de0fb41b2e223a8748d7a3f3ce6d3e77.ppt
- Количество слайдов: 31
 Recognizing and Minimizing Risks in VA Research Kevin L. Nellis, M. S. , M. T. (A. S. C. P. ) Program Analyst Program for Research Integrity Development and Education (PRIDE) March 28, 2011
Recognizing and Minimizing Risks in VA Research Kevin L. Nellis, M. S. , M. T. (A. S. C. P. ) Program Analyst Program for Research Integrity Development and Education (PRIDE) March 28, 2011
 Overview • Regulatory background • Individuals or populations vulnerable to risks • Types of risks in research • Minimizing risks • Potential risks based on types of research 2
Overview • Regulatory background • Individuals or populations vulnerable to risks • Types of risks in research • Minimizing risks • Potential risks based on types of research 2
 Minimization of Risks • Risks to human subjects are minimized by using procedures that: • Are consistent with sound research design • Do not unnecessarily expose subjects to risk • Are already being performed for diagnostic or treatment purposes 3
Minimization of Risks • Risks to human subjects are minimized by using procedures that: • Are consistent with sound research design • Do not unnecessarily expose subjects to risk • Are already being performed for diagnostic or treatment purposes 3
 Risks and Benefits Analysis • Risks to subjects must be reasonable in relation to • Anticipated benefits (outcome or advantage) • Importance of the knowledge that is expected as a result of the research • When analyzing the risks to benefits ratio • Consider only risks and benefits of the research • Do not to consider possible long-range effects of applying knowledge gained in the research 4
Risks and Benefits Analysis • Risks to subjects must be reasonable in relation to • Anticipated benefits (outcome or advantage) • Importance of the knowledge that is expected as a result of the research • When analyzing the risks to benefits ratio • Consider only risks and benefits of the research • Do not to consider possible long-range effects of applying knowledge gained in the research 4
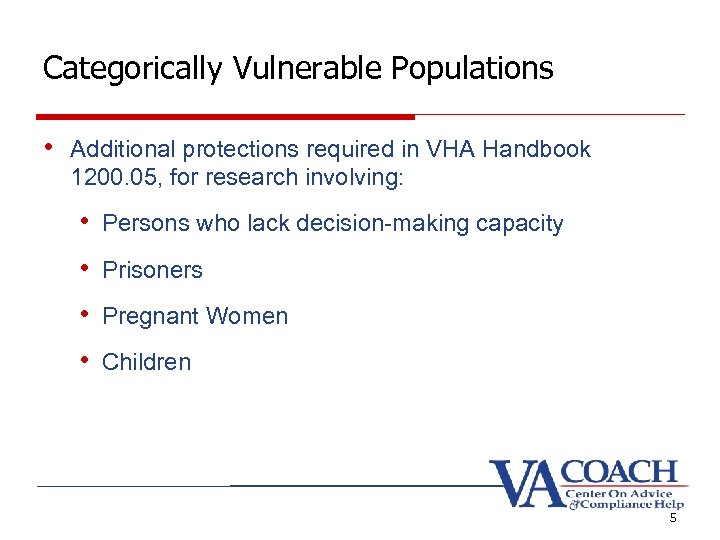 Categorically Vulnerable Populations • Additional protections required in VHA Handbook 1200. 05, for research involving: • Persons who lack decision-making capacity • Prisoners • Pregnant Women • Children 5
Categorically Vulnerable Populations • Additional protections required in VHA Handbook 1200. 05, for research involving: • Persons who lack decision-making capacity • Prisoners • Pregnant Women • Children 5
 Factors Contributing to Risk • Complicated lives or time constraints • Exposure to safety issues • The immature or emotionally fragile • Societal judgment • The financially insecure • Illegal behaviors • Legal actions • Certain populations 6
Factors Contributing to Risk • Complicated lives or time constraints • Exposure to safety issues • The immature or emotionally fragile • Societal judgment • The financially insecure • Illegal behaviors • Legal actions • Certain populations 6
 Factors Contributing to Risk • Employees • The homeless or displaced • Language or educational challenges • Mental or physical disabilities • Socio-economic disadvantages • Poor access to healthcare 7
Factors Contributing to Risk • Employees • The homeless or displaced • Language or educational challenges • Mental or physical disabilities • Socio-economic disadvantages • Poor access to healthcare 7
 Third Parties at Risk • Bystanders (family, community, social groups, caregivers, etc) • Consumers • Institutions 8
Third Parties at Risk • Bystanders (family, community, social groups, caregivers, etc) • Consumers • Institutions 8
 Possible Types of Risks • Physical (direct and indirect) • Psychological or emotional • Social or cultural • Economic • Legal 9
Possible Types of Risks • Physical (direct and indirect) • Psychological or emotional • Social or cultural • Economic • Legal 9
 Physical Risks • Injury • Pain • Fatigue • Side effects of drugs • Assault • Death 10
Physical Risks • Injury • Pain • Fatigue • Side effects of drugs • Assault • Death 10
 Protections From Physical Risks • Appropriate study design with appropriately trained individuals • Appropriate recruitment, advertising, and informed consent process • Careful monitoring of subjects • More frequent IRB review • Providing first aid or clinical care • Compensate for harms 11
Protections From Physical Risks • Appropriate study design with appropriately trained individuals • Appropriate recruitment, advertising, and informed consent process • Careful monitoring of subjects • More frequent IRB review • Providing first aid or clinical care • Compensate for harms 11
 Psychological Risks • Getting upset or worried • Anxiety or depression • Feelings of stress or guilt • Confusion or hallucination • Embarrassment or shame • Loss of self-esteem or self-confidence 12
Psychological Risks • Getting upset or worried • Anxiety or depression • Feelings of stress or guilt • Confusion or hallucination • Embarrassment or shame • Loss of self-esteem or self-confidence 12
 Protections From Psychological Risks • Adequately and sensitively administered informed consent • Respectful treatment • Remind participants of their right to withdraw or to limit their participation • When appropriate: • • Provide psychological support or counseling Thoroughly debrief subjects after participation Assure anonymity Provide contacts for suicide hotline 13
Protections From Psychological Risks • Adequately and sensitively administered informed consent • Respectful treatment • Remind participants of their right to withdraw or to limit their participation • When appropriate: • • Provide psychological support or counseling Thoroughly debrief subjects after participation Assure anonymity Provide contacts for suicide hotline 13
 Social Risks • Embarrassment • Stigma (ostracism) • Conflict • Scapegoating • Resentment • Discrimination 14
Social Risks • Embarrassment • Stigma (ostracism) • Conflict • Scapegoating • Resentment • Discrimination 14
 Protections From Social Risks • Adequately and sensitively administered informed consent • Assure privacy, confidentiality, and data security • Assure anonymity, when appropriate • Ensure researchers follow professional codes 15
Protections From Social Risks • Adequately and sensitively administered informed consent • Assure privacy, confidentiality, and data security • Assure anonymity, when appropriate • Ensure researchers follow professional codes 15
 Economic Risks • Loss of a money • Loss of financial opportunity • Loss of credit • Identity theft • Loss of insurability • Loss of wages • Loss of job or advancement 16
Economic Risks • Loss of a money • Loss of financial opportunity • Loss of credit • Identity theft • Loss of insurability • Loss of wages • Loss of job or advancement 16
 Protections From Economic Risks • Compensate for harms • Assure of privacy, confidentiality, and data security • Keep research data separate from medical records to prevent employers and insurance companies from obtaining information 17
Protections From Economic Risks • Compensate for harms • Assure of privacy, confidentiality, and data security • Keep research data separate from medical records to prevent employers and insurance companies from obtaining information 17
 Legal Risks • Lawsuits • Disclosure of illegal activities • Conviction of crime • Subpoena of damaging data 18
Legal Risks • Lawsuits • Disclosure of illegal activities • Conviction of crime • Subpoena of damaging data 18
 Protections From Legal Risks • Assure anonymity, when appropriate • Assure of privacy, confidentiality, and data security • Obtain Certificate of Confidentiality, if appropriate 19
Protections From Legal Risks • Assure anonymity, when appropriate • Assure of privacy, confidentiality, and data security • Obtain Certificate of Confidentiality, if appropriate 19
 Certificates of Confidentiality are Obtained From the NIH • Helps researchers protect the privacy of human research participants • Protect against compulsory legal demands (e. g. , court orders and subpoenas) • Does not protect against voluntary disclosures by the researcher (as specified in the informed consent form) • Subjects are protected permanently- even if the subject gave the researcher data before the Certificate is issued • NIH: http: //grants. nih. gov/grants/policy/coc/ 20
Certificates of Confidentiality are Obtained From the NIH • Helps researchers protect the privacy of human research participants • Protect against compulsory legal demands (e. g. , court orders and subpoenas) • Does not protect against voluntary disclosures by the researcher (as specified in the informed consent form) • Subjects are protected permanently- even if the subject gave the researcher data before the Certificate is issued • NIH: http: //grants. nih. gov/grants/policy/coc/ 20
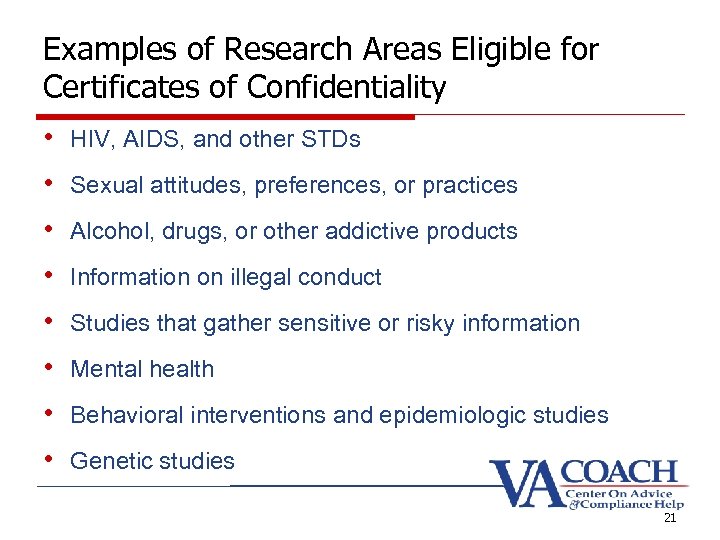 Examples of Research Areas Eligible for Certificates of Confidentiality • HIV, AIDS, and other STDs • Sexual attitudes, preferences, or practices • Alcohol, drugs, or other addictive products • Information on illegal conduct • Studies that gather sensitive or risky information • Mental health • Behavioral interventions and epidemiologic studies • Genetic studies 21
Examples of Research Areas Eligible for Certificates of Confidentiality • HIV, AIDS, and other STDs • Sexual attitudes, preferences, or practices • Alcohol, drugs, or other addictive products • Information on illegal conduct • Studies that gather sensitive or risky information • Mental health • Behavioral interventions and epidemiologic studies • Genetic studies 21
 22
22
 Potential Risks of Clinical Studies • Drugs and devices • Treatment-withholding studies • Placebo-controlled clinical trials • Phase I clinical trials in healthy subjects • Phase I oncology trials • Confusing language in informed consent • Conflicts of interests • High risk radioactive or other materials 23
Potential Risks of Clinical Studies • Drugs and devices • Treatment-withholding studies • Placebo-controlled clinical trials • Phase I clinical trials in healthy subjects • Phase I oncology trials • Confusing language in informed consent • Conflicts of interests • High risk radioactive or other materials 23
 Potential Risks of Genetic Testing • Psychological impact – emotions aroused by learning that one is (or is not) likely to develop a serious disease • Change in family dynamics • Involving minors or incapacitated • Privacy – will a predisposition remain a secret or slip out? • Incidental findings about paternity • Risks of sharing information • Impact on medical choices 24
Potential Risks of Genetic Testing • Psychological impact – emotions aroused by learning that one is (or is not) likely to develop a serious disease • Change in family dynamics • Involving minors or incapacitated • Privacy – will a predisposition remain a secret or slip out? • Incidental findings about paternity • Risks of sharing information • Impact on medical choices 24
 Potential Risks of Focus Groups, Questionnaires, or Surveys • Breach of confidentiality or violation of privacy • Inadvertent disclosures of media (audio, video, or photos) • Validation of inappropriate or undesirable behaviors • Presentation of results in a way that does not respect (or agree with) subjects’ interests • Damage to dignity, self-image, or innocence • Possible harm to secondary subjects • Reportable situations (e. g. , elderly or child abuse, threats, infectious disease, criminal activities) 25
Potential Risks of Focus Groups, Questionnaires, or Surveys • Breach of confidentiality or violation of privacy • Inadvertent disclosures of media (audio, video, or photos) • Validation of inappropriate or undesirable behaviors • Presentation of results in a way that does not respect (or agree with) subjects’ interests • Damage to dignity, self-image, or innocence • Possible harm to secondary subjects • Reportable situations (e. g. , elderly or child abuse, threats, infectious disease, criminal activities) 25
 Potential Risks of Focus Groups, Questionnaires, or Surveys • Three weeks after answering the questionnaire for a cancer study; a man in his early 20 s committed suicide, leaving a note stating that until he answered the questionnaire he did not really realize all of the experiences he had missed. • A survey researcher was investigating attitudes toward rape. When filling out questionnaires, several subjects became visibly emotionally distressed and asked the investigator for help. Ref: IRB Forum (January 2001) 26
Potential Risks of Focus Groups, Questionnaires, or Surveys • Three weeks after answering the questionnaire for a cancer study; a man in his early 20 s committed suicide, leaving a note stating that until he answered the questionnaire he did not really realize all of the experiences he had missed. • A survey researcher was investigating attitudes toward rape. When filling out questionnaires, several subjects became visibly emotionally distressed and asked the investigator for help. Ref: IRB Forum (January 2001) 26
 Potential Risks of Focus Groups, Questionnaires, or Surveys • A study involving adolescents with emotional difficulties included a few questions about sexual preferences. One subject interpreted the questions as if the researcher were suggesting she or he was a homosexual. The subject became very upset, attacked the researcher, and had to be restrained. Ref: IRB Forum (January 2001) 27
Potential Risks of Focus Groups, Questionnaires, or Surveys • A study involving adolescents with emotional difficulties included a few questions about sexual preferences. One subject interpreted the questions as if the researcher were suggesting she or he was a homosexual. The subject became very upset, attacked the researcher, and had to be restrained. Ref: IRB Forum (January 2001) 27
 Key Points • All those involved in research must understand various risks of research • Investigators must design the study to minimize risks • IRBs must ensure that risks are minimized and reasonable in relationship to anticipated benefits before approving the research 28
Key Points • All those involved in research must understand various risks of research • Investigators must design the study to minimize risks • IRBs must ensure that risks are minimized and reasonable in relationship to anticipated benefits before approving the research 28
 Key Points • Both the investigator and the IRB must ensure protections are specific to: • Nature of the risks • Individuals involved • Type of research • Location and culture • Monitor and modify the research to minimize risks 29
Key Points • Both the investigator and the IRB must ensure protections are specific to: • Nature of the risks • Individuals involved • Type of research • Location and culture • Monitor and modify the research to minimize risks 29
 References • Bankert and Amdur. IRB Management and Function. (2006) • • http: //books. google. com/books Risk and Harm: Social and Behavioral Sciences Working Group on Human Research Protections. (2004) http: //www. aera. net/humansubjects/risk-harm. pdf National Research Council. Protecting Participants and Facilitating Social and Behavioral Sciences Research. (2003) http: //www. nap. edu/catalog. php? record_id=10638 OHRP. IRB Guidebook. (1993) http: //www. hhs. gov/ohrp/archive/irb_guidebook. htm Clayton. Incidental Findings in Genetics Research Using Archived DNA. J. Law. Med. Ethics. 2008: 36(2): 286 -212 http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 2576744/ 30
References • Bankert and Amdur. IRB Management and Function. (2006) • • http: //books. google. com/books Risk and Harm: Social and Behavioral Sciences Working Group on Human Research Protections. (2004) http: //www. aera. net/humansubjects/risk-harm. pdf National Research Council. Protecting Participants and Facilitating Social and Behavioral Sciences Research. (2003) http: //www. nap. edu/catalog. php? record_id=10638 OHRP. IRB Guidebook. (1993) http: //www. hhs. gov/ohrp/archive/irb_guidebook. htm Clayton. Incidental Findings in Genetics Research Using Archived DNA. J. Law. Med. Ethics. 2008: 36(2): 286 -212 http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 2576744/ 30
 31
31


