rectification.pptx
- Количество слайдов: 11

Rectification { It’s a obtaining alcohol 96%

• Difference between the rectification and distillation The resulting alcohol is possible in two main ways by distillation (simple distillation) and rectification. For example: The fermented raw materials or finished wine is poured into a cube and is heated to vaporization temperature. The evolved vapors enter the cooler and under the influence of the temperature difference is converted into liquid. As it turns out raw alcohol strength of 40 -50 degrees. This product is not sufficiently separated from the impurities, it needs to further purification and additional deinking. Often, instead of insisting and extracts the distillate is distilled again - this increases its degree. If the output to get alcohol very high purity and strength, resorted to the second method - the process of rectification.
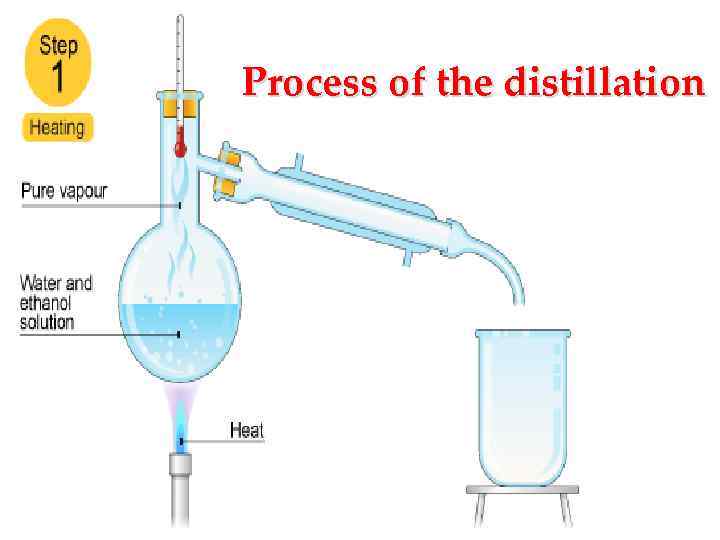
Process of the distillation
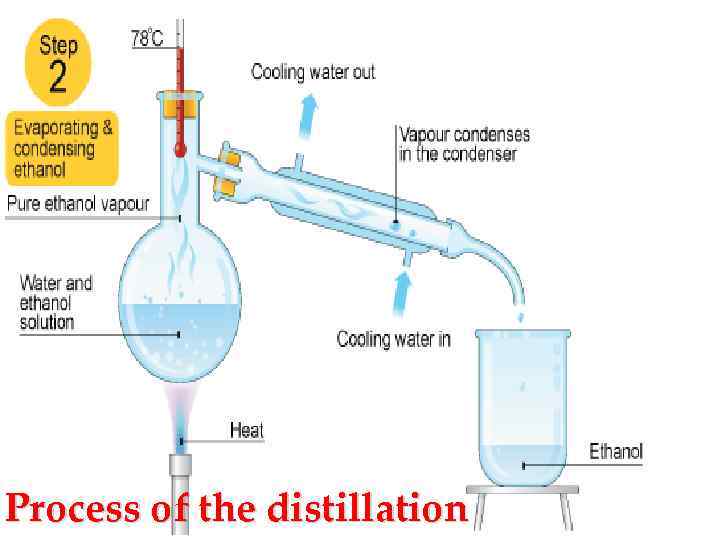
Process of the distillation

The high purification of crude alcohol occurs in the distillation columns consisting of a side-bar, reflux-cooler and the nozzle. It allocates heated feed steam which moves in the side-bar and interacts with a reflux liquid, covering the pipe wall. Part of the vapor deposited in a reflux condenses and drops in alcohol, the remainder is returned to the drawer side.
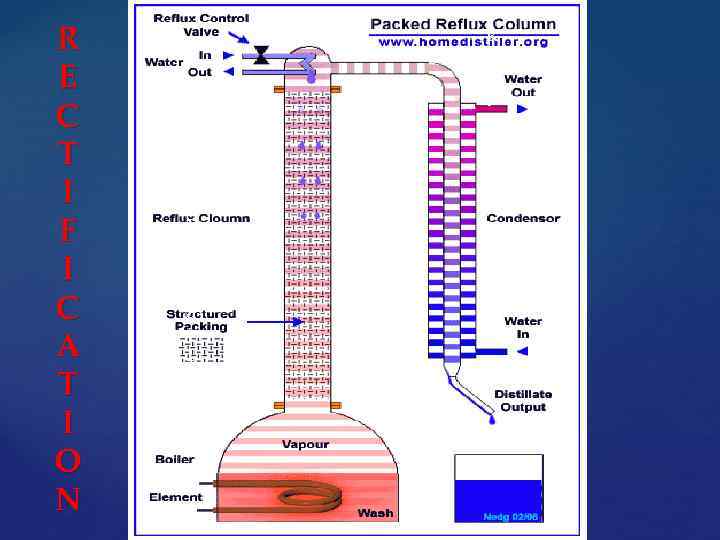
R E C T I F I C A T I O N R e c t I f I c a t I o n

Reflux - cooler with atmospheric sleeve Distillation column (side-bar)


Process of the rectification shows the expansion of the liquid in the heavy and light fractions, as a result of which the feed is the most cleaned of contaminants, suspensions, fusel oils.

Rectified spirit is of particular strength. It contains up to 96% alcohol, it is widely used in the technical, medical purposes, for preparing herbal balms and bitters. As you can see, the fundamental difference of distillation from the alcohol rectification, is not only in the techniques of distillation, but also in a final product.

That’s all!
rectification.pptx