016de658c7c00eca5b4fe44e5f43d8f1.ppt
- Количество слайдов: 22

Recombinant Factor VIIa in obstetric anaesthesia Dr Steve Thomas, Anaesthesia Fellow, BC Women’s hospital, 27 th November 2009

Learning objectives n By the end of this lecture you will have learnt: – How r. FVIIa is hypothesised to work – Potential benefits and hazards of r. FVIIa – Evidence for the use of r. FVIIa in massive haemorrhage – Evidence for the use of r. FVIIa within massive obstetric haemorrhage – Existing guidelines – Considerations of using r. FVIIa at BCWH

The clotting cascade n n n Exposed Tissue Factor (TF) on endothelial wall injury binds to endogenous FVII This complex generates FXa and subsequently small amounts of thrombin Thrombin activates FV, FVIII, FIX and platelets FVIII/FIX complex activates FX on platelets and binds to FVa Full thrombin burst mediated by FVa and FXa complex ‘Thrombin burst’ converts fibrinogen into fibrin to form stable clot
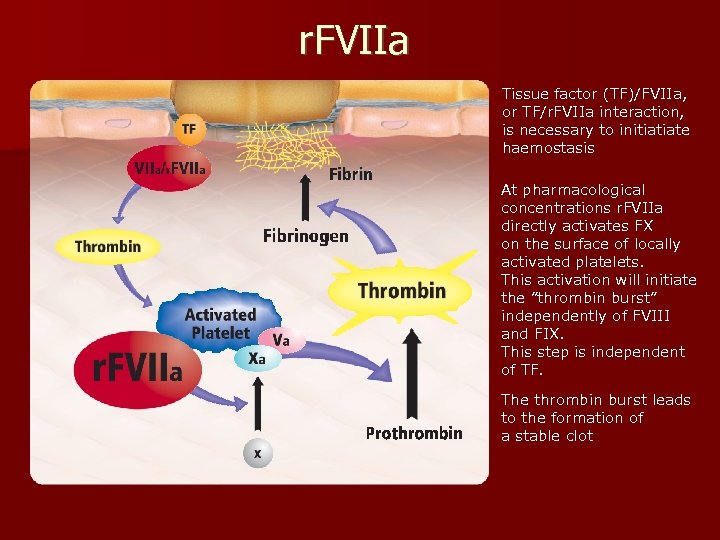
r. FVIIa Tissue factor (TF)/FVIIa, or TF/r. FVIIa interaction, is necessary to initiatiate haemostasis At pharmacological concentrations r. FVIIa directly activates FX on the surface of locally activated platelets. This activation will initiate the ”thrombin burst” independently of FVIII and FIX. This step is independent of TF. The thrombin burst leads to the formation of a stable clot
r. FVIIa n Nia. Stase (Canada) or Novoseven n Genetically engineered n Perceived benefits: – Does not contain human protein, therefore no risk of viral transmission – May halt/reduce bleeding – May reduce transfusion requirements n But…. . increased risk of thrombotic events
Current licensing (FDA) n Haemophilia A and B with: – Inhibitors to factors VIII or IX – Bleeding episodes during after surgery n ‘On label’ dosing: – 70 -90 mck/kg: Association of Haemophilia Clinic Directors of Canada (1999) – 90 mcg/kg: UK Haemophilia Centre Doctors Organisation (2006) n But has been used ‘off label’ in: – – – life threatening haemorrhage including PPH factor VII deficiency or platelet defects Reversal of anticoagulation e. g. warfarin
Off-licence use of r. FVIIa in nonobstetric haemorrhage Reduces blood product requirement in liver and cardiac surgery, vascular surgery, neurosurgery and trauma. n Cardiac: PRC, platelets, FFP and cryoprecipitate fell from 4, 15, 8 10 to 1, 0, 0, 0 respectively after administration of FVIIa. (Mc. Call P et al, CJA, n 2006) n Trauma: significant blood sparing effect, although no affect on mortality. (Boffard K et al, J Trauma, 2005)
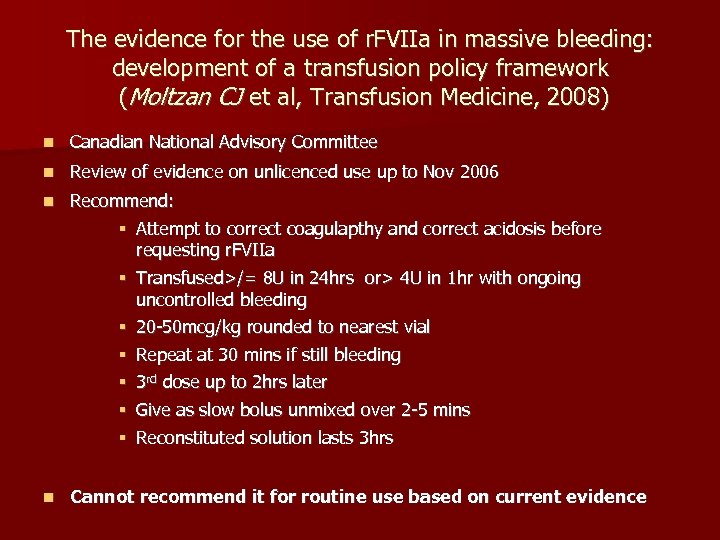
The evidence for the use of r. FVIIa in massive bleeding: development of a transfusion policy framework (Moltzan CJ et al, Transfusion Medicine, 2008) n Canadian National Advisory Committee n Review of evidence on unlicenced use up to Nov 2006 n Recommend: § Attempt to correct coagulapthy and correct acidosis before requesting r. FVIIa § Transfused>/= 8 U in 24 hrs or> 4 U in 1 hr with ongoing uncontrolled bleeding § 20 -50 mcg/kg rounded to nearest vial § § n Repeat at 30 mins if still bleeding 3 rd dose up to 2 hrs later Give as slow bolus unmixed over 2 -5 mins Reconstituted solution lasts 3 hrs Cannot recommend it for routine use based on current evidence

Thromboembolic adverse events after use of r. FVIIa (O’Connell KA et al, JAMA, 2006) n n n FDA’s Adverse Event Reporting System March 1999 -December 2004 Non-obstetric population US and non-US data, including off-licence use Clinician reporting is voluntary, therefore under reported 185 thromboembolic reports, 151 were ‘off label’ – – – – n CVA (39) MI (34) other arterial thromboembolism (26) DVT (42) PTE (32) clotting of indwelling devices (10) other venous thromboembolism (42) A further 61 thromboembolic reports were made in the following 10 months!

A Review: Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia (Cochrane Collaboration, Lin Y et al, 2009) 25 RCTs, 24 double-blinded, 3500 patients n Haemophiliacs excluded n In 14 trials of prophylactic use: n – – n No evidence of mortality benefit Decreased blood loss Decreased need for RBC transfusion (<1 U PRC!) Increased thromboembolic adverse events (not statistically significant) In 11 trials of therapeutic use: – – – No difference in transfusion requirements Reduction in mortality (not statistically significant) Increased thromboembolic adverse effects (not statistically significant)

Cochrane Collaboration “The effectiveness of r. FVIIa remains unproven. The use of r. FVIIa outside its current licensed indications should be restricted to clinical trials” (2007) “r. FVIIa is not effective in patients without haemophilia A” (2009)

‘Last ditch’ use of recombinant factor VIIa in patients with massive haemorrhage is ineffective (Clark AD et al, Vox Sanguinis, 2004) n n n 50 patients, retrospective over 1 year, >10 unit transfusions r. FVIIa used at 90 mcg/kg in 10 patients with retractable bleeding, despite pharmacological and blood product support and no foreseeable surgical correction of bleeding Transient reduction/cessation blood loss in 60% of treated patients. Not sustained. Mortality rates higher in the treated group: 70% died by day 7 r. FVIIa did not rescue these patients.

Timely intervention n A randomized trial of early vs late use of FVIIA in PPH in Nimes, France is not yet published (http: //clinicaltrials. gov/ct 2/show/NCT 00370877) n The main objectives of the study are: – to evaluate the reduction of the absolute risk of arterial embolization/surgery/hysterectomy in patients receiving a unique early infusion of r. FVIIa (60 µg/kg body weight); – to evaluate the number of women necessary to treat to avoid one arterial embolization/surgery/hysterectomy.

r. FVIIa in PPH described in 2001 (Moscardo et al) n Largely case reports. n First – r. FVIIa for life threatening PPH (Ahonen J et al, BJA, 2005): § § § 90 -120 mcg/kg 11/12 patients treated with FVIIa showed a response. Reduced PRC, FFP and platelets from 16, 5, 9 to 3, 1, 8 after FVIIa. Timing optimal when patient has lost 1. 5 blood volume. Consider before hysterectomy Better if diffuse bleeding, embolisation if localised.

Review Article: Recombinant factor VIIa in massive post partum haemorrhage (Karalapillai D et al, IJOA, 2007) n n n Dose of 90 -100 mcg/kg following conventional therapy although optimal dose unclear. Dose repeat after 2 hrs. May reduce blood product use ( Ahonen J et al, BJA, 2005) r. FVIIa requires other clotting factors and platelets to work therefore maintain platelets> 50 x 109/L, fibrinogen> 1 g/L (British and Israeli guidelines) Hypothermia and acidosis reduce factor VIIa activity significantly If localised, correct surgical bleeding (embolisation) FVIIa more effective in diffuse bleeding or as bridge to embolisation if off-site Administration should be considered before hysterectomy Resistance exists (up to 7%) Timing unclear Of use in Jehovah’s Witnesses as synthetic No data on thrombotic complication in obstetric haemorrhage

A Critical Review on the Use of Recombinant Factor VIIa in Life-Threatening Obstetric Postpartum Hemorrhage (Franchini M et al, Semin Thromb Hemost, 2008) n n n 2001 onwards Unlicensed use 31 studies. 118 cases. No RCTs or prospective clinical studies to date. All studies uncontrolled. Median dose 71. 6 mcg/kg Effective in stopping or reducing bleeding in 90% cases But…. . successful cases more likely to be reported Recommended use of 60 -90 mcg/kg repeated at 30 minutes Not as ‘last ditch’ but to be given before hysterectomy. It should not delay surgery or embolisation. Used with caution in sepsis, disseminated malignancy or after other coagulation bypassing agents due to thrombotic potential. More data needed!
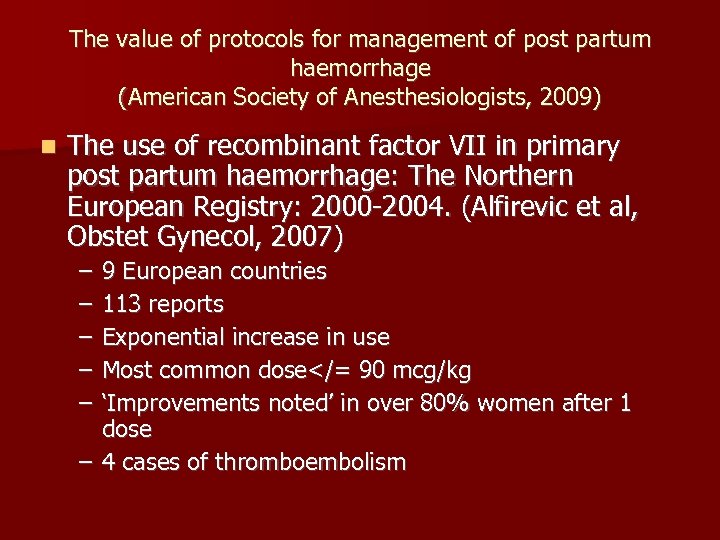
The value of protocols for management of post partum haemorrhage (American Society of Anesthesiologists, 2009) n The use of recombinant factor VII in primary post partum haemorrhage: The Northern European Registry: 2000 -2004. (Alfirevic et al, Obstet Gynecol, 2007) – – – 9 European countries 113 reports Exponential increase in use Most common dose</= 90 mcg/kg ‘Improvements noted’ in over 80% women after 1 dose – 4 cases of thromboembolism

The value of protocols for management of post partum haemorrhage (American Society of Anesthesiologists, 2009) n US vs. European Practice: – American Congress of Obstetricians and Gynaecologists (2006): Practice Bulletin; Postpartum haemorrhage. § No mention of r. FVIIa. – World Health Organisation (2009): ’Guidelines for the management of PPH and retained placenta. ’ § No mention of r. FVIIa.

Guidelines for the use of recombinant factor VII in massive obstetric haemorrhage (Welsh A, Mc. Lintock C, Gatt S et al, 2008) n Only following blood component therapy and medical and surgical intervention, consider r. FVIIa. This includes: – uterotonics, uterine massage – B-lynch suture, arterial ligation, balloon catheters – radiological embolisation n Transfusion targets: platelets>50, a. PTT ratio>1. 5, Hb>7, fibronogen>1 n 90 mcg/kg as single bolus over 3 -5 mins n After 20 mins if no response: n Optimise temperature, acidaemia, serum calcium, platelets, fibrinogen, then. . . 2 nd dose of 90 mcg/kg n Consider hysterectomy if bleeding persists n 24 hrs after bleeding stops: – Calf compression/stockings – LMWH
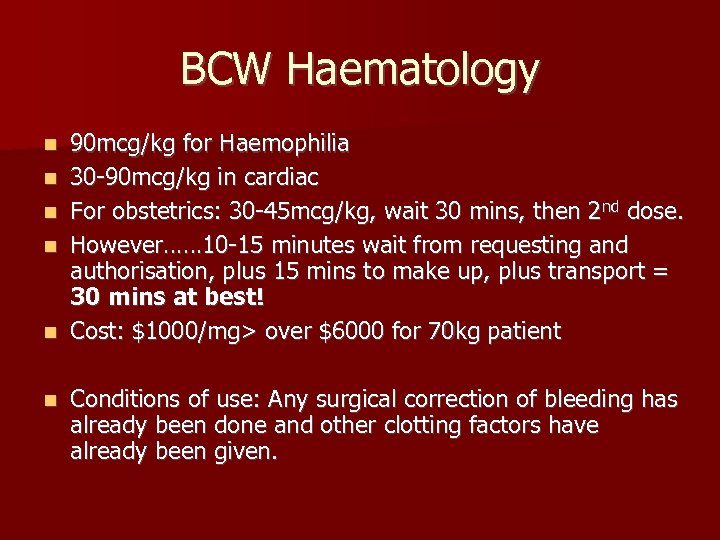
BCW Haematology n n n 90 mcg/kg for Haemophilia 30 -90 mcg/kg in cardiac For obstetrics: 30 -45 mcg/kg, wait 30 mins, then 2 nd dose. However…… 10 -15 minutes wait from requesting and authorisation, plus 15 mins to make up, plus transport = 30 mins at best! Cost: $1000/mg> over $6000 for 70 kg patient Conditions of use: Any surgical correction of bleeding has already been done and other clotting factors have already been given.

In summary It is associated with thrombotic events in a non-obstetric population. n It’s use has been successful outwith obstetrics n Little strong evidence supports it’s use in obstetrics………. Is a RCT ever going to be possible anyway? n It is being used widely across Europe and in Australia/New Zealand. n Guidelines are beginning to emerge. n If given, there may be benefit in using it early rather than as last ditch. n Acidosis and hypothermia should be corrected first n Surgical correction and clotting factors should be given first n It’s use should be considered prior to hysterectomy n Although available at BCW it takes a long time to become available n

Questions 22
016de658c7c00eca5b4fe44e5f43d8f1.ppt