9571532975b776f3864208372b7cb8f0.ppt
- Количество слайдов: 66


REFERENCE DOCUMENT: Cleaning Validation A practical approach by Gil Bismuth and Shosh Neumann PDA Journal of Pharmaceutical Science and Technology Establishing Scientifically Justified Acceptance Criteria for Cleaning Validation of Finished Drug Products by Desitin A. Le. Blanc 2

Objective of Training To impart the basics and fundamentals of Cleaning validation in general. 3

CLEANING VALIDATION The validation of cleaning method is an important element for both qualification and process validation of drug substance and drug product manufacturing. 4

Objective: To attain documented evidence, which provides a high degree of assurance that the Cleaning procedure can effectively remove residues of a product and a cleaning agent from the manufacturing equipment, to a level that does not raise patient safety concerns. 5

Advantages of Validation a. Reduction of quality costs. b. Assurance of Quality & Safety. c. Government regulations. d. Making good business sense. e. Less down time, fewer batch failures and may operate / clean more efficiently. 6

Limitations a. Cost incurred. b. People. c. Delays. d. Inadequate equipments. 7
Elements / components of validation a. Analytical test procedure. b. Calibration of instruments. c. Operator qualification. d. Equipment qualification. 8

REGULATORY REQUIREMENTS • As per the FDA, 21 CFR part 211, subpart D, “Equipments and utensils shall be cleaned, maintained and sanitized at appropriate intervals to prevent malfunctions or contamination that would alter the safety, identity, strength, quality or purity of the drug product beyond the official or other established requirement. Written procedures shall be established and followed for cleaning”. 9

CLEANING MECHANISMS • Several basic mechanisms exist to remove residues from equipment, including mechanical action, dissolution, detergency and chemical reaction. 10

CLEANING MECHANISMS • Mechanical action refers to physical actions such as brushing, scrubbing and pressurized water to remove particulates. 11

CLEANING MECHANISMS • Dissolution involves dissolving residues with a suitable solvent. • The most common and practical solvent is water because of its advantages: water is non-toxic, cheap, does not leave residues, and is environment friendly. 12

CLEANING MECHANISMS However, in some cases it may be preferable to use a non-aqueous solvent or a combination of both aqueous and non-aqueous solvents due to the solubility characteristics of the materials. Alkaline or acidic solvents, for example, can enhance dissolution of the materials and could be 13 advantageous.

CLEANING MECHANISMS • Detergency requires the use of surfactant, usually in an aqueous system. Detergents act in four different ways: wetting agents, solubilizers, emulsifiers, and dispersants. Usually detergents posses all these properties which broaden their action. 14

CLEANING MECHANISMS • Chemical reactions, such as oxidation and hydrolysis in which the residues are chemically changed. Example: Sodium Hypochloride • During cleaning validation, the effectiveness of these mechanisms must be challenged and checked as a whole in the cleaning procedure. 15

CLEANING PROCEDURE (SOP) Standard Operating Procedure (SOP) should be required duly approved by approving authority and it should specify the following: 1. Precautions and safety warning. 2. Cleaning tools and materials with their name, concentration, their dilution instruction, volume requirement, storage period and 16 requirements.

CLEANING PROCEDURE (SOP) 3. Time limitations: a. time between end of manufacturing and start of cleaning. b. time between final rinse and drying. c. frequency of major cleaning for mfg. batches of the same product in campaign. d. time until additional cleaning is performed for unused clean equipment. 17

CLEANING PROCEDURE (SOP) 4. Cleaning level: MINOR between two batches of same product or between different strengths (inter convertible formula) of the same product. For minor cleaning, cleaning validation is not required, since cross contamination is not an issue. MAJOR between two products. In this case, validation of the effectiveness of the cleaning procedure in removing residues to the required level is mandatory. 18

CLEANING PROCEDURE (SOP) 5. Cleaning procedure covers following steps… Dismantling, Initial washing, Final washing & Parameters such as Time, Temperature, Volume, Flow rate etc. should be mentioned. 19

CLEANING PROCEDURE (SOP) 6. Drying : is very important to prevent microbiological proliferation. Sometimes the final rinse is conducted with hot purified water to facilitate evaporation of the water. Drying time and temperature should defined. 7. Visual inspection : No traces or particles visible to the naked eye should be observed after the cleaning. 20

CLEANING PROCEDURES 8. Cleaned status has to be indicated by putting a label or a card on the clean equipment. 9. Storage place of cleaned equipment / utensils with proper wrapping & instructions clearly mentioned. 10. Cleaning log should be maintained. 21

THE CLEANING VALIDATION PROGRAMME 1. Selection of cleaning method 2. Selecting the Scientific basis for the contamination limit 3. Selecting the Worst case related to the equipment 4. Selecting the Worst case related to the product 5. Establishing the storage period after cleaning. 6. Selecting the sampling method 7. Selecting the analytical method 8. Documentation 22

1. SELECTION OF CLEANING METHOD A. CLEAN-IN-PLACE ( CIP ) METHOD v Cleaning of the equipment is performed in place without disassembling v Cleaning process may be controlled manually or by an automated program. v Very consistent and reproducible cleaning method. v Can be validated readily. v Being a closed system visual inspection of all components is difficult. 23

1. SELECTION OF CLEANING METHOD B. CLEAN-OUT-OF-PLACE (COP) METHOD v Cleaning of disassembled equipment is performed in a central washing machine. v The washing machine also requires validation such as the temperature, ultrasonic activity, cycle time, cleaning operation sequence, detergent quantity dispensed etc. 24

1. SELECTION OF CLEANING METHOD C. MANUAL CLEANING METHOD v Difficult to validate v Most extensive and elaborate cleaning procedures are required. v A high quality and extensive training program is required. 25

1. SELECTION OF CLEANING METHOD Torrent Pharmaceuticals Limited have designed the facility with manual cleaning operations. Following were taken into consideration for selecting manual cleaning method. § “Seeing is Believing” § Product diversity / range § Risk of failure of cleaning equipments § Validation of automated cleaning equipments § Trained and experienced working staff 26

1. SELECTION OF CLEANING METHOD • The equipment design and manual cleaning method are taken into consideration for selection of equipment. All equipments selected viewing following cleaning considerations: § Ease of disassembling of contact parts § All contact surfaces are non-reactive to cleaning method § Dedicated disposable materials where difficult to clean e. g. FBD bags, filters, Disposable bags in transit containers Least chance of contamination from equipment noncontact parts e. g. lubricants, gaskets, drive system, mechanical seal etc. 27

1. SELECTION OF CLEANING METHOD The risk involved in manual cleaning processes is taken care of with following: § Proper washroom design with drying, protection and storage requirement. § Detailed cleaning SOP § Training of cleaning operators 28
2. SELECTING THE SCIENTIFIC BASIS FOR THE CONTAMINATION LIMIT • Limit calculation is done on the basis of smallest therapeutic dose: Factors such as the batch size of the next product, the route of administration, and the largest daily dose of subsequent product, which might be administered, are important in the calculation. All of these factors mentioned previously are usually summarized in an equation, which may take the following general form: 29

2. SELECTING THE SCIENTIFIC BASIS FOR THE CONTAMINATION LIMIT MAR = TD x BS x SF LDD Where, MAR = the max. allowable Residue TD = Smallest therapeutic dose amongst all products. BS = Batch size of the next product to be manufactured in the same equipment, which has the least value of BS/LDD within the group, is taken SF = The safety factor LDD = The largest daily dose amongst the next product 30 to be manufactured in the same equipment.
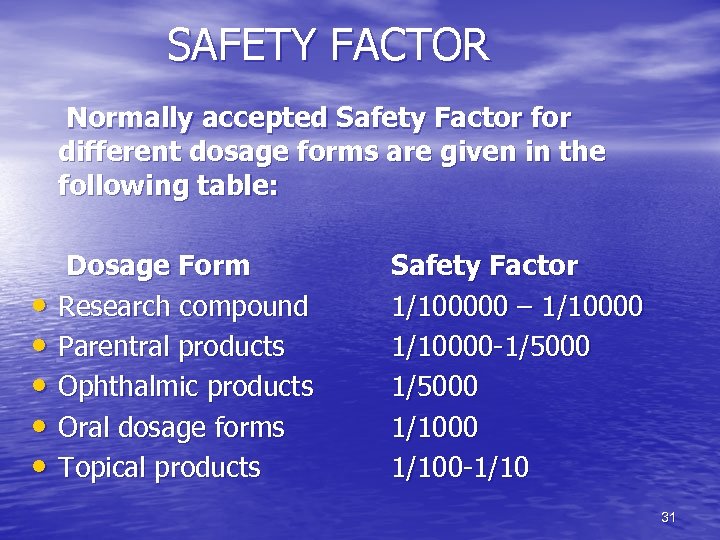
SAFETY FACTOR Normally accepted Safety Factor for different dosage forms are given in the following table: Dosage Form • Research compound • Parentral products • Ophthalmic products • Oral dosage forms • Topical products Safety Factor 1/100000 – 1/10000 -1/5000 1/1000 1/100 -1/10 31

2. SELECTING THE SCIENTIFIC BASIS FOR THE CONTAMINATION LIMIT • Contamination Limit Based on Other Considerations: · Not Detectable This type of limit refers to a specific analytical method. · Absolute Limit An absolute limit also termed “single limit” or “blanket specification” means that the same limit is set for any product, without consideration to toxicological data or to the detection level of the analytical method, and is usually expressed in parts per million (ppm) 32
2. SELECTING THE SCIENTIFIC BASIS FOR THE CONTAMINATION LIMIT • Acceptance Criterion based on Visual Inspection: The visual detection limits of most active ingredients is approximately 4 g / cm 2 33

2. SELECTING THE SCIENTIFIC BASIS FOR THE CONTAMINATION LIMIT THE APPROACH TAKEN Dose related contamination limit shall be determined. If this limit is more than 10 ppm, then a value of less than 10 ppm shall be the acceptance limit. Samples for testing the residue shall be taken only if the equipment surface is visually clean. 34

MAR LIMIT IN PPM To determine the MAR in ppm would be = MAR limit in milligram . B. Size of subsequent product in kg If this calculation gives a value more than 10 ppm, equivalent value of 10 ppm in milligram has to be calculated, this would be ……. 10 x MAR limit in subsequent batch in units of milligram MAR limit in subsequent batch in units of ppm 35

MAR LIMIT CALCULATION Following is the relevant data for calculation. Ø Previous Product : · Active ingredient : Alprazolam. · Strength / tablet : 0. 25 mg. · Minimum Dose / day : 1 tablet. Ø Next product tablet. · Average weight of tablet : 742 mg. · Maximum dose /day : 9 tablets. · Batch Size of T 500 tablet : 48. 23 kg. 36
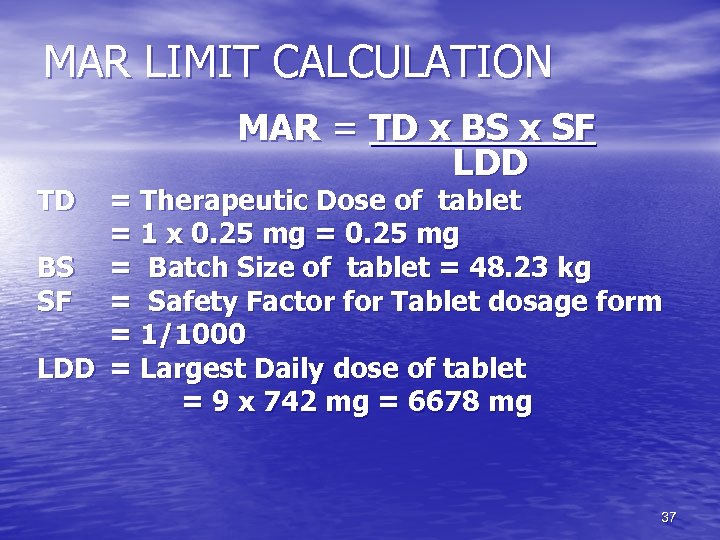
MAR LIMIT CALCULATION MAR = TD x BS x SF LDD TD = Therapeutic Dose of tablet = 1 x 0. 25 mg = 0. 25 mg BS = Batch Size of tablet = 48. 23 kg SF = Safety Factor for Tablet dosage form = 1/1000 LDD = Largest Daily dose of tablet = 9 x 742 mg = 6678 mg 37

MAR LIMIT CALCULATION • MAR = 0. 25 x 48. 23 x 1000 / 6678 x 1000 = 1. 8 mg. • Therefore, 1. 8 mg of Alprazolam in 48. 23 kg of tablet is the MAR limit. • This, in units of ppm, is equivalent to 1. 8 mg / 48. 23 kg = 0. 0373 ppm. • Since the value of 0. 0373 is less than the 10 ppm criteria, 1. 8 mg is considered as the acceptance criteria. 38

3. SELECTING THE WORST CASE RELATED TO THE EQUIPMENT • Identical, interchangeable piece of equipment with the same cleaning procedure can be grouped together. • Equipment with the same operating principle and the same cleaning procedure, but with different product contact surface area, can be grouped, if they can be interchanged. 39

3. SELECTING THE WORST CASE RELATED TO THE EQUIPMENT • The worst case for a group of equipment is represented by the equipment with the larger product contact surface and the hardest-toclean locations. 40

4. SELECTING THE WORST CASE RELATED TO THE PRODUCT: Only one product out of a group of product processed in a piece of equipment is selected for the cleaning validation study, based on the lowest solubility of the active ingredient and its therapeutic dose. 41

4. SELECTING THE WORST CASE RELATED TO THE PRODUCT: • To arrive at the worst case equipment and worst case product we need Equipment Database and Product Database. 42

5. ESTABLISHING THE STORAGE PERIOD AFTER CLEANING The objective for establishing time limit between equipment cleaning and reuse is to ensure that the equipment remains clean till the next use. This needs demonstration that there is no microbial proliferation in cleaned equipments during storage. 43

5. ESTABLISHING THE STORAGE PERIOD AFTER CLEANING For establishing the time limit, the equipment should be dried. Initial swab samples for surface should be taken. Thereafter, the equipment should be protected as prescribed in the SOP and stored in its designated area. Periodic samples of product contact surface for microbiological contamination should be taken. (1 st day, 2 nd day, 3 rd day etc. ) Based on the data generated establish the acceptable time limit. 44

5. ESTABLISHING THE STORAGE PERIOD AFTER CLEANING • Cleaned equipment surface sample (contact surface only) test results should demonstrate absence of specified micro organisms (E. Coli, Salmonella etc. ) • The acceptance level for surface sampling is 25 CFU per 25 sq. cm. • Representative colonies of the microorganisms isolated should be identified in order to build a plant microbial flora baseline with the aim of locating and eliminating potential contamination sources. 45
6. SELECTING THE SAMPLING METHOD The two main sampling methods are: 1. Swab sampling. 2. Rinse sampling. 46
6. SELECTING THE SAMPLING METHOD 6. 1 Swab sampling method • This method is based on the physical removal of residue left over on a piece of equipment after it has been cleaned and dried. A swab wetted with a solvent is rubbed over a previously determined sample surface area to remove any potential residue, and thereafter extracted into a known volume of solvent in which the contaminant active ingredient residue is soluble. The amount of contaminant per swab is then determined by an analytical method of adequate sensitivity. 47
6. SELECTING THE SAMPLING METHOD Advantages of swab sampling method. • Direct evaluation of surface contamination. • Insoluble or poorly soluble substances may be physically removed from the equipment surfaces. • Hard-to-clean but accessible areas are easily incorporated into the final evaluation. Disadvantages of swab sampling method. • Difficult to implement in large-scale manufacturing equipment. • Extrapolation of results obtained for a small sample surface area to the whole product contact surface area. 48

6. SELECTING THE SAMPLING METHOD 6. 2 Rinse sampling method • This method is based on the analytical determination of a sample of the last rinsing solvent (generally water) used in the cleaning procedure. The volume of solvent used for the last rinse must be known to allow for the quantitative determination of the contamination. 49
6. SELECTING THE SAMPLING METHOD • • Advantages of Rinse sample method. Ease of sampling. Evaluation of entire product contact surface. Accessibility of all equipment parts to the rinsing solvent. Best fitted to sealed or large scale equipment and equipment which is not easily or routinely disassembled. Disadvantages of Rinse sample method. No physical removal of the contaminant. The rinsing solvent may not reach inaccessible part of equipment. Use of organic solvents for water insoluble 50 materials.
6. SELECTING THE SAMPLING METHOD Looking at the advantages and disadvantages of both the sampling methods swab sampling method was selected. The cleaning procedure uses water as a solvent and we have dosage forms having active ingredient which is insoluble in water. 51

SAMPLING LOCATIONS & NUMBER OF SAMPLES The sample locations are dictated by worst-case conditions. The equipment’s hard to clean locations are identified based on cleaning experience and the design of equipment. The number of samples should take into consideration the equipment surface area, design, shape, operating principle and construction material. 52
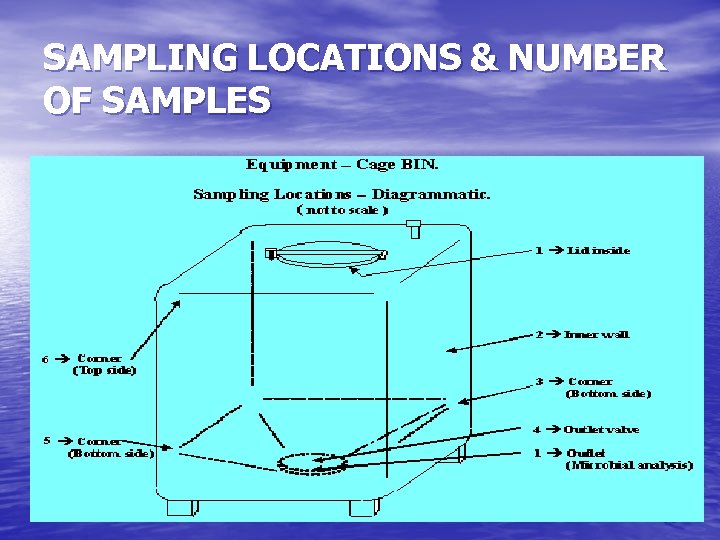
SAMPLING LOCATIONS & NUMBER OF SAMPLES 53

SAMPLE SURFACE AREA Sample surface areas usually vary from 25 sq. cm to 100 sq. cm. The swab area chosen is 100 sq. cm. SWAB RECOVERY STUDY A swab recovery study is performed to determine the ability of the swab to quantitatively remove the contaminant from the surface sampled. The swab taken is Whatman– Carbon free paper. 54

7. SELECTING METHOD THE ANALYTICAL • The Basic Requirements for the Analytical Method. 1. The sensitivity of the method shall be appropriate to the calculated contamination limit. 2. The method shall be practical and rapid, and, as much as possible use instrumentation existing in the company. 3. The method shall be validated in accordance with ICH, USP and EP requirements. 4. The analytical development shall include a recovery study to challenge the sampling and testing methods. 55

7. SELECTING METHOD THE ANALYTICAL • SPECIFIC METHODS ØChromatographic methods such as LC/MS, GC/MS, and HPLC Ø Thin layer chromatography Ø Specific ion meter Of the above methods, chromatography methods are the methods of choice, as they separate analytes, are highly specific, highly sensitive, and quantitative. But the methods are costly and time consuming. 56

7. SELECTING METHOD THE ANALYTICAL The chromatography method (HPLC) was selected for cleaning validation studies because of their sensitivity, specificity, and ability to quantify. The method should be validated. 57
7. SELECTING METHOD THE ANALYTICAL • NON-SPECIFIC METHODS. Ø Spectrophotometric methods in the visible, infrared, or UV ranges Ø Total organic carbon (TOC) Ø Other Methods For monitoring cleaning procedure TOC method is used. It offers at a moderate cost and in addition to its rapidity, a detection capability down to the ppb range. 58

8. DOCUMENTATION • Validation Master Plan • Technical Transfer Information such as solubility of product ingredients, therapeutic dose, equipments to be used and its contact surface area, batch size, minimum daily dose, maximum daily dose etc for the preparation of Equipment Matrix & Product Matrix. • Equipment Qualification Reports (DQ/IQ/OQ/PQ) 59

8. DOCUMENTATION • Equipment logs • Training records • Method validation of analytical test method. • Cleaning Validation Protocol • Cleaning Validation Reports 60

8. DOCUMENTATION Cleaning Validation Protocol contains: Protocol pre-approvals Objective of the validation process. Scope Responsibility for performing & approving validation study. Product details Acceptance criteria 61

8. DOCUMENTATION Cleaning procedure (Ref. SOP) for each equipment. Sampling location for chemical and microbial testing Sampling procedure and sampling plan for chemical and microbial testing. Verification and Revalidation criteria. Sampling location – Diagrammatic Test data slip for chemical and microbial analysis. 62

8. DOCUMENTATION Summary report contains Summary Report approval. Equipments used Study performed ( Including recovery studies where appropriate. Analytical Methods including the limit of detection and limit of quantitation of those methods. ) Acceptance criteria Observation and Results Evaluation Conclusion and recommendation 63

At least three consecutive applications of the cleaning procedure should be performed and shown to be successful in order to prove that the method is validated. It is usually not considered acceptable to “test until clean”. 64

The validation of cleaning method is only meaningful if the written cleaning procedures (on which the validation is based) are always meticulously followed. 65

Thanks 66
9571532975b776f3864208372b7cb8f0.ppt