbs the UV-B rays while allowing the heat generating infra-red rays to reach the earth. This heat is lifesustaining on the planet keeeping the temperatures of planet at desired levels. • It influences the weather by stabilizing the stratosphere which acts as a cap to the turbulent weather system in the troposphere.
OZONE LAYER Contd. IMPORTANCE OF OZONE LAYER • It absorbs the UV-B rays while allowing the heat generating infra-red rays to reach the earth. This heat is lifesustaining on the planet keeeping the temperatures of planet at desired levels. • It influences the weather by stabilizing the stratosphere which acts as a cap to the turbulent weather system in the troposphere.
 FORMATION OF OZONE LAYER • Ultra Violet Radiation below 190 nm is removed before it reaches Stratosphere by atmospheric nitrogen and oxygen. • U V Radiation between 190 nm and 340 nm is removed in Stratosphere by the reaction O 2 + h. V = O + O* • There is continuous cyclic formation and destruction of ozone due to foll: reactions O + O* =O 3 + M*; O 3 + h. V = O 3 + O 2 = 2 O 2
FORMATION OF OZONE LAYER • Ultra Violet Radiation below 190 nm is removed before it reaches Stratosphere by atmospheric nitrogen and oxygen. • U V Radiation between 190 nm and 340 nm is removed in Stratosphere by the reaction O 2 + h. V = O + O* • There is continuous cyclic formation and destruction of ozone due to foll: reactions O + O* =O 3 + M*; O 3 + h. V = O 3 + O 2 = 2 O 2
 • FORMATION OF OZONE LAYER in Contd. The absorption of UVRadiation Stratosphere prevents virtually all radiation below 290 nm and mostly between 290 nm and 320 nm from reaching the earth. • Above the Stratosphere, the density of gases is so low that O 2 atoms rarely find other molecules to collide with; so ozone is not found in abundance. • Below the ozone layer, too little solar radiation penetrates to allow appreciable
• FORMATION OF OZONE LAYER in Contd. The absorption of UVRadiation Stratosphere prevents virtually all radiation below 290 nm and mostly between 290 nm and 320 nm from reaching the earth. • Above the Stratosphere, the density of gases is so low that O 2 atoms rarely find other molecules to collide with; so ozone is not found in abundance. • Below the ozone layer, too little solar radiation penetrates to allow appreciable
 DEPLETION OF OZONE LAYER Contd. Process • Chlorine in Stratosphere becomes trapped in so called reservoir compounds such as Hydrogen Chloride and Chlorine Nitrate which themselves do not destroy Ozone. • Once Stratosphere becomes cold enough to freeze closed particles, ice crystals provide surfaces on which reactions can occur.
DEPLETION OF OZONE LAYER Contd. Process • Chlorine in Stratosphere becomes trapped in so called reservoir compounds such as Hydrogen Chloride and Chlorine Nitrate which themselves do not destroy Ozone. • Once Stratosphere becomes cold enough to freeze closed particles, ice crystals provide surfaces on which reactions can occur.
 DEPLETION OF OZONE LAYER Contd. Process • Chlorine Nitrate Cl. ONO 2 reacts with Hydrochloric Acid present on ice surface, producing molecular chlorine and Nitric Acid. • Nitric Acid remains bonded to the ice and molecular Chlorine is quickly broken down into Atomic Chlorine which reacts with Ozone destroying it through production of chlorine Monoxide and Molecular Oxygen.
DEPLETION OF OZONE LAYER Contd. Process • Chlorine Nitrate Cl. ONO 2 reacts with Hydrochloric Acid present on ice surface, producing molecular chlorine and Nitric Acid. • Nitric Acid remains bonded to the ice and molecular Chlorine is quickly broken down into Atomic Chlorine which reacts with Ozone destroying it through production of chlorine Monoxide and Molecular Oxygen.
 DEPLETION OF OZONE LAYER Contd. Process Contd. • Chlorine Monoxide undergoes further reactions that reform a chlorine atom which is free to destroy another Ozone molecule. • The Ozone depleted is transported from the polar regions to lower latitudes through wind in the late spring when antarctic Vortex breaks up.
DEPLETION OF OZONE LAYER Contd. Process Contd. • Chlorine Monoxide undergoes further reactions that reform a chlorine atom which is free to destroy another Ozone molecule. • The Ozone depleted is transported from the polar regions to lower latitudes through wind in the late spring when antarctic Vortex breaks up.
 DEPLETION OF OZONE LAYER Contd. Process Contd. • Ozone depletion process is accelerated during presence of sunlight and low temperatures.
DEPLETION OF OZONE LAYER Contd. Process Contd. • Ozone depletion process is accelerated during presence of sunlight and low temperatures.
 DEPLETION OF OZONE LAYER Contd. Where does the Chlorine come from? • CFCs are one particular class of Refrigerants and Fire Extinguishing Mediums containing Chlorine and Fluorine Atoms. Ex. R-12, R-11. • They are chemically very stable and remain unchanged in the lower atmospheric layer (Troposphere). • Upon reaching Stratosphere, they encounter high energy Ultra Violet light which breaks them releasing Chlorine atoms.
DEPLETION OF OZONE LAYER Contd. Where does the Chlorine come from? • CFCs are one particular class of Refrigerants and Fire Extinguishing Mediums containing Chlorine and Fluorine Atoms. Ex. R-12, R-11. • They are chemically very stable and remain unchanged in the lower atmospheric layer (Troposphere). • Upon reaching Stratosphere, they encounter high energy Ultra Violet light which breaks them releasing Chlorine atoms.
 DEPLETION OF OZONE LAYER Contd. Chlorine Levels in Stratosphere • 1970 - 1. 2 parts per billion • 1985 - 3. 0 parts per billion • 2050 projected - 8. 2 parts per billion 1 Chlorine Atom can destroy upto 100000 ozone molecules before the chemical processes remove the Chlorine from the Atmosphere.
DEPLETION OF OZONE LAYER Contd. Chlorine Levels in Stratosphere • 1970 - 1. 2 parts per billion • 1985 - 3. 0 parts per billion • 2050 projected - 8. 2 parts per billion 1 Chlorine Atom can destroy upto 100000 ozone molecules before the chemical processes remove the Chlorine from the Atmosphere.
 DEPLETION OF OZONE LAYER Contd. • Ozone Hole keeps forming over Antarctica during certain months of the year. • The lowest ozone levels were recorded over South Pole in the first 2 weeks of October 1987.
DEPLETION OF OZONE LAYER Contd. • Ozone Hole keeps forming over Antarctica during certain months of the year. • The lowest ozone levels were recorded over South Pole in the first 2 weeks of October 1987.
 DEPLETION OF OZONE LAYER Contd. What happens if the atmospheric Ozone Layer gets depleted? • Ultra Violet rays from sunlight can reach the Earth can damage human immune system, can cause cataracts, increase incidence of skin cancer. • Every 1% depletion of Stratospheric Ozone, results in 2% rise in skin cancer. • In USA, 3000000 to 4000000 new cases of skin cancer are reported every year.
DEPLETION OF OZONE LAYER Contd. What happens if the atmospheric Ozone Layer gets depleted? • Ultra Violet rays from sunlight can reach the Earth can damage human immune system, can cause cataracts, increase incidence of skin cancer. • Every 1% depletion of Stratospheric Ozone, results in 2% rise in skin cancer. • In USA, 3000000 to 4000000 new cases of skin cancer are reported every year.
 DEPLETION OF OZONE LAYER Contd. • Effects on Plants: Reduced leaf size, stunted growth, poor seed quality, susceptibility to weeds, disease and pests. • Marine Food Chain can be killed with significant UV Radiation. • Lesser amounts of UV Radiation causes slowdown of Photosynthesis.
DEPLETION OF OZONE LAYER Contd. • Effects on Plants: Reduced leaf size, stunted growth, poor seed quality, susceptibility to weeds, disease and pests. • Marine Food Chain can be killed with significant UV Radiation. • Lesser amounts of UV Radiation causes slowdown of Photosynthesis.
 OBLIGATIONS OF MARINE FRATERNITY • Do not vent out CFCs and HCFCs into the atmosphere. • Use CFC to HFC conversion kits that are available for existing equipment. • When ordering for new Equipment /Machinery for Refrigeration and Air Conditioning, ensure to use only HFC Refrigerant based Machinery. • Educate and Train the floating staff regarding the importance of these steps.
OBLIGATIONS OF MARINE FRATERNITY • Do not vent out CFCs and HCFCs into the atmosphere. • Use CFC to HFC conversion kits that are available for existing equipment. • When ordering for new Equipment /Machinery for Refrigeration and Air Conditioning, ensure to use only HFC Refrigerant based Machinery. • Educate and Train the floating staff regarding the importance of these steps.


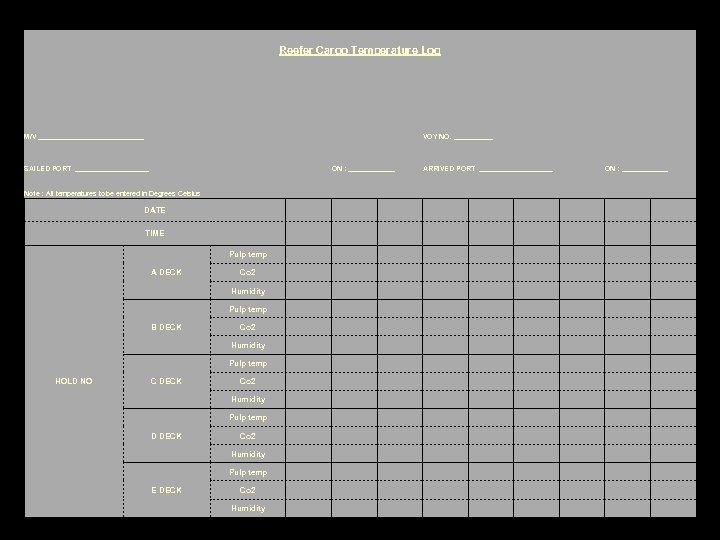 Reefer Cargo Temperature Log M/V ______________ SAILED PORT __________ ON : ______ VOY NO. _____ ARRIVED PORT __________ ON : ______ Note : All temperatures to be entered in Degrees Celsius DATE TIME Pulp temp A DECK Co 2 Humidity Pulp temp B DECK Co 2 Humidity Pulp temp HOLD NO C DECK Co 2 Humidity Pulp temp D DECK Co 2 Humidity Pulp temp E DECK Co 2 Humidity
Reefer Cargo Temperature Log M/V ______________ SAILED PORT __________ ON : ______ VOY NO. _____ ARRIVED PORT __________ ON : ______ Note : All temperatures to be entered in Degrees Celsius DATE TIME Pulp temp A DECK Co 2 Humidity Pulp temp B DECK Co 2 Humidity Pulp temp HOLD NO C DECK Co 2 Humidity Pulp temp D DECK Co 2 Humidity Pulp temp E DECK Co 2 Humidity



