e8daf28e95a6a8d529168539b7a2ad61.ppt
- Количество слайдов: 22

Radiocarbon Dating -----Natural Clock

• “seldom has a single discovery in chemistry had such an impact on the thinking of so many fields of human endeavor. Seldom has a single discovery generated such wide public interest”----one of the scientists who proposed Libby for the Nobel laureate characterized the significance of the C 14 method. W. F. Libby, Nobel Prize for Chemistry in 1960 Radiocarbon after four decades, Springer-Verlag, 1992
Outline • • • Principles Sample and measurement Application Advantages and limitations Future developments
• • Principle First developed by J. R. Arnold and W. F. Libby in 1949; In laboratory, many studies of neutrons produced by cosmic radiation on all the ordinary elements and especially on nitrogen and oxygen ( the constituents of the air) Oxygen is extraordinarily inert, nitrogen is reactive; dominant reaction N 14 +n=C 14+H 1 Radiocarbon dating by W. F. Libby, 1955
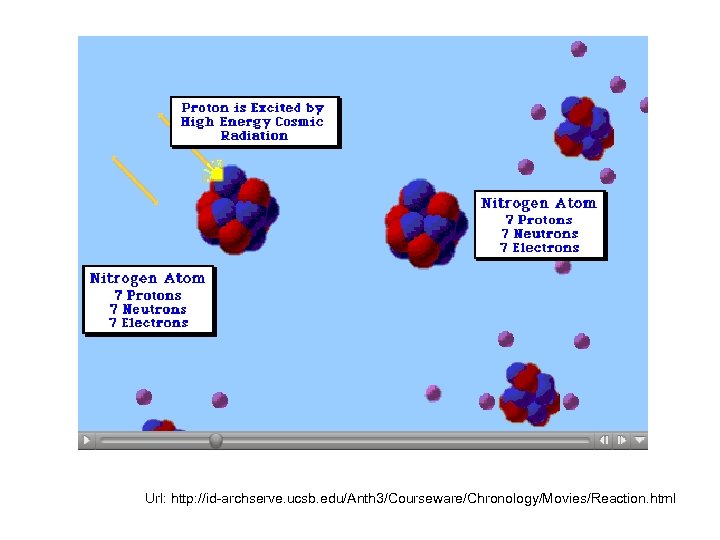
Url: http: //id-archserve. ucsb. edu/Anth 3/Courseware/Chronology/Movies/Reaction. html
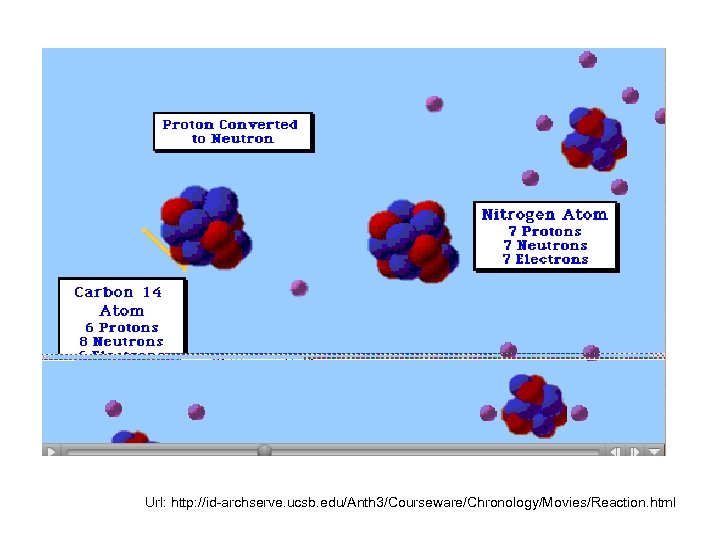
Url: http: //id-archserve. ucsb. edu/Anth 3/Courseware/Chronology/Movies/Reaction. html
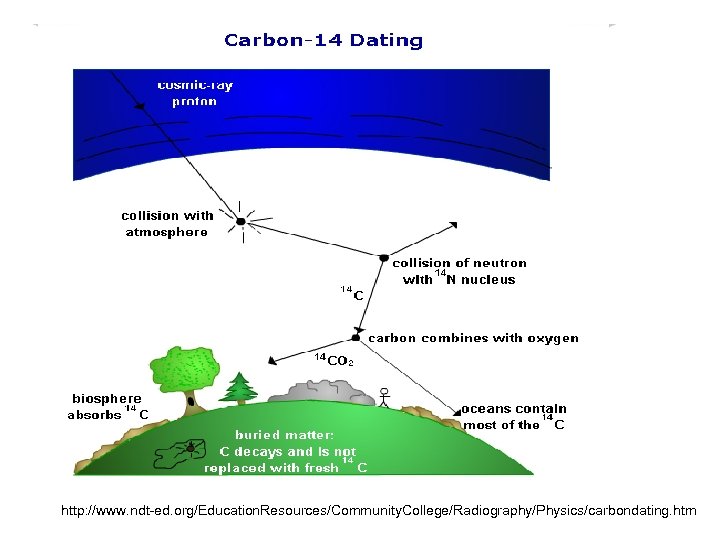
http: //www. ndt-ed. org/Education. Resources/Community. College/Radiography/Physics/carbondating. htm

• The unstable isotope C-14 is brought to Earth. • attached to complex organic molecules through photosynthesis in plants and becomes part of their molecular makeup. • Animals eating those plants in turn absorb Carbon-14 as well as the stable isotopes. • This process of ingesting C-14 continues as long as the plant or animal remains alive.
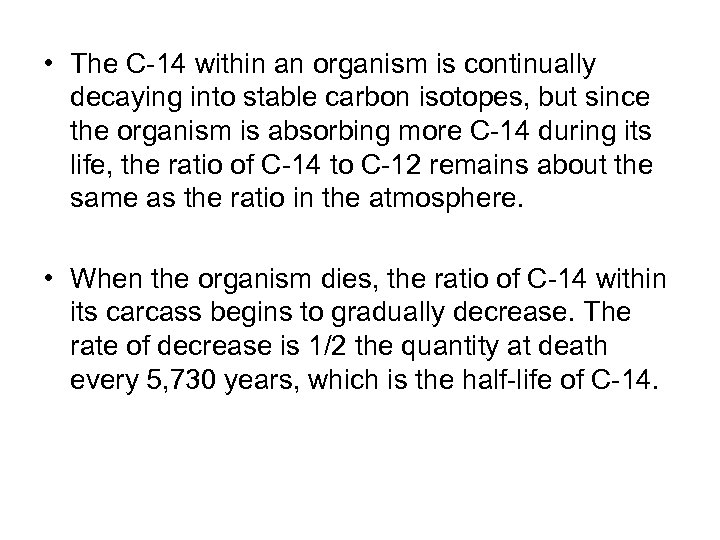
• The C-14 within an organism is continually decaying into stable carbon isotopes, but since the organism is absorbing more C-14 during its life, the ratio of C-14 to C-12 remains about the same as the ratio in the atmosphere. • When the organism dies, the ratio of C-14 within its carcass begins to gradually decrease. The rate of decrease is 1/2 the quantity at death every 5, 730 years, which is the half-life of C-14.
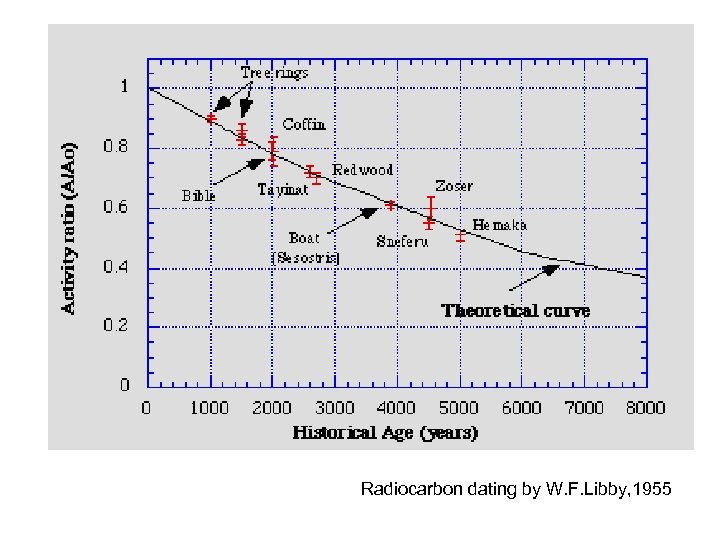
Radiocarbon dating by W. F. Libby, 1955

• Samples and measurements Sampling order: 1. Charcoal or charred organic material such as heavily burned bone; 2. Well-preserved wood; 3. Grasses, cloth, and peat; 4. Well-preserved antler and similar hairy structures 5. Well-preserved shell; Sample should contain the original carbon atoms at the time it died. large molecular structures, such as cellulose molecules, resist putrefaction and chemical alteration well.
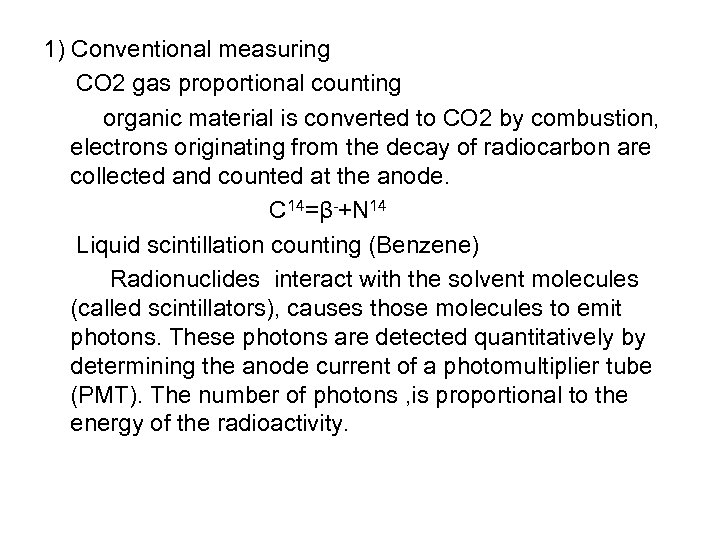
1) Conventional measuring CO 2 gas proportional counting organic material is converted to CO 2 by combustion, electrons originating from the decay of radiocarbon are collected and counted at the anode. C 14=β-+N 14 Liquid scintillation counting (Benzene) Radionuclides interact with the solvent molecules (called scintillators), causes those molecules to emit photons. These photons are detected quantitatively by determining the anode current of a photomultiplier tube (PMT). The number of photons , is proportional to the energy of the radioactivity.
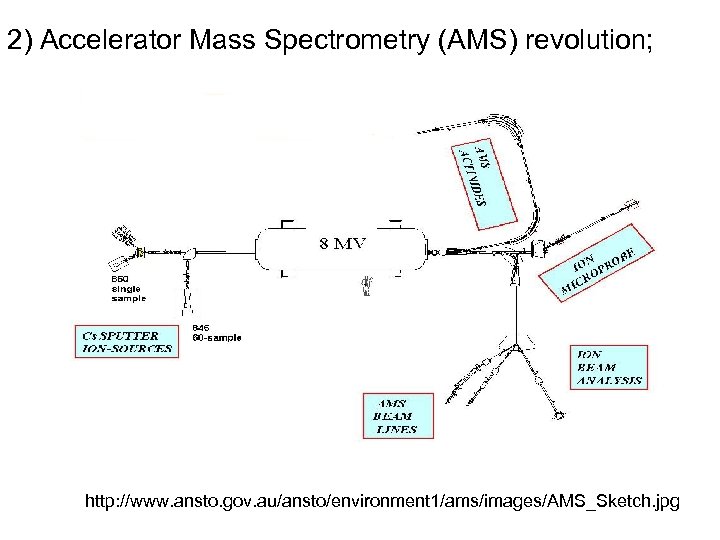
2) Accelerator Mass Spectrometry (AMS) revolution; http: //www. ansto. gov. au/ansto/environment 1/ams/images/AMS_Sketch. jpg

• The ion source produces a beam of negative ions from a few milligrams of solid material by the cesium sputter source ; • Low energy mass analysis select the mass of interest, a radioisotope of the element inserted in the sample holder, and reject the much-more-intense neighboring stable isotopes. • Accelerators: accelerate ions, remove several electrons, turning the negative carbon ions into positive ones. The accelerator functions as a molecular dissociator. The final velocity is a few percent of the speed of light or about 50 million miles per hour. • High energy mass analysis: counts C 14 ions with a gas ionization detector. measures Isotope ratios • Measuring time just a few hours

• Application Archaeology Earth sciences Environmental sciences Biomedical applications Hydrology: groundwater dating

• The Shroud of Turin • “ The results of radiocarbon measurements at Arizona, Oxford and Zurich yield a calibrated calendar age range with at least 95% confidence for the linen of the Shroud of Turin of AD 1260 - 1390 (rounded down/up to nearest 10 yr). These results therefore provide conclusive evidence that the linen of the Shroud of Turin is mediaeval ” • (P. E. Damon, etal. , Radiocarbon Dating of the Shroud of Turin, Nature 337: 6208, 16 February 1989, pp 611 -615)

• Basic two assumptions in radiocarbon dating : • 1. the planetary distribution of C 14 in the biosphere is uniform in time and space; • 2. the sample, as measured, contains carbon that come only from a living organism, and that living organism took its carbon only from the biosphere;

• Things That Can't Be Carbon-Dated 1 things which didn't get their carbon from the air. aquatic creatures, their carbon might (for example) come from dissolved carbonate rock. any animal that eats seafood. 2 things that are too old. After about ten half-lives, there's very little C 14 left. So, anything more than about 50, 000 years old probably can't be dated at all. 3 oil paints, because their oil is "old" carbon from petroleum. 4 fossils, for three reasons. First, they are almost always too old. Second, they rarely contain any of the original carbon. And third, it is common to soak new-found fossils in a preservative, such as shellac. 5 things that are too young. The nuclear tests of the 1950's created a lot of C 14. Also, humans are now burning large amounts of "fossil fuel". As the name suggests, fossil fuel is old, and no longer contains C 14. Both of these man-made changes are a nuisance to carbon dating.

• Calibration and tree-ring correction • Measure the C 14 activity of wood samples that have known ages ( dendrochronologically determined). • Create the calibration curves by plotting the wood radiocarbon ages versus the calibrated (cal) ages. (dated by another radioactive material, Thorium-230 or by ice layer

Jim Parks prepares to analyze one of hundreds of core samples collected at Mesa Verde National Park, Colorado. (Photo by John Florence. ) http: //www. ltrr. arizona. edu/archaeology/progandhist. htm

AMS Radiocarbon Dating of Bones At LSCE N Tisnérat-Laborde; H Valladas; E Kaltnecker; M Arnold Development of an Automated System for Preparation of Organic Samples Christine Hatté; Jean-Jacques Poupeau; Jean-François Tannau; Martine Paterne Environmental Studies Bomb Radiocarbon in Tree Rings from Northern New South Wales, Australia: Implications for Dendochronology, Atmospheric Transport, and Air-Sea Exchange of CO 2 Quan Hua; Mike Barbetti; Ugo Zoppi; David M Chapman; Bruce Thomson Freshwater Reservoir Effect in 14 C Dates of Food Residue on Pottery Anders Fischer; Jan Heinemeier Soils and Sediments 14 C Ages of a Varved Last Glacial Maximum Section Off Pakistan Ulrich von Rad; Michael Sarnthein; Pieter M Grootes; Heidi Doose-Rolinski; Jochen Erbacher AMS Dating of Pollen Concentrates - A Methodological Study of Late Quaternary Sediments from South Westland, New Zealand Marcus J Vandergoes; Christine A Prior
• Thank you
e8daf28e95a6a8d529168539b7a2ad61.ppt