7b9a6bf01851257796a61b65bbe8d60c.ppt
- Количество слайдов: 35
RABIES IMMUNOGLOBULINS Dr. P. SANGRAM, MBBS, MD; MRC PATH (LONDON)
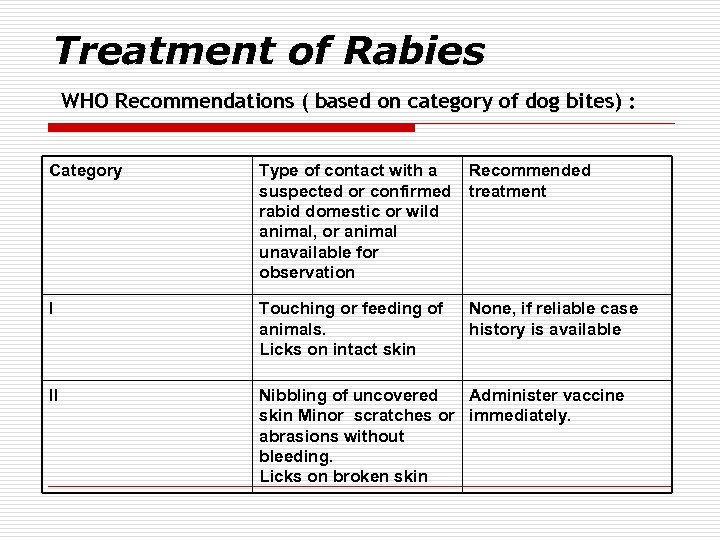
Treatment of Rabies WHO Recommendations ( based on category of dog bites) : Category Type of contact with a suspected or confirmed rabid domestic or wild animal, or animal unavailable for observation Recommended treatment I Touching or feeding of animals. Licks on intact skin None, if reliable case history is available II Nibbling of uncovered Administer vaccine skin Minor scratches or immediately. abrasions without bleeding. Licks on broken skin
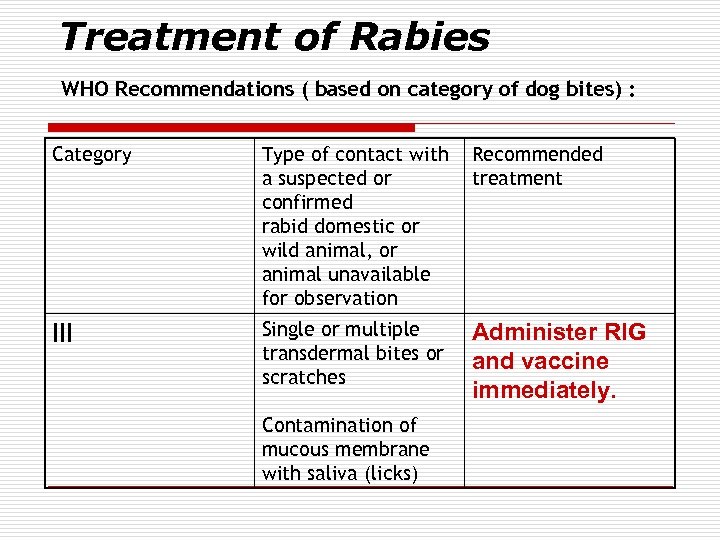
Treatment of Rabies WHO Recommendations ( based on category of dog bites) : Category Type of contact with a suspected or confirmed rabid domestic or wild animal, or animal unavailable for observation Recommended treatment III Single or multiple transdermal bites or scratches Administer RIG and vaccine immediately. Contamination of mucous membrane with saliva (licks)
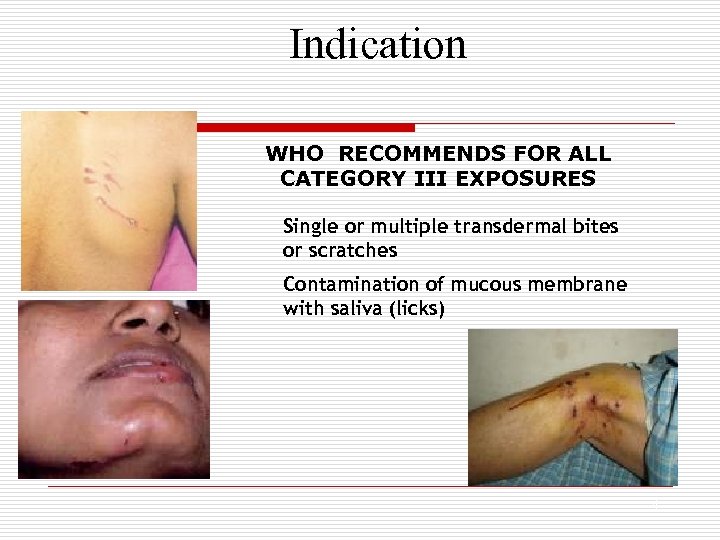
Indication WHO RECOMMENDS FOR ALL CATEGORY III EXPOSURES Single or multiple transdermal bites or scratches Contamination of mucous membrane with saliva (licks) 4

RIG in India o Rabies takes 20000 lives every year. o More than 70% victims are of category-III bites o Awareness of RIG is still poor o Only 6% victims gets complete treatment
RABIES IMMUNOGLOBULINS o o Rabies Immunoglobulins are special neutralizing antibodies that immediately neutralize virus on contact. Rabies Immunoglobulin gives a coating to the virus so that it cannot enter the nerve ending resulting in reduction or total obliteration of inoculated virus.
RABIES IMMUNOGLOBULINS o There are two types of specific rabies immunoglobulins: 1. Human Rabies Immunoglobulin (HRIG) 2. Equine Rabies Immunoglobulin (ERIG)
HUMAN RABIES IMMUNOGLOBULINS o o o It is a liquid of Freeze-Dried preparation containing – Immunoglobulins mainly Ig. G obtained from plasma or serum of donors immunized against rabies and contains specific antibodies that neutralize the rabies virus. It is prepared from plasma of more than 1000 donors. It provides passive protection when given immediately to individuals exposed to rabies virus.
EQUINE RABIES IMMUNOGLOBULINS o o o It is produced by immunizing horses with low concentrations of rabies vaccines. The antibodies produced in the blood of these horses are then purified and filtered. The modern automated ultra filtration technology reduces the time it takes to purify immunoglobulins from one week to one day – while increasing yield levels. Thailand (Thai Red Cross) produces and distributes over 100, 000 doses of ERIGS per year
GLOBAL STANDARDS o A combination of rabies immunoglobulin and vaccine together with prompt and appropriate wound care, has become the Global Standard for prevention after human exposure. o Administration of rabies immunoglobulin as a part of routine post-exposure treatment is intended to provide a passive source of antibodies before endogenous development by vaccination. o The need for rabies immunoglobulin appears to be on the rise, because of the ubiquity of human exposure to rabies virus from uncontrolled canine sources in developing countries.
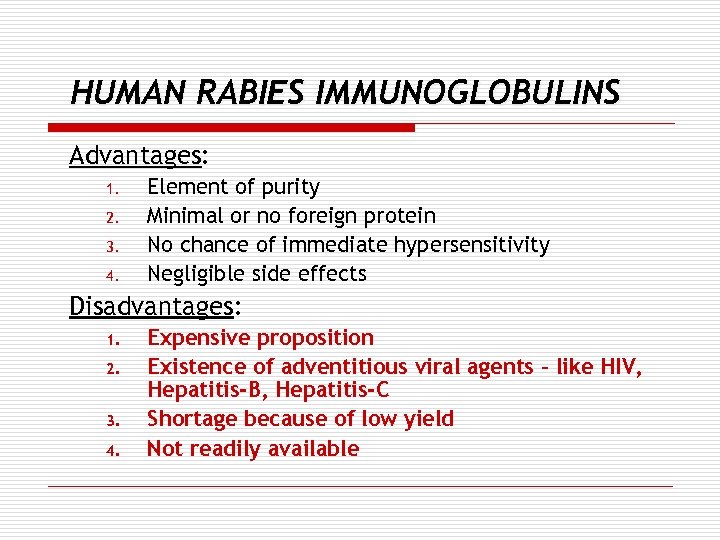
HUMAN RABIES IMMUNOGLOBULINS Advantages: 1. 2. 3. 4. Element of purity Minimal or no foreign protein No chance of immediate hypersensitivity Negligible side effects Disadvantages: 1. 2. 3. 4. Expensive proposition Existence of adventitious viral agents – like HIV, Hepatitis-B, Hepatitis-C Shortage because of low yield Not readily available
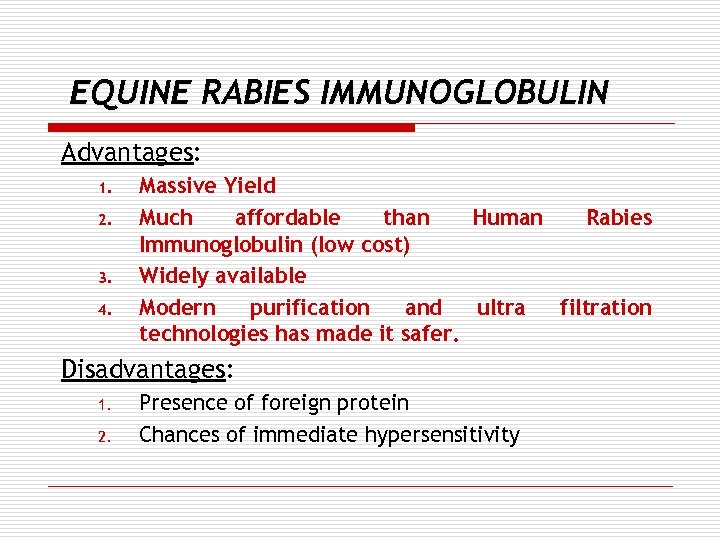
EQUINE RABIES IMMUNOGLOBULIN Advantages: 1. 2. 3. 4. Massive Yield Much affordable than Human Rabies Immunoglobulin (low cost) Widely available Modern purification and ultra filtration technologies has made it safer. Disadvantages: 1. 2. Presence of foreign protein Chances of immediate hypersensitivity

INDICATIONS FOR USE OF RABIES IMMUNOGLOBULINS 1. 2. 3. 4. 5. 6. 7. 8. All category III bites (WHO classification) Licks on mucous membranes by wild or pet animals Category II & III bites in case AIDS patients with grossly reduced CD 4 count Varicella (can cause transient immunodeficiency in children) Patients on long term Corticosteroids Long term Chemotherapy Radiation Therapy Wound suturing (pre-infiltration)
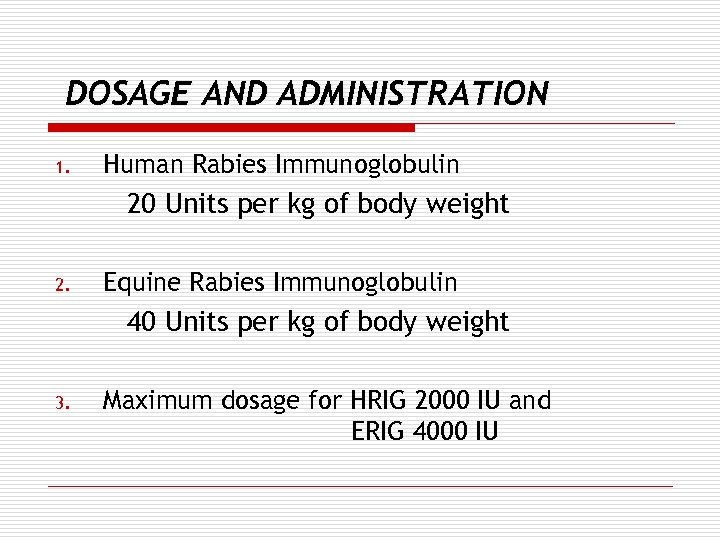
DOSAGE AND ADMINISTRATION 1. Human Rabies Immunoglobulin 20 Units per kg of body weight 2. Equine Rabies Immunoglobulin 40 Units per kg of body weight 3. Maximum dosage for HRIG 2000 IU and ERIG 4000 IU
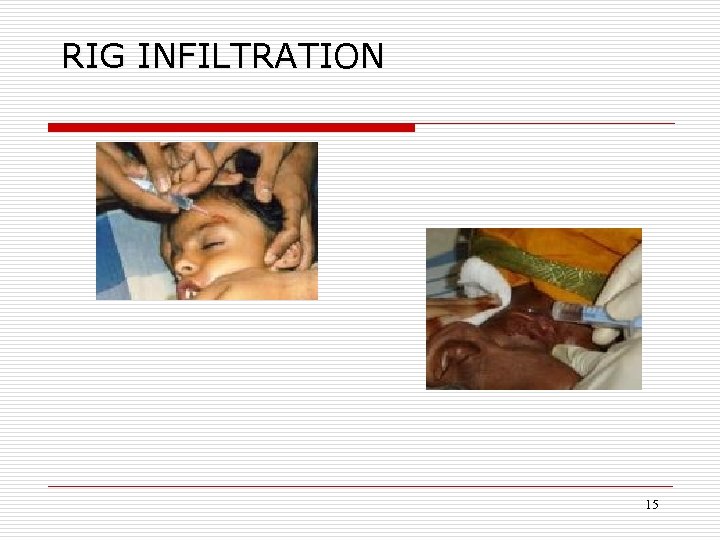
RIG INFILTRATION 15
DOSAGE AND ADMINISTRATION o o o As much as anatomically feasible the RIG should be infiltrated into and around the wound. The remaining portion of the calculated amount of the RIG, whatever is left over, to be injected in the deltoid / gluteal region away from the site of vaccine administration to prevent on site neutralization of vaccine antigen. RIGs should never be administered intravenously because of the potential for serious reactions.
DOSAGE AND ADMINISTRATION o o The dose should not exceed the calculated recommended dose since RIG may partially suppress active production of rabies antibody. RIG should be administered only once usually at the beginning of the post-exposure regimen and beyond the 7 th day RIG is not indicated as it may interfere with endogenous antibody response.
ADMINISTRATION – PRECAUTIONS o o Warm the ampoule to body temperature before intramuscular injection. Do not administer with the same syringe or into the same anatomical site as the rabies vaccine, discard any unused portion. Care is needed to avoid RIG seeping out of wounds during infiltration. If such a loss does occur, the volume should be estimated and replaced. Caution is needed if injecting into a tissue e. g. finger pulp, excess fluid can result in increased compartmental pressure and lead to necrosis.

RIG TREATMENT - CHILDREN o o o For small children – The calculated dosage of RIG may be insufficient to infiltrate all wounds. Sterile saline can be used to dilute the volume 2 or 3 fold to permit thorough effective infiltration. Under any circumstances the total dose of RIG should not be increased for the fear of reducing the vaccine induced response.
RABIES IMMUNOGLOBULINS – MECHANISM OF ACTION EFFECT o o Following intramuscular administration – Rabies immunoglobulin provides immediate passive antibodies for a short period of time. This protects the patient until the patient can produce antibodies from the rabies vaccine. An adequate titre of passive antibody is present 24 hours after RIG administration. Duration of Protective Effect: Short: Rabies immunoglobulin has a half-life of approximately 21 days. Cross sensitivity and/or related problems: Patients sensitive to other human immune globulin products may be sensitive to Rabies Immunoglobulin also.

RABIES IMMUNOGLOBULINS – PREGNANCY AND LACTATION o Pregnancy has contraindication not been considered to be a o No harmful effect on the foetus or neonate. o A study done on 202 women and their infants at Queen Saovobha Memorial Institute, Bangkok, Thailand (WHO collaborating center for Rabies) by Henry Wilde confirms this finding (during 1987 -1989 – 2 year study). o Breastfeeding: No problems have been documented in Humans. In fact Immunoglobulins are excreted into the milk and may contribute to the transfer of protective antibodies to the neonate.
RABIES IMMUNOGLOBULINS – SIDE / ADVERSE EFFECTS o o o Severe adverse systemic effects to Rabies Immunoglobulin are rare. Side effects of Less Frequency: 1. Local: Pain, tenderness may occur at injection site; cutaneous reactions. 2. Systemic: Headache, Fever, Chills, Flushing, Backache, Nausea – have been reported. Persons with selective Ig. A deficiency may develop antibodies to the small amount of Ig. A in this preparation, leading to sensitization and subsequent reaction to Ig. A containing material, such as blood and plasma derived medical products.
DRUG INTERACTIONS AND/OR RELATED PROBLEMS o o Immunoglobulin administration may impair for a period of at least 6 weeks and up to 3 months, the efficacy of live attenuated virus vaccines such as Measles, Mumps, Rubella and Varicella. Hence these vaccines should be administered at least 14 days prior to or 3 months after administration of Rabies Immunoglobulin. Over dosage may result in significant depression of active immunity. Repeated doses should not be administered once the rabies vaccine treatment has been started, because this could prevent full development of the active immunity expected from the vaccine.
STORAGE AND OTHER PRECAUTIONS o o o Store between 20 C - 80 C (350 F - 460 F) Do not freeze The solution should not be used if it is discoloured or contains particulate matter RIG should not be heated RIG should not be administered in the same syringe or into the same body site as the rabies vaccine.
ABHAYRIG ADMINISTRATION – INTRADERMAL TEST o Since Abhay. RIG is Equine Rabies Immunoglobulins, it is mandatory to carry out an intradermal test to know the sensitivity status of the individual. o Test: 0. 1 ml of 1: 10 diluted Abhay. RIG with normal saline is given intradermally on the left forearm. o Observation: A patient is observed for 15 minutes for local /systemic reactions.
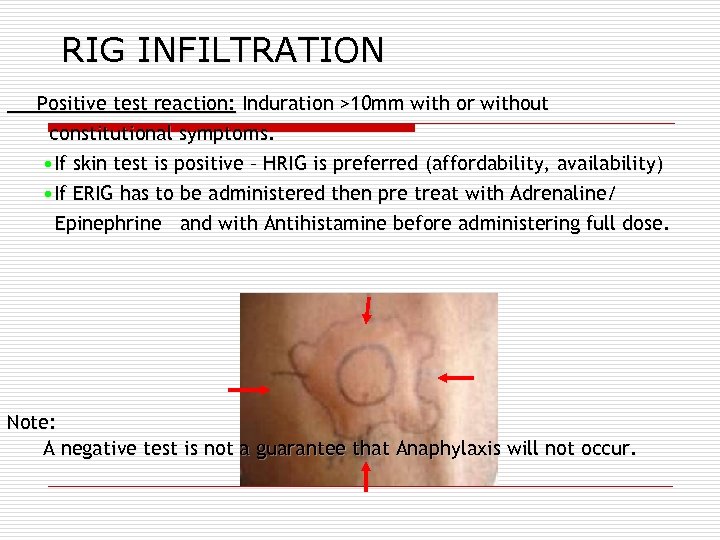
RIG INFILTRATION Positive test reaction: Induration >10 mm with or without constitutional symptoms. • If skin test is positive – HRIG is preferred (affordability, availability) • If ERIG has to be administered then pre treat with Adrenaline/ Epinephrine and with Antihistamine before administering full dose. Note: A negative test is not a guarantee that Anaphylaxis will not occur.
ABHAYRIG ADMINISTRATION – INTRADERMAL TEST o o o Local: Erythema, Wheal, Severe pain at the local site. Systemic: Giddiness may lead to mild circulatory collapse with fall of BP. Precaution: Patients giving history of sensitization to other vaccines or immunoglobulins may be given antihistamines prior to administration.
ABHAYRIG ADMINISTRATION – INTRADERMAL TEST o Management – Post Reaction: 1) Mild Local Reaction: Anti histamines 2) Systemic reaction EPINEPHRINE 1: 1000 (Aqueous) – 0. 01 ml / Kg per dose repeated every 10 – 20 minutes. o Usual Dose: Infants – 0. 05 ml – 1 ml Children – 0. 1 - 0. 3 ml Adolescents – 0. 3 – 0. 5 ml
ABHAYRIG ADMINISTRATION – INTRADERMAL TEST 3) SEVERE CASES: HYDROCORTISONE : I. V 100 – 200 Mg / Kg

Therefore, Provide Complete Treatment and Protection against deadly Rabies with ARV (Abhayrab) and RIG (Abhay RIG) and save lives !!

Human Biologicals Institute Biotechnology in healthcare made affordable and accessible

Human Biologicals Institute - Lineage Established by National Dairy Development Board White revolution and operation flood Cooperative movement in India-largest producer of Milk and milk products in the World

Human Biologicals Institute Division of Indian Immunologicals Limited : Experience and expertise of two decades in vaccine production India’s first tissue culture rabies vaccine for veterinary use Asia’s largest and World’s second largest manufacturers of veterinary vaccines State-of-the-art manufacturing facilities-WHO and ISO-9001 approved

Human Biologicals Institute An ISO-9001 company World class manufacturing facilities at Ooty ISO-9001 and WHO GMP approved India’s first and most affordable PVRV World’s second manufacturing facility of PVRV

THANK YOU. . !
7b9a6bf01851257796a61b65bbe8d60c.ppt