a4a5d2520cf7301eb5b36ceb781e13ee.ppt
- Количество слайдов: 46
 Radiation Defects in Alkali Halides and Oxides A. I. Popov Institute of Solid State Physics, University of Latvia, LV
Radiation Defects in Alkali Halides and Oxides A. I. Popov Institute of Solid State Physics, University of Latvia, LV
 REI-15, Padova, Sept. 1, 2009 Basic Properties of Radiation-Induced Point Defects in Halides and Oxides A. I. Popov, Max Planck Institute, Stuttgart and Institute of Solid State Physics, University of Latvia, LV E. A. Kotomin, Max Planck Institute, Stuttgart and Institute of Solid State Physics, University of Latvia J. Maier, Max Planck Institute, Stuttgart
REI-15, Padova, Sept. 1, 2009 Basic Properties of Radiation-Induced Point Defects in Halides and Oxides A. I. Popov, Max Planck Institute, Stuttgart and Institute of Solid State Physics, University of Latvia, LV E. A. Kotomin, Max Planck Institute, Stuttgart and Institute of Solid State Physics, University of Latvia J. Maier, Max Planck Institute, Stuttgart
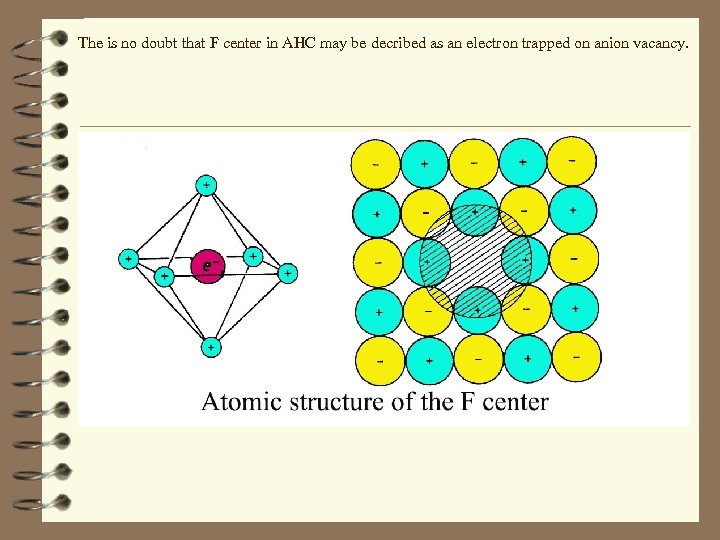 The is no doubt that F center in AHC may be decribed as an electron trapped on anion vacancy.
The is no doubt that F center in AHC may be decribed as an electron trapped on anion vacancy.
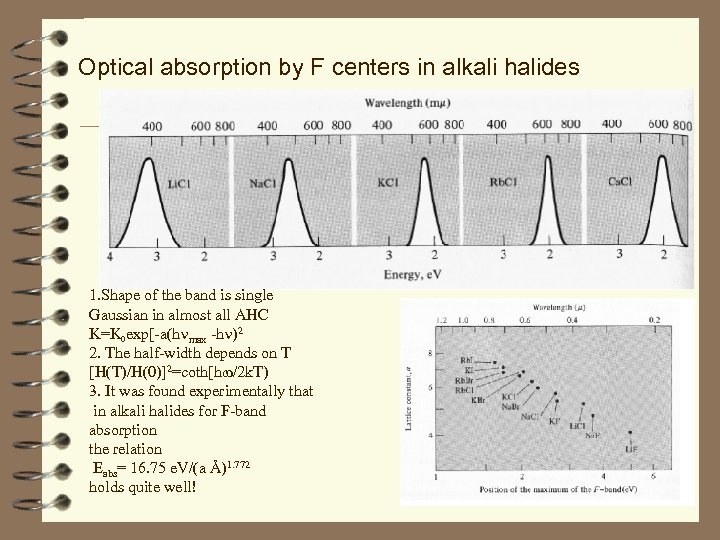 Optical absorption by F centers in alkali halides 1. Shape of the band is single Gaussian in almost all AHC K=K 0 exp[-a(h max -h )2 2. The half-width depends on T [H(T)/H(0)]2=coth[h /2 k. T) 3. It was found experimentally that in alkali halides for F-band absorption the relation Eabs= 16. 75 e. V/(a Å)1. 772 holds quite well!
Optical absorption by F centers in alkali halides 1. Shape of the band is single Gaussian in almost all AHC K=K 0 exp[-a(h max -h )2 2. The half-width depends on T [H(T)/H(0)]2=coth[h /2 k. T) 3. It was found experimentally that in alkali halides for F-band absorption the relation Eabs= 16. 75 e. V/(a Å)1. 772 holds quite well!
 Radiation Defects Ionizing radiation produces a variety of vacancy and intersitial type of point defects: In alkali halides: vacancy defects includes bare cation and anion vacancy, as well as halogen vacancy with one electron ( or F center). KCl - The activation energy for diffusion is found to increase monotonically in the series Vc, Va and F center 1. 19 e. V, 1. 44 e. V and 1. 64 e. V In simple oxides: vacancy defects includes bare cation and oxygen vacancy, as well as oxygen vacancy with one or two electrons (F+ and F center). Mg. O- The activation energy for diffusion is found to increase monotonically in the series Vc, Va, F+ and F center (2. 43, 2. 50, 2. 72, and 3. 13 e. V, respectively).
Radiation Defects Ionizing radiation produces a variety of vacancy and intersitial type of point defects: In alkali halides: vacancy defects includes bare cation and anion vacancy, as well as halogen vacancy with one electron ( or F center). KCl - The activation energy for diffusion is found to increase monotonically in the series Vc, Va and F center 1. 19 e. V, 1. 44 e. V and 1. 64 e. V In simple oxides: vacancy defects includes bare cation and oxygen vacancy, as well as oxygen vacancy with one or two electrons (F+ and F center). Mg. O- The activation energy for diffusion is found to increase monotonically in the series Vc, Va, F+ and F center (2. 43, 2. 50, 2. 72, and 3. 13 e. V, respectively).
 Radiation Damage Processes 1. Electronic processes 2. Elastic collisions Five types of radiation may produce displaced atom or ions (1) - rays, (2) energetic electrons, (3) thermal neutrons, (4) fast neutrons, (5) energetic atoms or ions 3. Radiolysis (1) Electronic excitation creation of an electronic defects (2) Conversion of this energy into kinetic energy of a lattice ion moves (3) The motion and stabilization of the ion The available energy, Egap (in fact Ex < Egap) > the formation energy of the Frenkel pair. the radiolysis can only occurs in insulators or wide band-gap semiconductors. The excitation must be localised on one atomic (or molecular) site Non-radiative transitions, allowing an efficient kinetic energy transfer to an atom, must prevail over radiative transitions
Radiation Damage Processes 1. Electronic processes 2. Elastic collisions Five types of radiation may produce displaced atom or ions (1) - rays, (2) energetic electrons, (3) thermal neutrons, (4) fast neutrons, (5) energetic atoms or ions 3. Radiolysis (1) Electronic excitation creation of an electronic defects (2) Conversion of this energy into kinetic energy of a lattice ion moves (3) The motion and stabilization of the ion The available energy, Egap (in fact Ex < Egap) > the formation energy of the Frenkel pair. the radiolysis can only occurs in insulators or wide band-gap semiconductors. The excitation must be localised on one atomic (or molecular) site Non-radiative transitions, allowing an efficient kinetic energy transfer to an atom, must prevail over radiative transitions
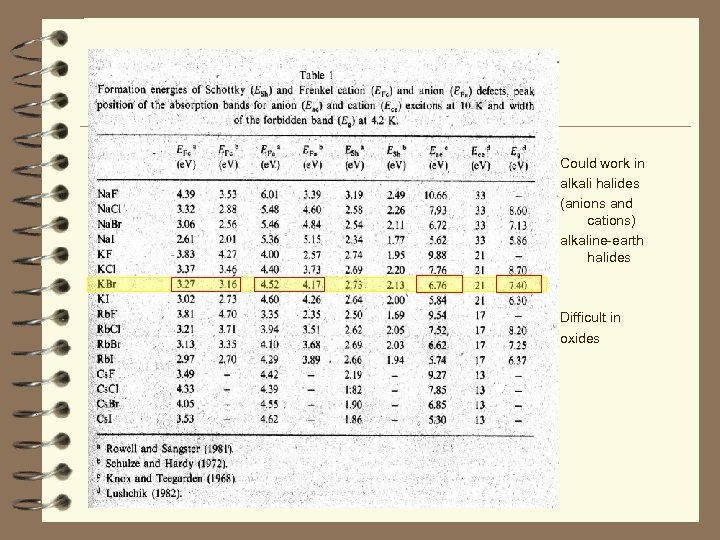 Could work in alkali halides (anions and cations) alkaline-earth halides Difficult in oxides
Could work in alkali halides (anions and cations) alkaline-earth halides Difficult in oxides
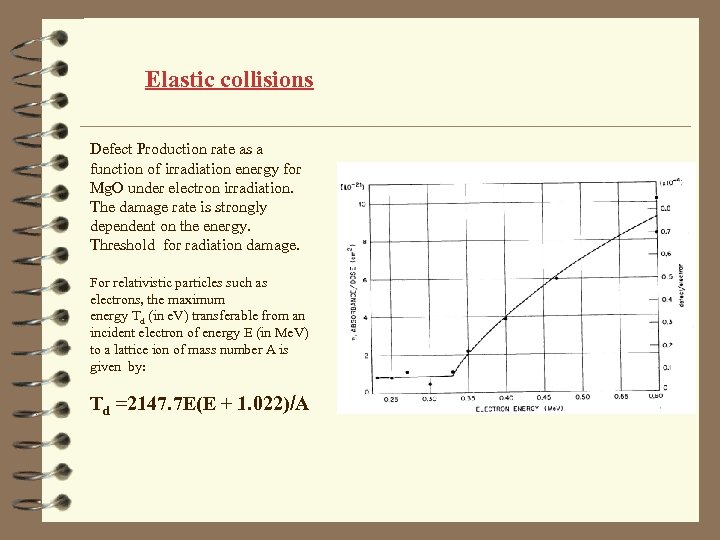 Elastic collisions Defect Production rate as a function of irradiation energy for Mg. O under electron irradiation. The damage rate is strongly dependent on the energy. Threshold for radiation damage. For relativistic particles such as electrons, the maximum energy Td (in e. V) transferable from an incident electron of energy E (in Me. V) to a lattice ion of mass number A is given by: Td =2147. 7 E(E + 1. 022)/A
Elastic collisions Defect Production rate as a function of irradiation energy for Mg. O under electron irradiation. The damage rate is strongly dependent on the energy. Threshold for radiation damage. For relativistic particles such as electrons, the maximum energy Td (in e. V) transferable from an incident electron of energy E (in Me. V) to a lattice ion of mass number A is given by: Td =2147. 7 E(E + 1. 022)/A
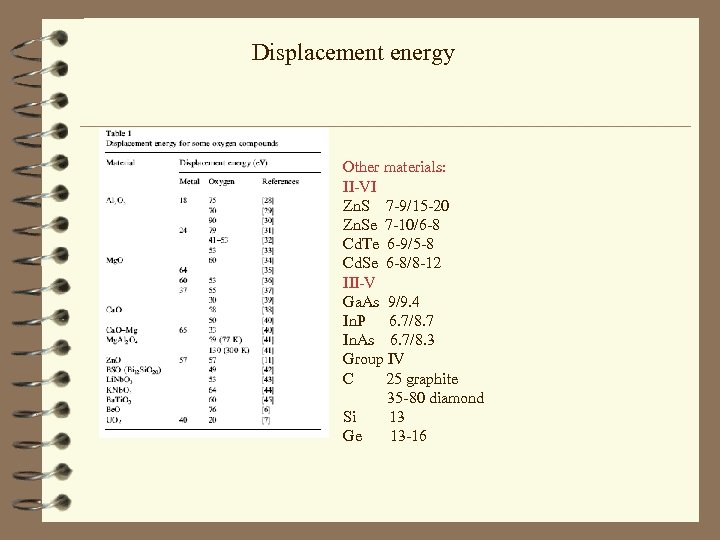 Displacement energy Other materials: II-VI Zn. S 7 -9/15 -20 Zn. Se 7 -10/6 -8 Cd. Te 6 -9/5 -8 Cd. Se 6 -8/8 -12 III-V Ga. As 9/9. 4 In. P 6. 7/8. 7 In. As 6. 7/8. 3 Group IV C 25 graphite 35 -80 diamond Si 13 Ge 13 -16
Displacement energy Other materials: II-VI Zn. S 7 -9/15 -20 Zn. Se 7 -10/6 -8 Cd. Te 6 -9/5 -8 Cd. Se 6 -8/8 -12 III-V Ga. As 9/9. 4 In. P 6. 7/8. 7 In. As 6. 7/8. 3 Group IV C 25 graphite 35 -80 diamond Si 13 Ge 13 -16
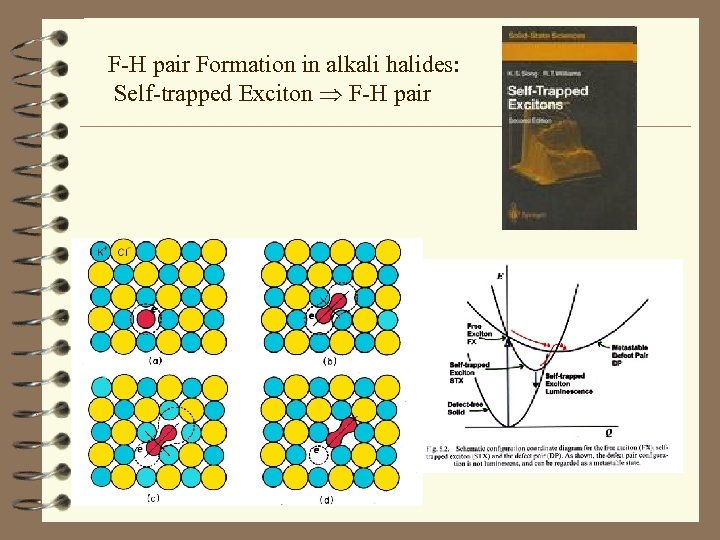 F-H pair Formation in alkali halides: Self-trapped Exciton F-H pair
F-H pair Formation in alkali halides: Self-trapped Exciton F-H pair
 Resistant and sensitive materials 4 Resistant: 4 Metals, semi-conductors. 4 crystalline Oxides: 4 metastables (Sr. Ti. O 3, Mg. O, Al 2 O 3, c-Si. O 2) 4 Sensitive: 4 Alkali halides 4 4 Alkaline-earth halides Ca. F 2, Mg. F 2, Sr. F 2 : 4 KMg. F 3, Ba. FBr, Li. YF 4: 4 Silver halides Ag. Cl; Ag. Br 4 Amorphous solids a-Si. O 2 , a-As 2 Se 3, a-As 2 S 3, a-Se, a-As 4 Water and organic mater (bio matter)
Resistant and sensitive materials 4 Resistant: 4 Metals, semi-conductors. 4 crystalline Oxides: 4 metastables (Sr. Ti. O 3, Mg. O, Al 2 O 3, c-Si. O 2) 4 Sensitive: 4 Alkali halides 4 4 Alkaline-earth halides Ca. F 2, Mg. F 2, Sr. F 2 : 4 KMg. F 3, Ba. FBr, Li. YF 4: 4 Silver halides Ag. Cl; Ag. Br 4 Amorphous solids a-Si. O 2 , a-As 2 Se 3, a-As 2 S 3, a-Se, a-As 4 Water and organic mater (bio matter)
 Radiolysis versus ballistic damage 4 Radiolysis is not universal, not easily predictable 4 2) Is in essence temperature dependent 4 3) Spans over a wide time scale 4 4) Occurs generally on one sub-lattice (anions) 4 5) Radiolysis occurs occasionally 4 when it occurs, it is with a good energetic efficiency. 4 Elastic damage occurs every time but with a relatively poor energetic efficiency.
Radiolysis versus ballistic damage 4 Radiolysis is not universal, not easily predictable 4 2) Is in essence temperature dependent 4 3) Spans over a wide time scale 4 4) Occurs generally on one sub-lattice (anions) 4 5) Radiolysis occurs occasionally 4 when it occurs, it is with a good energetic efficiency. 4 Elastic damage occurs every time but with a relatively poor energetic efficiency.
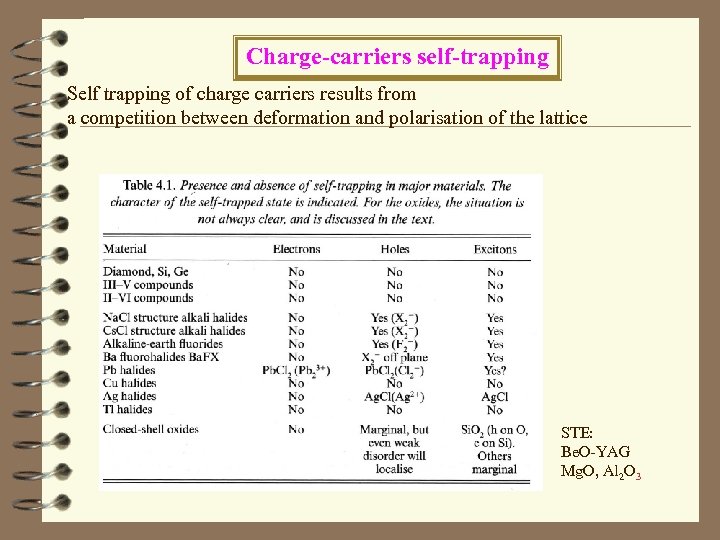 Charge-carriers self-trapping Self trapping of charge carriers results from a competition between deformation and polarisation of the lattice STE: Be. O-YAG Mg. O, Al 2 O 3
Charge-carriers self-trapping Self trapping of charge carriers results from a competition between deformation and polarisation of the lattice STE: Be. O-YAG Mg. O, Al 2 O 3
 Radiation Defects 1. Electronic defects, which involve changes in valence states Examples: KCl: Tl+ + hole Tl 2+ Tl+ + electron Tl 0 Mg. O: Fe etc Fe 2+ + hole Fe 3+ + electron Fe 2+ n-irradiated Mg. O
Radiation Defects 1. Electronic defects, which involve changes in valence states Examples: KCl: Tl+ + hole Tl 2+ Tl+ + electron Tl 0 Mg. O: Fe etc Fe 2+ + hole Fe 3+ + electron Fe 2+ n-irradiated Mg. O
 In this talk: 1. 2. 3. F center production in Cs-halides. Show the extension of Rabin. Klick diagram for all AHC. Discuss differences between F center in AHC and F+ and F center in oxide materials (Mg. O as an example) Discuss whether common and famous Mollwo-Ivey rule could be extended for oxide materials Type Self-trapping Formation of defects Some exciton hole Single excitation Dense excitation examples 1 no no no yes Mg. O, Ca. O 2 yes yes Alkali halides
In this talk: 1. 2. 3. F center production in Cs-halides. Show the extension of Rabin. Klick diagram for all AHC. Discuss differences between F center in AHC and F+ and F center in oxide materials (Mg. O as an example) Discuss whether common and famous Mollwo-Ivey rule could be extended for oxide materials Type Self-trapping Formation of defects Some exciton hole Single excitation Dense excitation examples 1 no no no yes Mg. O, Ca. O 2 yes yes Alkali halides
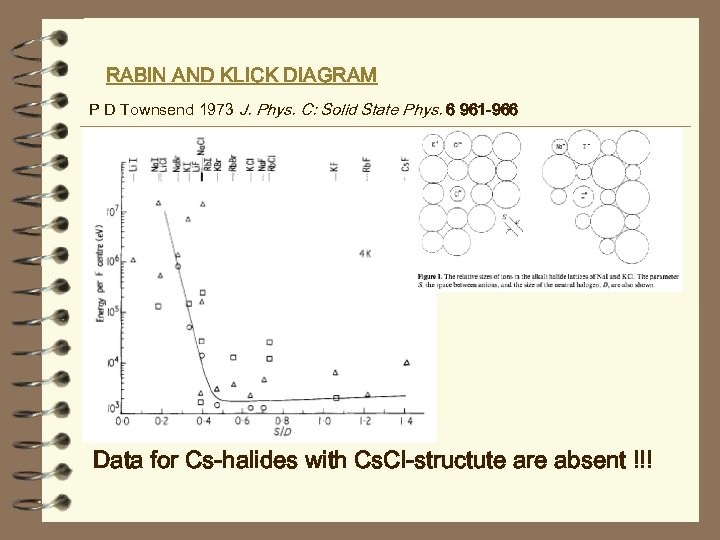 RABIN AND KLICK DIAGRAM P D Townsend 1973 J. Phys. C: Solid State Phys. 6 961 -966 Data for Cs-halides with Cs. Cl-structute are absent !!!
RABIN AND KLICK DIAGRAM P D Townsend 1973 J. Phys. C: Solid State Phys. 6 961 -966 Data for Cs-halides with Cs. Cl-structute are absent !!!
 Cs. I Three different types of Cs. I crystals were studied in this paper. Nominally pure Cs. I crystals have been grown in the Laboratoire de Spectroscopie Atomique (CNRS/ISMRA, Caen). The low-doped Cs. I–Tl crystals with Tl+ ion concentration of about 1017 ion/cm 3 have been supplied by Dr. P. Schotanus (SCIONIX, Holland). The highly doped Cs. I–Tl with Tl+ ion concentration of about 1019 ion/cm 3 was obtained Institute of Solid State Physics, University of Latvia. Crystals have been irradiated at GANIL on the medium-energy beam line (SME) with 86 Kr ions (8. 63 Me. V/amu). In this study, both the irradiation and in-situ measurements were done at 15 K.
Cs. I Three different types of Cs. I crystals were studied in this paper. Nominally pure Cs. I crystals have been grown in the Laboratoire de Spectroscopie Atomique (CNRS/ISMRA, Caen). The low-doped Cs. I–Tl crystals with Tl+ ion concentration of about 1017 ion/cm 3 have been supplied by Dr. P. Schotanus (SCIONIX, Holland). The highly doped Cs. I–Tl with Tl+ ion concentration of about 1019 ion/cm 3 was obtained Institute of Solid State Physics, University of Latvia. Crystals have been irradiated at GANIL on the medium-energy beam line (SME) with 86 Kr ions (8. 63 Me. V/amu). In this study, both the irradiation and in-situ measurements were done at 15 K.
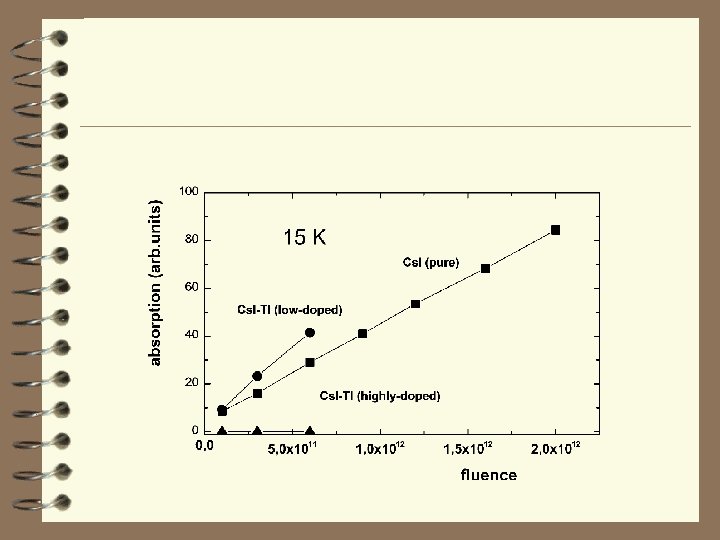
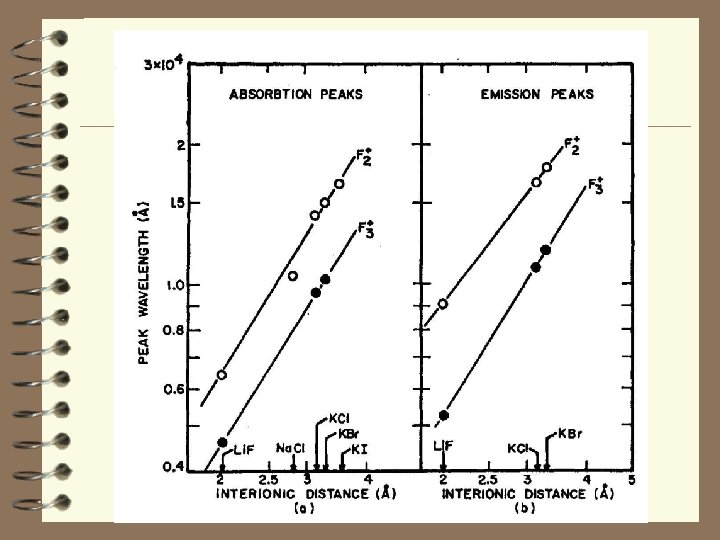
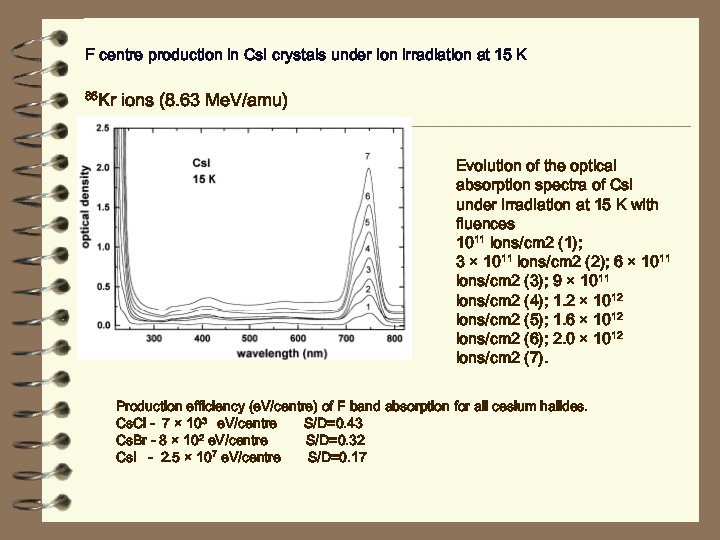 F centre production in Cs. I crystals under ion irradiation at 15 K 86 Kr ions (8. 63 Me. V/amu) Evolution of the optical absorption spectra of Cs. I under irradiation at 15 K with fluences 1011 ions/cm 2 (1); 3 × 1011 ions/cm 2 (2); 6 × 1011 ions/cm 2 (3); 9 × 1011 ions/cm 2 (4); 1. 2 × 1012 ions/cm 2 (5); 1. 6 × 1012 ions/cm 2 (6); 2. 0 × 1012 ions/cm 2 (7). Production efficiency (e. V/centre) of F band absorption for all cesium halides. Cl - 7 × 103 e. V/centre S/D=0. 43 2 e. V/centre Cs. Br - 8 × 10 S/D=0. 32 7 e. V/centre Cs. I - 2. 5 × 10 S/D=0. 17
F centre production in Cs. I crystals under ion irradiation at 15 K 86 Kr ions (8. 63 Me. V/amu) Evolution of the optical absorption spectra of Cs. I under irradiation at 15 K with fluences 1011 ions/cm 2 (1); 3 × 1011 ions/cm 2 (2); 6 × 1011 ions/cm 2 (3); 9 × 1011 ions/cm 2 (4); 1. 2 × 1012 ions/cm 2 (5); 1. 6 × 1012 ions/cm 2 (6); 2. 0 × 1012 ions/cm 2 (7). Production efficiency (e. V/centre) of F band absorption for all cesium halides. Cl - 7 × 103 e. V/centre S/D=0. 43 2 e. V/centre Cs. Br - 8 × 10 S/D=0. 32 7 e. V/centre Cs. I - 2. 5 × 10 S/D=0. 17
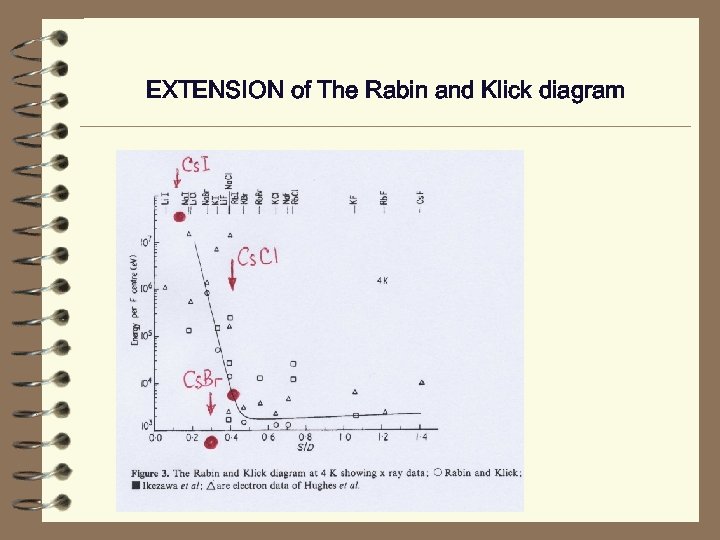 EXTENSION of The Rabin and Klick diagram
EXTENSION of The Rabin and Klick diagram
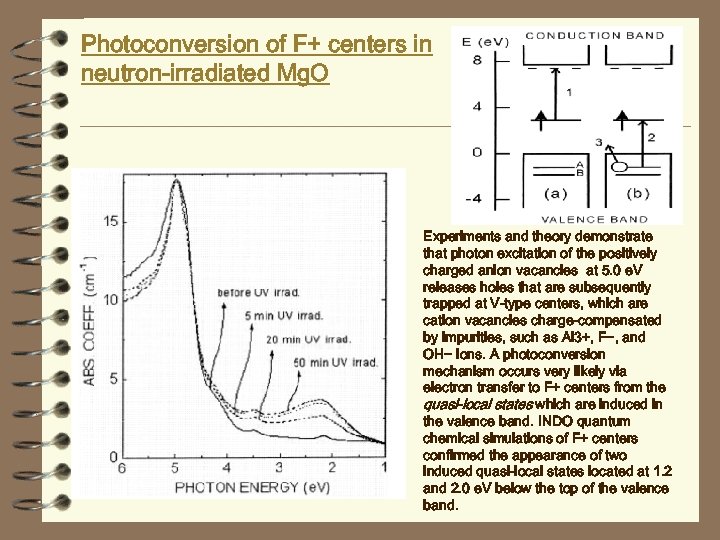 Photoconversion of F+ centers in neutron-irradiated Mg. O Experiments and theory demonstrate that photon excitation of the positively charged anion vacancies at 5. 0 e. V releases holes that are subsequently trapped at V-type centers, which are cation vacancies charge-compensated by impurities, such as Al 3+, F−, and OH− ions. A photoconversion mechanism occurs very likely via electron transfer to F+ centers from the quasi-local states which are induced in the valence band. INDO quantum chemical simulations of F+ centers confirmed the appearance of two induced quasi-local states located at 1. 2 and 2. 0 e. V below the top of the valence band.
Photoconversion of F+ centers in neutron-irradiated Mg. O Experiments and theory demonstrate that photon excitation of the positively charged anion vacancies at 5. 0 e. V releases holes that are subsequently trapped at V-type centers, which are cation vacancies charge-compensated by impurities, such as Al 3+, F−, and OH− ions. A photoconversion mechanism occurs very likely via electron transfer to F+ centers from the quasi-local states which are induced in the valence band. INDO quantum chemical simulations of F+ centers confirmed the appearance of two induced quasi-local states located at 1. 2 and 2. 0 e. V below the top of the valence band.
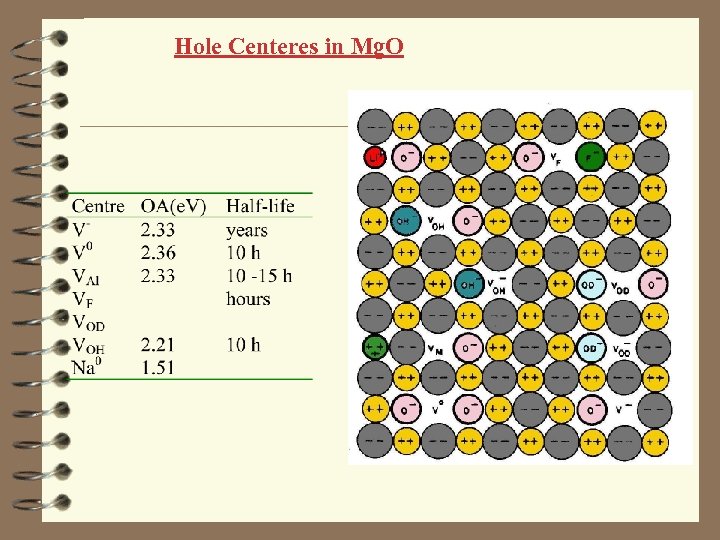
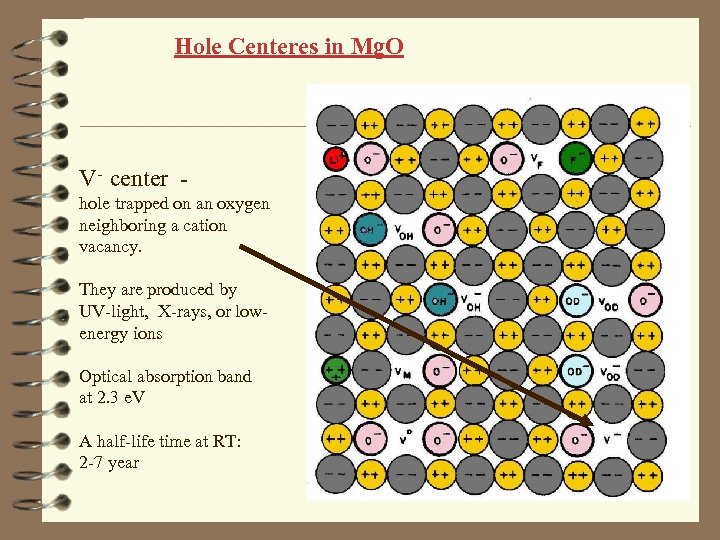 Hole Centeres in Mg. O V- center hole trapped on an oxygen neighboring a cation vacancy. They are produced by UV-light, X-rays, or lowenergy ions Optical absorption band at 2. 3 e. V A half-life time at RT: 2 -7 year
Hole Centeres in Mg. O V- center hole trapped on an oxygen neighboring a cation vacancy. They are produced by UV-light, X-rays, or lowenergy ions Optical absorption band at 2. 3 e. V A half-life time at RT: 2 -7 year
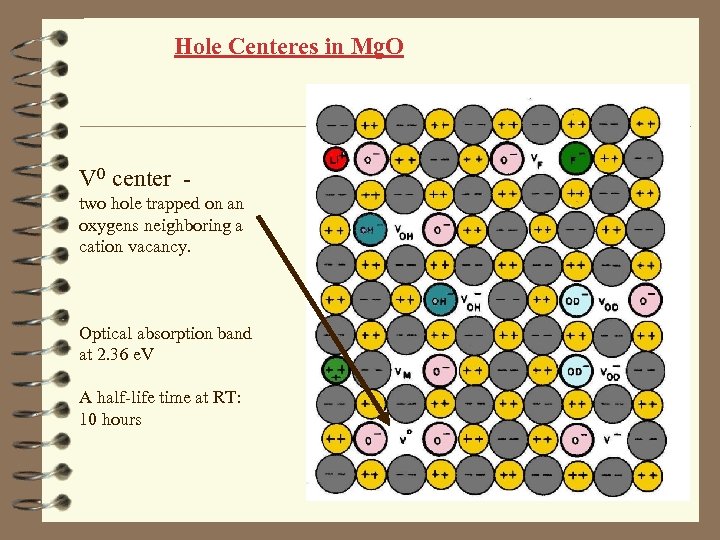 Hole Centeres in Mg. O V 0 center two hole trapped on an oxygens neighboring a cation vacancy. Optical absorption band at 2. 36 e. V A half-life time at RT: 10 hours
Hole Centeres in Mg. O V 0 center two hole trapped on an oxygens neighboring a cation vacancy. Optical absorption band at 2. 36 e. V A half-life time at RT: 10 hours
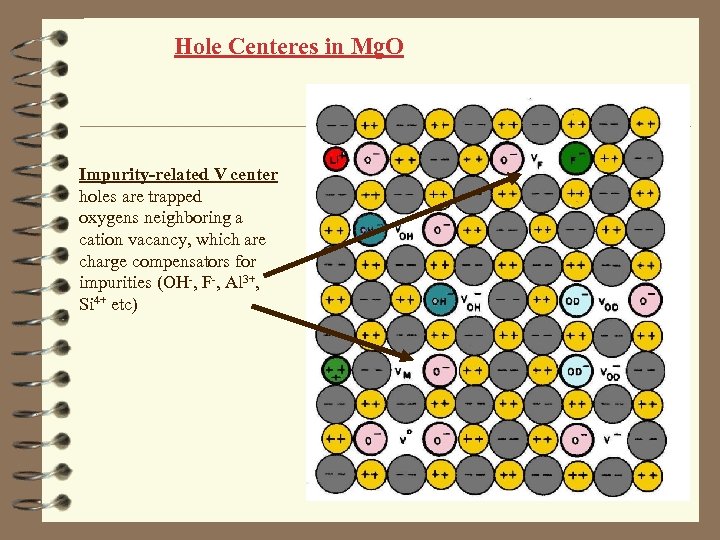 Hole Centeres in Mg. O Impurity-related V center holes are trapped oxygens neighboring a cation vacancy, which are charge compensators for impurities (OH-, F-, Al 3+, Si 4+ etc)
Hole Centeres in Mg. O Impurity-related V center holes are trapped oxygens neighboring a cation vacancy, which are charge compensators for impurities (OH-, F-, Al 3+, Si 4+ etc)
 Hole Centeres in Mg. O
Hole Centeres in Mg. O
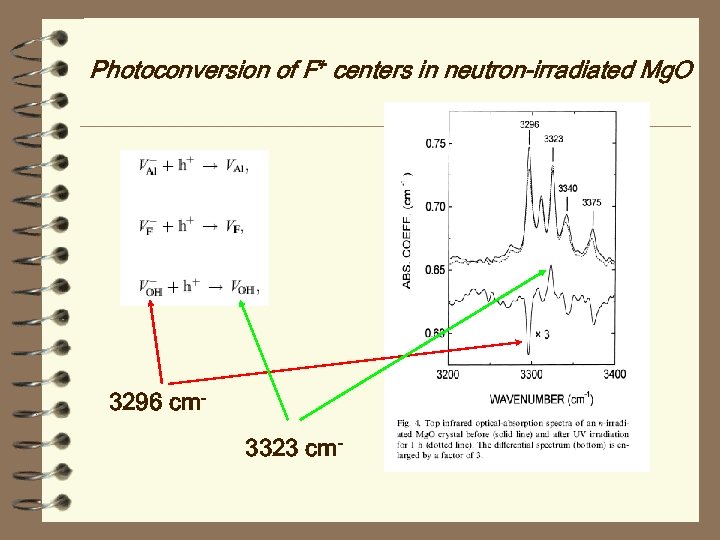 Photoconversion of F+ centers in neutron-irradiated Mg. O 3296 cm 3323 cm-
Photoconversion of F+ centers in neutron-irradiated Mg. O 3296 cm 3323 cm-
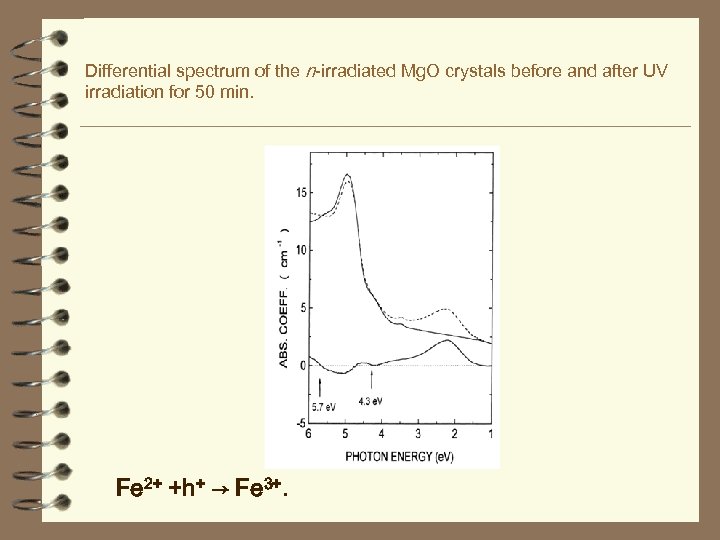 Differential spectrum of the n-irradiated Mg. O crystals before and after UV irradiation for 50 min. Fe 2+ +h+ → Fe 3+.
Differential spectrum of the n-irradiated Mg. O crystals before and after UV irradiation for 50 min. Fe 2+ +h+ → Fe 3+.
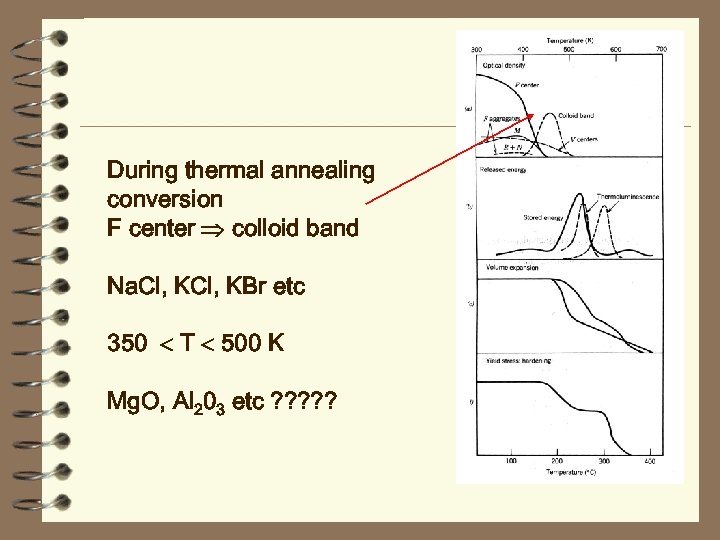 During thermal annealing conversion F center colloid band Na. Cl, KBr etc 350 T 500 K Mg. O, Al 203 etc ? ? ?
During thermal annealing conversion F center colloid band Na. Cl, KBr etc 350 T 500 K Mg. O, Al 203 etc ? ? ?
 Mg. O TCR samples The Mg. O crystals used were grown at the Oak Ridge National Laboratory using the arc fusion technique. The starting material was Mg. O powder from the Kanto Chemical Company, Japan. TCR was performed in a tantalum chamber at 2000 K and 7 atmospheres of magnesium vapor, followed by rapid cooling. This process produces anion oxygen vacancies, due to a stoichiometric excess of cations.
Mg. O TCR samples The Mg. O crystals used were grown at the Oak Ridge National Laboratory using the arc fusion technique. The starting material was Mg. O powder from the Kanto Chemical Company, Japan. TCR was performed in a tantalum chamber at 2000 K and 7 atmospheres of magnesium vapor, followed by rapid cooling. This process produces anion oxygen vacancies, due to a stoichiometric excess of cations.
 Mg. O: vacancy diffusion Mg. O- The activation energy for diffusion is found to increase monotonically in the series Vc, Va, F+ and F center (2. 43, 2. 50, 2. 72, and 3. 13 e. V, respectively).
Mg. O: vacancy diffusion Mg. O- The activation energy for diffusion is found to increase monotonically in the series Vc, Va, F+ and F center (2. 43, 2. 50, 2. 72, and 3. 13 e. V, respectively).
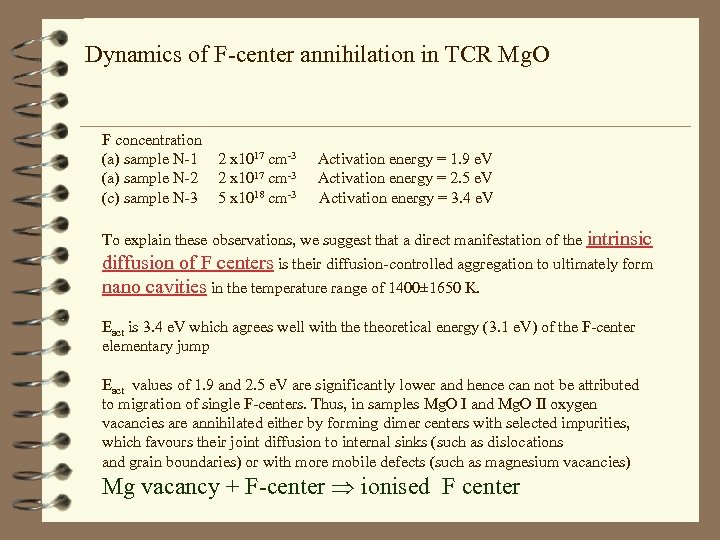 Dynamics of F-center annihilation in TCR Mg. O F concentration (a) sample N-1 (a) sample N-2 (c) sample N-3 2 x 1017 cm-3 5 x 1018 cm-3 Activation energy = 1. 9 e. V Activation energy = 2. 5 e. V Activation energy = 3. 4 e. V To explain these observations, we suggest that a direct manifestation of the intrinsic diffusion of F centers is their diffusion-controlled aggregation to ultimately form nano cavities in the temperature range of 1400± 1650 K. Eact is 3. 4 e. V which agrees well with theoretical energy (3. 1 e. V) of the F-center elementary jump Eact values of 1. 9 and 2. 5 e. V are significantly lower and hence can not be attributed to migration of single F-centers. Thus, in samples Mg. O I and Mg. O II oxygen vacancies are annihilated either by forming dimer centers with selected impurities, which favours their joint diffusion to internal sinks (such as dislocations and grain boundaries) or with more mobile defects (such as magnesium vacancies) Mg vacancy + F-center ionised F center
Dynamics of F-center annihilation in TCR Mg. O F concentration (a) sample N-1 (a) sample N-2 (c) sample N-3 2 x 1017 cm-3 5 x 1018 cm-3 Activation energy = 1. 9 e. V Activation energy = 2. 5 e. V Activation energy = 3. 4 e. V To explain these observations, we suggest that a direct manifestation of the intrinsic diffusion of F centers is their diffusion-controlled aggregation to ultimately form nano cavities in the temperature range of 1400± 1650 K. Eact is 3. 4 e. V which agrees well with theoretical energy (3. 1 e. V) of the F-center elementary jump Eact values of 1. 9 and 2. 5 e. V are significantly lower and hence can not be attributed to migration of single F-centers. Thus, in samples Mg. O I and Mg. O II oxygen vacancies are annihilated either by forming dimer centers with selected impurities, which favours their joint diffusion to internal sinks (such as dislocations and grain boundaries) or with more mobile defects (such as magnesium vacancies) Mg vacancy + F-center ionised F center
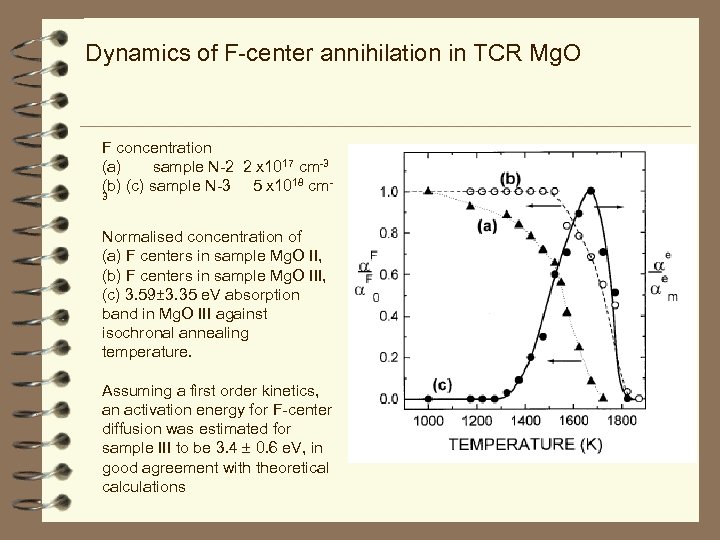 Dynamics of F-center annihilation in TCR Mg. O F concentration (a) sample N-2 2 x 1017 cm-3 (b) (c) sample N-3 5 x 1018 cm 3 Normalised concentration of (a) F centers in sample Mg. O II, (b) F centers in sample Mg. O III, (c) 3. 59± 3. 35 e. V absorption band in Mg. O III against isochronal annealing temperature. Assuming a first order kinetics, an activation energy for F-center diffusion was estimated for sample III to be 3. 4 0. 6 e. V, in good agreement with theoretical calculations
Dynamics of F-center annihilation in TCR Mg. O F concentration (a) sample N-2 2 x 1017 cm-3 (b) (c) sample N-3 5 x 1018 cm 3 Normalised concentration of (a) F centers in sample Mg. O II, (b) F centers in sample Mg. O III, (c) 3. 59± 3. 35 e. V absorption band in Mg. O III against isochronal annealing temperature. Assuming a first order kinetics, an activation energy for F-center diffusion was estimated for sample III to be 3. 4 0. 6 e. V, in good agreement with theoretical calculations
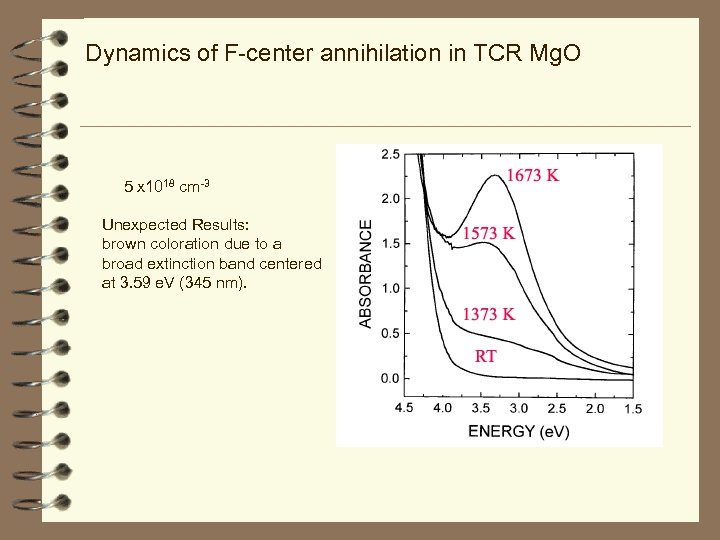 Dynamics of F-center annihilation in TCR Mg. O 5 x 1018 cm-3 Unexpected Results: brown coloration due to a broad extinction band centered at 3. 59 e. V (345 nm).
Dynamics of F-center annihilation in TCR Mg. O 5 x 1018 cm-3 Unexpected Results: brown coloration due to a broad extinction band centered at 3. 59 e. V (345 nm).
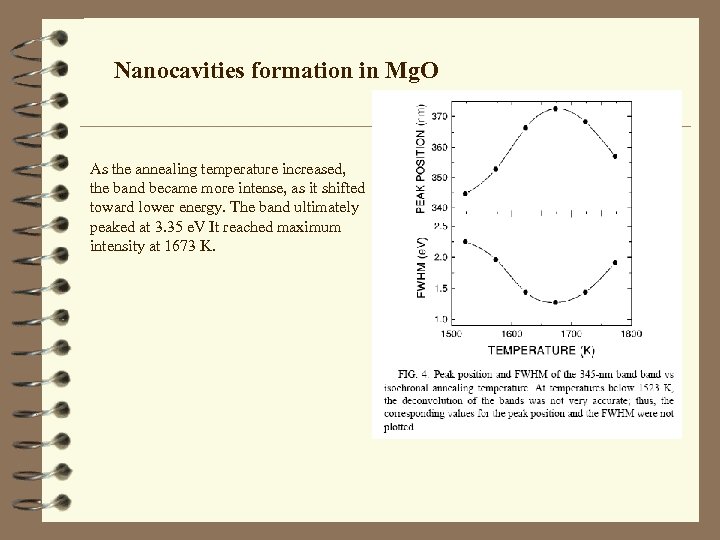 Nanocavities formation in Mg. O As the annealing temperature increased, the band became more intense, as it shifted toward lower energy. The band ultimately peaked at 3. 35 e. V It reached maximum intensity at 1673 K.
Nanocavities formation in Mg. O As the annealing temperature increased, the band became more intense, as it shifted toward lower energy. The band ultimately peaked at 3. 35 e. V It reached maximum intensity at 1673 K.
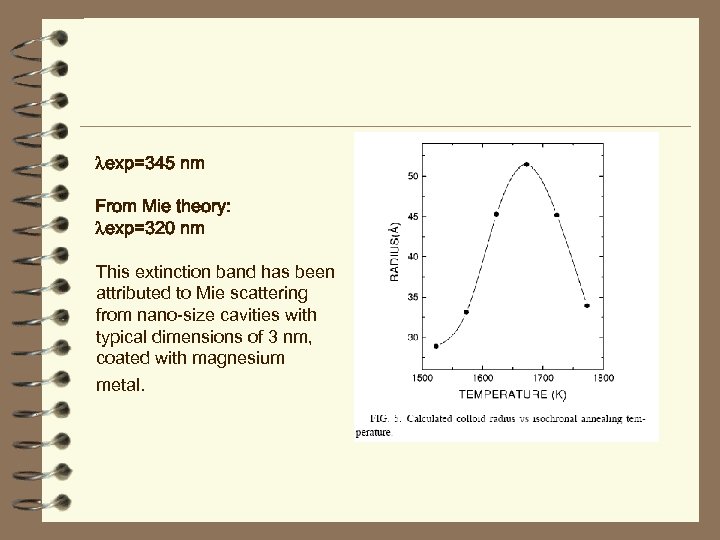 exp=345 nm From Mie theory: exp=320 nm This extinction band has been attributed to Mie scattering from nano-size cavities with typical dimensions of 3 nm, coated with magnesium metal.
exp=345 nm From Mie theory: exp=320 nm This extinction band has been attributed to Mie scattering from nano-size cavities with typical dimensions of 3 nm, coated with magnesium metal.
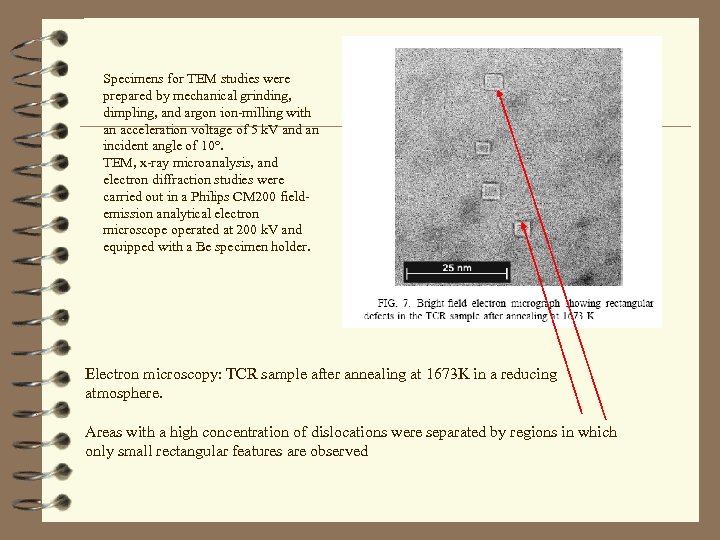 Specimens for TEM studies were prepared by mechanical grinding, dimpling, and argon ion-milling with an acceleration voltage of 5 k. V and an incident angle of 10°. TEM, x-ray microanalysis, and electron diffraction studies were carried out in a Philips CM 200 fieldemission analytical electron microscope operated at 200 k. V and equipped with a Be specimen holder. Electron microscopy: TCR sample after annealing at 1673 K in a reducing atmosphere. Areas with a high concentration of dislocations were separated by regions in which only small rectangular features are observed
Specimens for TEM studies were prepared by mechanical grinding, dimpling, and argon ion-milling with an acceleration voltage of 5 k. V and an incident angle of 10°. TEM, x-ray microanalysis, and electron diffraction studies were carried out in a Philips CM 200 fieldemission analytical electron microscope operated at 200 k. V and equipped with a Be specimen holder. Electron microscopy: TCR sample after annealing at 1673 K in a reducing atmosphere. Areas with a high concentration of dislocations were separated by regions in which only small rectangular features are observed
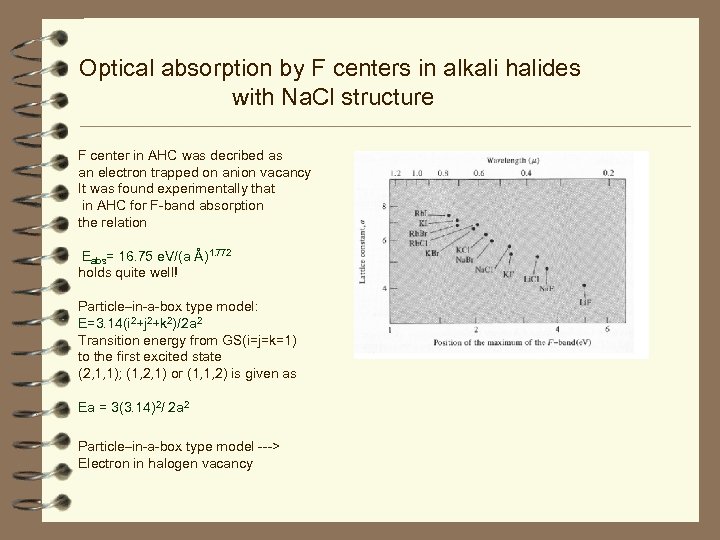 Optical absorption by F centers in alkali halides with Na. Cl structure F center in AHC was decribed as an electron trapped on anion vacancy It was found experimentally that in AHC for F-band absorption the relation Eabs= 16. 75 e. V/(a Å)1. 772 holds quite well! Particle–in-a-box type model: E=3. 14(i 2+j 2+k 2)/2 a 2 Transition energy from GS(i=j=k=1) to the first excited state (2, 1, 1); (1, 2, 1) or (1, 1, 2) is given as Ea = 3(3. 14)2/ 2 a 2 Particle–in-a-box type model ---> Electron in halogen vacancy
Optical absorption by F centers in alkali halides with Na. Cl structure F center in AHC was decribed as an electron trapped on anion vacancy It was found experimentally that in AHC for F-band absorption the relation Eabs= 16. 75 e. V/(a Å)1. 772 holds quite well! Particle–in-a-box type model: E=3. 14(i 2+j 2+k 2)/2 a 2 Transition energy from GS(i=j=k=1) to the first excited state (2, 1, 1); (1, 2, 1) or (1, 1, 2) is given as Ea = 3(3. 14)2/ 2 a 2 Particle–in-a-box type model ---> Electron in halogen vacancy
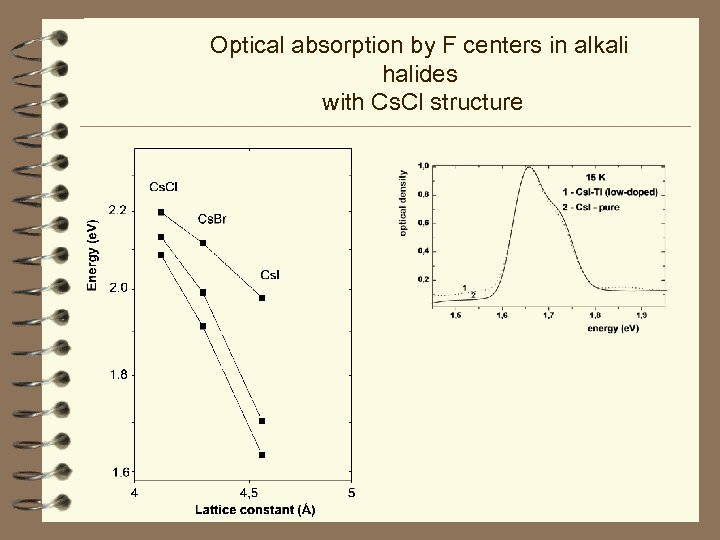 Optical absorption by F centers in alkali halides with Cs. Cl structure
Optical absorption by F centers in alkali halides with Cs. Cl structure
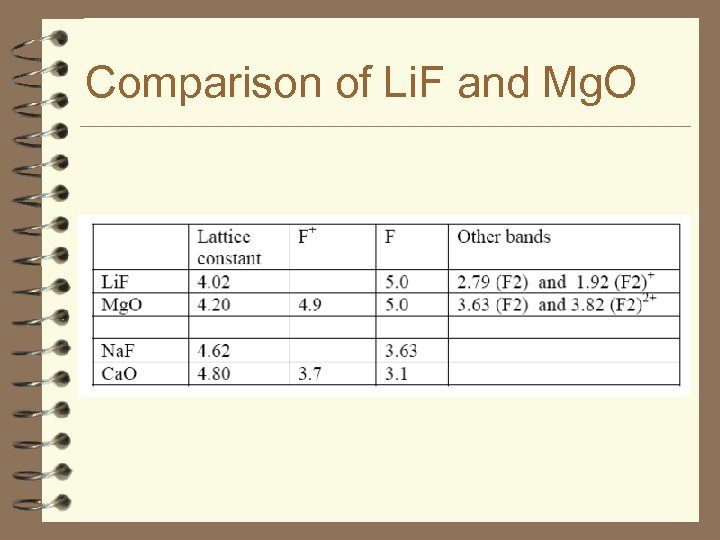 Comparison of Li. F and Mg. O
Comparison of Li. F and Mg. O
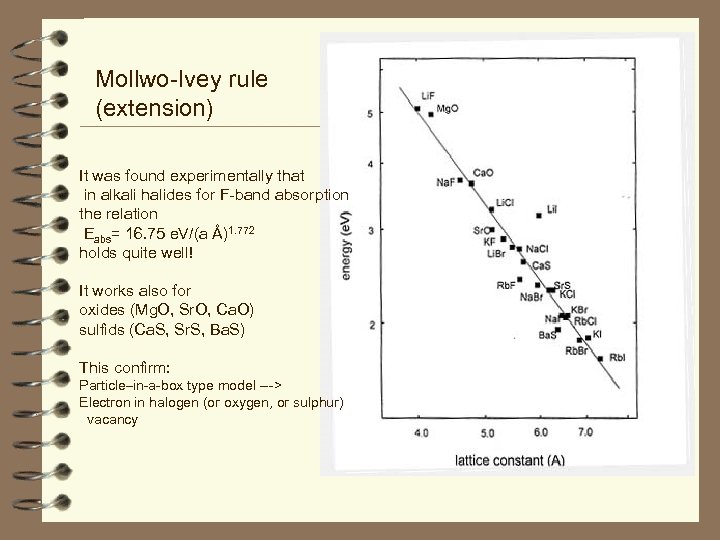 Mollwo-Ivey rule (extension) It was found experimentally that in alkali halides for F-band absorption the relation Eabs= 16. 75 e. V/(a Å)1. 772 holds quite well! It works also for oxides (Mg. O, Sr. O, Ca. O) sulfids (Ca. S, Sr. S, Ba. S) This confirm: Particle–in-a-box type model ---> Electron in halogen (or oxygen, or sulphur) vacancy
Mollwo-Ivey rule (extension) It was found experimentally that in alkali halides for F-band absorption the relation Eabs= 16. 75 e. V/(a Å)1. 772 holds quite well! It works also for oxides (Mg. O, Sr. O, Ca. O) sulfids (Ca. S, Sr. S, Ba. S) This confirm: Particle–in-a-box type model ---> Electron in halogen (or oxygen, or sulphur) vacancy
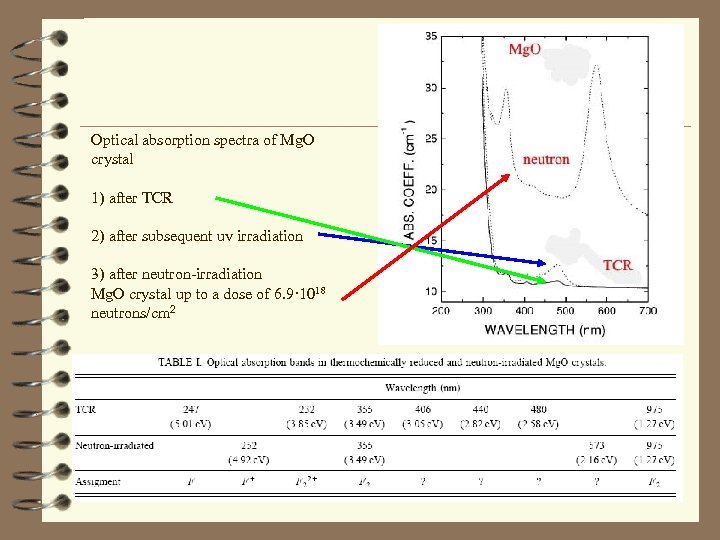 Optical absorption spectra of Mg. O crystal 1) after TCR 2) after subsequent uv irradiation 3) after neutron-irradiation Mg. O crystal up to a dose of 6. 9· 1018 neutrons/cm 2
Optical absorption spectra of Mg. O crystal 1) after TCR 2) after subsequent uv irradiation 3) after neutron-irradiation Mg. O crystal up to a dose of 6. 9· 1018 neutrons/cm 2
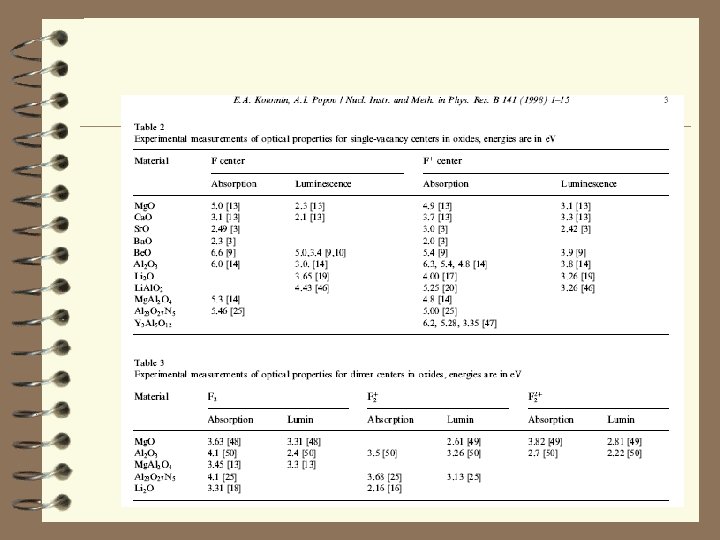
 Conclusion: 1. 2. 3. F center production in Cs-halides. Show the extension of Rabin. Klick diagram for all AHC. Discuss differences between F center in AHC and F+ and F center in oxide materials (Mg. O as an example) Show that famous Mollwo-Ivey rule could be extended for some simple oxide and sulfide materials with Na. Cl structure
Conclusion: 1. 2. 3. F center production in Cs-halides. Show the extension of Rabin. Klick diagram for all AHC. Discuss differences between F center in AHC and F+ and F center in oxide materials (Mg. O as an example) Show that famous Mollwo-Ivey rule could be extended for some simple oxide and sulfide materials with Na. Cl structure
 Thank you very much for your attention
Thank you very much for your attention


