0288006f86df645bce761d1e71ba24b6.ppt
- Количество слайдов: 62
 QUALITY MANAGEMENT SOLUTIONs Quality Management Systems
QUALITY MANAGEMENT SOLUTIONs Quality Management Systems
 Our Mission Our mission is to Improve and Enhance the Quality and Delivery of Healthcare Services in the World through Effective Collaboration with Each Client to Design and Implement their Customized Quality Management System SOLUTION.
Our Mission Our mission is to Improve and Enhance the Quality and Delivery of Healthcare Services in the World through Effective Collaboration with Each Client to Design and Implement their Customized Quality Management System SOLUTION.
 Our Vision Our vision is to become recognized as the Healthcare Consulting firm of choice to design and implement Medical Laboratory Quality Management System SOLUTIONS.
Our Vision Our vision is to become recognized as the Healthcare Consulting firm of choice to design and implement Medical Laboratory Quality Management System SOLUTIONS.
 Our Values = ARCHITECT Accuracy Respect Collaboration: Our consulting is collaborative and our Architects’ SOLUTIONS are applied upon each Client’s strengths within an appreciative inquiry model. Honesty Innovation: Our Architects infuse innovation and creativity, through new, focused, evidence-based, and consistent Quality Management, into each and every SOLUTION that is created. Teamwork Excellence: Our consulting methods and SOLUTIONS are infused with excellence, quality, consistency, and strict attention to detail. Confidentiality: Our Healthcare SOLUTION Architects have lived and breathed Quality and Confidentiality their entire careers. Truthfulness
Our Values = ARCHITECT Accuracy Respect Collaboration: Our consulting is collaborative and our Architects’ SOLUTIONS are applied upon each Client’s strengths within an appreciative inquiry model. Honesty Innovation: Our Architects infuse innovation and creativity, through new, focused, evidence-based, and consistent Quality Management, into each and every SOLUTION that is created. Teamwork Excellence: Our consulting methods and SOLUTIONS are infused with excellence, quality, consistency, and strict attention to detail. Confidentiality: Our Healthcare SOLUTION Architects have lived and breathed Quality and Confidentiality their entire careers. Truthfulness
 Our Services & Support SPENALEX SOLUTIONS – based in Ontario, Canada. Robert D. Scheuermann: President & Principal SOLUTION Architect ü Sustainable Quality Management Systems üHealthcare Budgets and Business Cases üLaboratory Operation Management üQuality Performance Indicators üOccurrence/Defect Reporting and Management üPolicy, Process, Procedure & Forms Development & Control üQuality Assurance Program Development
Our Services & Support SPENALEX SOLUTIONS – based in Ontario, Canada. Robert D. Scheuermann: President & Principal SOLUTION Architect ü Sustainable Quality Management Systems üHealthcare Budgets and Business Cases üLaboratory Operation Management üQuality Performance Indicators üOccurrence/Defect Reporting and Management üPolicy, Process, Procedure & Forms Development & Control üQuality Assurance Program Development
 Our Services & Support (Cont. ) üManual & Automated Method Validation üTraining and Development of Quality Strategies üEffective Blood Utilization Strategies üLaboratory and Blood Transfusion Safety üLaboratory Management Succession Planning üTo Chair Advisory Boards and Working Groups OUR NEWEST website holds the documents you need available at: www. iso 15189 medlabmanual. com to help you become ISO 15189 certified.
Our Services & Support (Cont. ) üManual & Automated Method Validation üTraining and Development of Quality Strategies üEffective Blood Utilization Strategies üLaboratory and Blood Transfusion Safety üLaboratory Management Succession Planning üTo Chair Advisory Boards and Working Groups OUR NEWEST website holds the documents you need available at: www. iso 15189 medlabmanual. com to help you become ISO 15189 certified.
 What's the Difference between a Quality Manual and a Quality Management System? A quality manual is just a huge book of documents in written or electronic form. A quality management system is the complete network of documents in ACTION. A processbased QMS is a network of many interrelated and interconnected processes (elements)
What's the Difference between a Quality Manual and a Quality Management System? A quality manual is just a huge book of documents in written or electronic form. A quality management system is the complete network of documents in ACTION. A processbased QMS is a network of many interrelated and interconnected processes (elements)
 What's the Difference between a Quality Manual and a Quality Management System? (cont. ) Your quality management system does not sit on your shelf, nor does it live inside your computer.
What's the Difference between a Quality Manual and a Quality Management System? (cont. ) Your quality management system does not sit on your shelf, nor does it live inside your computer.
 I. Quality Management System Any business shall operate efficiently and effectively if it meets regulatory requirements and is managed with the interests of stakeholders, while continually striving to improve its performance by means of a quality management system. It is important to identify (audit), measure, analyze, improve, and communicate those instances where practice does not comply with the quality management system and/or documented operational policies, processes and procedures (problem identification). Documents and records shall be controlled, contracts reviewed, and referral services managed.
I. Quality Management System Any business shall operate efficiently and effectively if it meets regulatory requirements and is managed with the interests of stakeholders, while continually striving to improve its performance by means of a quality management system. It is important to identify (audit), measure, analyze, improve, and communicate those instances where practice does not comply with the quality management system and/or documented operational policies, processes and procedures (problem identification). Documents and records shall be controlled, contracts reviewed, and referral services managed.
 What's the difference between a Process and a Procedure? A process is any series of actions or operations viewed as a whole, with a defined start/input and a finish/output. A procedure is a series of actions or operations viewed as discrete steps.
What's the difference between a Process and a Procedure? A process is any series of actions or operations viewed as a whole, with a defined start/input and a finish/output. A procedure is a series of actions or operations viewed as discrete steps.
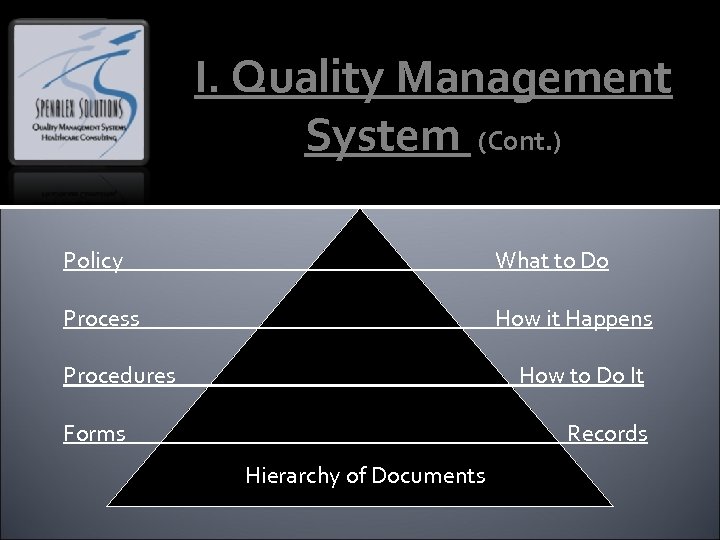 I. Quality Management System (Cont. ) Policy What to Do Process How it Happens Procedures How to Do It Forms Records Hierarchy of Documents
I. Quality Management System (Cont. ) Policy What to Do Process How it Happens Procedures How to Do It Forms Records Hierarchy of Documents
 What is ISO? Background “The International Organization for Standardization (ISO) was established in 1947 and since then as been developing voluntary technical standards over sectors of business, industry and technology. The work is conducted by a worldwide federation of national standards bodies from more than 140 countries. With the exception of ISO 9000 and ISO 14000, the vast majority of ISO standards are highly specific and technical in nature. The ISO 9000 series was first published in 1987, and was the first ISO document to focus on management principles for a much wider business community. In 2000, the series was rewritten.
What is ISO? Background “The International Organization for Standardization (ISO) was established in 1947 and since then as been developing voluntary technical standards over sectors of business, industry and technology. The work is conducted by a worldwide federation of national standards bodies from more than 140 countries. With the exception of ISO 9000 and ISO 14000, the vast majority of ISO standards are highly specific and technical in nature. The ISO 9000 series was first published in 1987, and was the first ISO document to focus on management principles for a much wider business community. In 2000, the series was rewritten.
 What is ISO? Background (Cont. ) It consists of three basic documents: · ISO 9000: 2005. Quality management systems—Fundamentals and vocabulary · ISO 9001: 2008. Quality management systems—Requirements · ISO 9004: 2000. Quality management systems—Guidelines for performance improvements The concept of a quality management system is based on principles described in ISO 9001: 2008, Quality management systems requirements. It describes eight quality management principles that can be applied to any business or service. Five hundred thousand organizations are already registered to ISO 9001.
What is ISO? Background (Cont. ) It consists of three basic documents: · ISO 9000: 2005. Quality management systems—Fundamentals and vocabulary · ISO 9001: 2008. Quality management systems—Requirements · ISO 9004: 2000. Quality management systems—Guidelines for performance improvements The concept of a quality management system is based on principles described in ISO 9001: 2008, Quality management systems requirements. It describes eight quality management principles that can be applied to any business or service. Five hundred thousand organizations are already registered to ISO 9001.
 “The Basics” 1 basic element that must apply to each and every component of your Quality Management System: "SAY what you DO - then, DO what you SAY you DO Each & Every time without exception!" Therefore, all personnel and other stakeholders that use the QMS must be educated to every part of the Quality Management System that they are responsible for.
“The Basics” 1 basic element that must apply to each and every component of your Quality Management System: "SAY what you DO - then, DO what you SAY you DO Each & Every time without exception!" Therefore, all personnel and other stakeholders that use the QMS must be educated to every part of the Quality Management System that they are responsible for.
 ISO Definition of a Quality Manual “A document specifying the quality management system of an organization. ” (However, the structure and setup of an organization’s Quality manual can diverge from the “ISO QMS standard format” to take into consideration the size and complexity of an individual organization. 2) ISO 9001: 2008 contains the following clause (4. 2. 2) regarding the quality manual: The organization shall establish and maintain a quality manual that includes a) The scope of the quality management system, including details of and justifications for any exclusions; b) The documented procedures or reference to them; c) A description and interaction between the processes of the QMS. 3
ISO Definition of a Quality Manual “A document specifying the quality management system of an organization. ” (However, the structure and setup of an organization’s Quality manual can diverge from the “ISO QMS standard format” to take into consideration the size and complexity of an individual organization. 2) ISO 9001: 2008 contains the following clause (4. 2. 2) regarding the quality manual: The organization shall establish and maintain a quality manual that includes a) The scope of the quality management system, including details of and justifications for any exclusions; b) The documented procedures or reference to them; c) A description and interaction between the processes of the QMS. 3
 Creating a Quality Manual The following points should be considered when creating a quality manual: 1. A Quality Manual describes an organization’s quality management system QMS) through a series of policies and processes. 2. A Quality Manual is the basis for all operations and, therefore, must provide a thorough description of an organization’s system. 3. The expected length is from 30 -70 pages although this could vary depending on the size and scope of the facility, and the amount of information a facility chooses to include. 4. It will usually include management processes, but does not usually include any technical procedures. Standard Operating Procedures are referenced where appropriate.
Creating a Quality Manual The following points should be considered when creating a quality manual: 1. A Quality Manual describes an organization’s quality management system QMS) through a series of policies and processes. 2. A Quality Manual is the basis for all operations and, therefore, must provide a thorough description of an organization’s system. 3. The expected length is from 30 -70 pages although this could vary depending on the size and scope of the facility, and the amount of information a facility chooses to include. 4. It will usually include management processes, but does not usually include any technical procedures. Standard Operating Procedures are referenced where appropriate.
 Creating a Quality Manual (Cont. ) 5. It is a road map to the rest of a laboratory’s documentation, and will refer to a myriad of supporting documentation: procedures (work instructions), records, forms, charts, aids, etc. 6. Some of the supporting documentation may be within the manual or included as appendices, but usually it will be kept elsewhere. The quality manual should indicate where supporting documentation can be found by electronic file ID (hyperlinked if possible). 7. The medium can be either electronic or paper. 8. It must be easy for authorized personnel to update and easy for staff to access.
Creating a Quality Manual (Cont. ) 5. It is a road map to the rest of a laboratory’s documentation, and will refer to a myriad of supporting documentation: procedures (work instructions), records, forms, charts, aids, etc. 6. Some of the supporting documentation may be within the manual or included as appendices, but usually it will be kept elsewhere. The quality manual should indicate where supporting documentation can be found by electronic file ID (hyperlinked if possible). 7. The medium can be either electronic or paper. 8. It must be easy for authorized personnel to update and easy for staff to access.
 Creating a Quality Manual (Cont. ) 9. Typically, it is maintained and reviewed by a quality manager. 10. Everyone in the organization must be encouraged and have the ability to provide input into the development of the quality manual. 11. It is essential for the entire staff to be educated and trained to fully understand use the Quality Manual and its related documentation
Creating a Quality Manual (Cont. ) 9. Typically, it is maintained and reviewed by a quality manager. 10. Everyone in the organization must be encouraged and have the ability to provide input into the development of the quality manual. 11. It is essential for the entire staff to be educated and trained to fully understand use the Quality Manual and its related documentation
 I. Quality Management System (Cont. ) Procedure Development Identified from process mapping Templates developed based on ISO guidelines Develop Standardized templates for all areas of operations to use
I. Quality Management System (Cont. ) Procedure Development Identified from process mapping Templates developed based on ISO guidelines Develop Standardized templates for all areas of operations to use
 I. Quality Management System (Cont. ) Document Management System Create Identify Change Approve File Distribute Archive
I. Quality Management System (Cont. ) Document Management System Create Identify Change Approve File Distribute Archive
 I. Quality Management System (Cont. ) Service & Satisfaction Customer Satisfaction Feedback from Stakeholders
I. Quality Management System (Cont. ) Service & Satisfaction Customer Satisfaction Feedback from Stakeholders
 Quality Management System Principles Principle 1: Customer Focus Principle 2: Leadership Principle 3: Involvement of People Principle 4: Process Approach Principle 5: System Approach to Management Principle 6: Continual Improvement Principle 7: Factual approach to Decision Making Principle 8: Mutually Beneficial Supplier Relationships
Quality Management System Principles Principle 1: Customer Focus Principle 2: Leadership Principle 3: Involvement of People Principle 4: Process Approach Principle 5: System Approach to Management Principle 6: Continual Improvement Principle 7: Factual approach to Decision Making Principle 8: Mutually Beneficial Supplier Relationships
 Quality Management System Principles (Cont. ) Principle 1: Customer Focus Organizations depend on their customers Key Benefits: • Increased revenue and market share obtained through flexible and fast responses to market opportunities • Increased effectiveness in the use of the organization’s resources to enhance customer satisfaction • Improved customer loyalty leading to more interactive and collaborative projects, working groups, etc.
Quality Management System Principles (Cont. ) Principle 1: Customer Focus Organizations depend on their customers Key Benefits: • Increased revenue and market share obtained through flexible and fast responses to market opportunities • Increased effectiveness in the use of the organization’s resources to enhance customer satisfaction • Improved customer loyalty leading to more interactive and collaborative projects, working groups, etc.
 Quality Management System Principles (Cont. ) Principle 2: Leadership Leaders establish unity of purpose and direction Key Benefits: • People will understand be motivated towards the organization’s goals and objectives • Activities are evaluated, aligned and implemented in a unified and collaborative way • Miscommunication between levels of the organization will be minimized (eg. MOH-LTC, Coordinators, Field Officers, Regions) within defined and agreeable parameters of action
Quality Management System Principles (Cont. ) Principle 2: Leadership Leaders establish unity of purpose and direction Key Benefits: • People will understand be motivated towards the organization’s goals and objectives • Activities are evaluated, aligned and implemented in a unified and collaborative way • Miscommunication between levels of the organization will be minimized (eg. MOH-LTC, Coordinators, Field Officers, Regions) within defined and agreeable parameters of action
 Quality Management System Principles (Cont. ) Principle 3: Involvement of People at all levels are the essence of the organization Key Benefits: • Empowerment and involvement of all personnel in an organization lends itself to a more motivated and committed team • Innovation and creativity in furthering the organization’s objectives is fostered in this type of culture • People being accountable for their own performance • People being eager to participate and contribute to continual improvement
Quality Management System Principles (Cont. ) Principle 3: Involvement of People at all levels are the essence of the organization Key Benefits: • Empowerment and involvement of all personnel in an organization lends itself to a more motivated and committed team • Innovation and creativity in furthering the organization’s objectives is fostered in this type of culture • People being accountable for their own performance • People being eager to participate and contribute to continual improvement
 Quality Management System Principles (Cont. ) Principle 4: Process Approach A desired result is achieve more efficiently when activities and related resources are managed as a process Key Benefits: • Lower costs and shorter cycle times through effective use of resources • Improved, consistent and predictable results • Focused and prioritized improvement activities • Process review is more focused within RCA groups
Quality Management System Principles (Cont. ) Principle 4: Process Approach A desired result is achieve more efficiently when activities and related resources are managed as a process Key Benefits: • Lower costs and shorter cycle times through effective use of resources • Improved, consistent and predictable results • Focused and prioritized improvement activities • Process review is more focused within RCA groups
 Quality Management System Principles (Cont. ) Principle 5: System Approach to Management Identifying, understanding and managing interrelated processes as a system contributes to the organization’s effectiveness and efficiency in achieving its objectives. Key Benefits: • Integration and alignment of the processes that will best achieve the desired results • Ability to focus effort on the key processes • Providing confidence to interested parties as to the consistency, effectiveness, and efficiency of the organization
Quality Management System Principles (Cont. ) Principle 5: System Approach to Management Identifying, understanding and managing interrelated processes as a system contributes to the organization’s effectiveness and efficiency in achieving its objectives. Key Benefits: • Integration and alignment of the processes that will best achieve the desired results • Ability to focus effort on the key processes • Providing confidence to interested parties as to the consistency, effectiveness, and efficiency of the organization
 Quality Management System Principles (Cont. ) Principle 6: Continual Improvement of the organization’s overall performance should be a permanent objective of the organization Key Benefits: • Performing advantage through improved organizational capabilities • Alignment of improvement activities at all levels to an organization’s strategic intent • High-Performance organization is confident in its actions and is Flexible to react quickly to opportunities
Quality Management System Principles (Cont. ) Principle 6: Continual Improvement of the organization’s overall performance should be a permanent objective of the organization Key Benefits: • Performing advantage through improved organizational capabilities • Alignment of improvement activities at all levels to an organization’s strategic intent • High-Performance organization is confident in its actions and is Flexible to react quickly to opportunities
 Quality Management System Principles (Cont. ) Principle 7: Factual Approach to Decision Making Effective decisions are based on the analysis of data and information Key Benefits: • Regular feedback from stakeholders allows Informed decisions to be made based on data analysis • An increased ability to demonstrate the effectiveness of past decisions through reference to factual records • Increased ability to review, challenge and change opinions and decisions in a participative model
Quality Management System Principles (Cont. ) Principle 7: Factual Approach to Decision Making Effective decisions are based on the analysis of data and information Key Benefits: • Regular feedback from stakeholders allows Informed decisions to be made based on data analysis • An increased ability to demonstrate the effectiveness of past decisions through reference to factual records • Increased ability to review, challenge and change opinions and decisions in a participative model
 Quality Management System Principles (Cont. ) Principle 8: Mutually beneficial supplier relationships An organization and its suppliers are interdependent and a mutually beneficial relationship enhances the ability of both to create value Key Benefits: • Increased ability to create value for both parties • Flexibility and speed of joint responses to changing market or customer needs and expectations • Optimization of costs and resources
Quality Management System Principles (Cont. ) Principle 8: Mutually beneficial supplier relationships An organization and its suppliers are interdependent and a mutually beneficial relationship enhances the ability of both to create value Key Benefits: • Increased ability to create value for both parties • Flexibility and speed of joint responses to changing market or customer needs and expectations • Optimization of costs and resources
 What is ISO? Background (Cont. ) ISO 17025: 2005 (General requirements for the competence of testing and calibration laboratories) and ISO 15189: 2007 (Medical laboratories - Particular requirements for quality and competence) are more technical documents written specifically for laboratories by international committees. ISO 22870: 2006 Point-of-care Testing (POCT) Requirements for Quality and Competence Example: The OLA Program Requirements are based on ALL of the laboratory ISO standards: ISO 17025: 2005 and ISO 15189: 2007, & ISO 22870: 2006, which emphasize quality management system implementation. In addition, further requirements were added based on Ontario law. Also included were requirements that represent generally accepted principles of good laboratory practice, usually consensus guidelines from professional societies and institutes. ”
What is ISO? Background (Cont. ) ISO 17025: 2005 (General requirements for the competence of testing and calibration laboratories) and ISO 15189: 2007 (Medical laboratories - Particular requirements for quality and competence) are more technical documents written specifically for laboratories by international committees. ISO 22870: 2006 Point-of-care Testing (POCT) Requirements for Quality and Competence Example: The OLA Program Requirements are based on ALL of the laboratory ISO standards: ISO 17025: 2005 and ISO 15189: 2007, & ISO 22870: 2006, which emphasize quality management system implementation. In addition, further requirements were added based on Ontario law. Also included were requirements that represent generally accepted principles of good laboratory practice, usually consensus guidelines from professional societies and institutes. ”
 ISO vs. QMPLS “Ontario is the first and only North American Accreditation Authority to assess Medical Laboratories against ISO 15189: 2003. To date, Ontario Laboratories demonstrate that they can meet this rigorous international standard” (QMP-LS. org, 2005). Version 4 of the QMP-LS Supporting References for Guidelines was released in December 2007 in response to ISO 15189: 2007 (Appendix 1: References for OLA Requirements).
ISO vs. QMPLS “Ontario is the first and only North American Accreditation Authority to assess Medical Laboratories against ISO 15189: 2003. To date, Ontario Laboratories demonstrate that they can meet this rigorous international standard” (QMP-LS. org, 2005). Version 4 of the QMP-LS Supporting References for Guidelines was released in December 2007 in response to ISO 15189: 2007 (Appendix 1: References for OLA Requirements).
 ISO vs. QMPLS (Cont. ) OLA is responsible for setting and maintaining accreditation criteria (requirements) for Ontario's licensed medical laboratories. Accreditation requirements are based on the following International Organization for Standardization (ISO) standards: ISO 15189: 2007 Medical Laboratories Particular Requirements for Quality and Competence ISO 15190: 2003 Medical Laboratories Requirements for Safety ISO 22870: 2006 Point-of-care Testing (POCT) Requirements for Quality and Competence
ISO vs. QMPLS (Cont. ) OLA is responsible for setting and maintaining accreditation criteria (requirements) for Ontario's licensed medical laboratories. Accreditation requirements are based on the following International Organization for Standardization (ISO) standards: ISO 15189: 2007 Medical Laboratories Particular Requirements for Quality and Competence ISO 15190: 2003 Medical Laboratories Requirements for Safety ISO 22870: 2006 Point-of-care Testing (POCT) Requirements for Quality and Competence
 The Caribbean’s Desire to Implement Quality Standards Caribbean, May 1, 2006: Senator the Honourable Dr. Bernard Nottage praised Caribbean countries for making positive and progressive steps in healthcare on Monday, May 1, 2006. “It is intended to assure both Caribbean nationals and those from outside the region that the same quality control and accuracy can be obtained from our national and regional laboratories whether in Bermuda, Belize, The Bahamas, Barbados or Anguilla and Barbuda as from first world laboratories” (Nottage, 2006). He added that they will be adept in budgeting, laboratory design, workplace safety, writing skills and communication and all the other requisites from the proper functioning of a first class facility – which will be quite an achievement and long overdue (Bahamas Information Services, 2006).
The Caribbean’s Desire to Implement Quality Standards Caribbean, May 1, 2006: Senator the Honourable Dr. Bernard Nottage praised Caribbean countries for making positive and progressive steps in healthcare on Monday, May 1, 2006. “It is intended to assure both Caribbean nationals and those from outside the region that the same quality control and accuracy can be obtained from our national and regional laboratories whether in Bermuda, Belize, The Bahamas, Barbados or Anguilla and Barbuda as from first world laboratories” (Nottage, 2006). He added that they will be adept in budgeting, laboratory design, workplace safety, writing skills and communication and all the other requisites from the proper functioning of a first class facility – which will be quite an achievement and long overdue (Bahamas Information Services, 2006).
 Quality System Essentials ISO and the Ontario Laboratory Accreditation (OLA) programs have based the requirements for Quality Management System Organization/Personnel/Lab accreditation on twelve QSE: Management Path of Workflow Physical Facilities Equipment/Reagents/Purchasing Pre-Analytical to Post /Inventory Analytical Pre-Analytical & Quality Assurance Post Analytical/Process Control Laboratory Information System Safety Point of Care Testing Quality system essentials apply to all operations in the path of workflow
Quality System Essentials ISO and the Ontario Laboratory Accreditation (OLA) programs have based the requirements for Quality Management System Organization/Personnel/Lab accreditation on twelve QSE: Management Path of Workflow Physical Facilities Equipment/Reagents/Purchasing Pre-Analytical to Post /Inventory Analytical Pre-Analytical & Quality Assurance Post Analytical/Process Control Laboratory Information System Safety Point of Care Testing Quality system essentials apply to all operations in the path of workflow
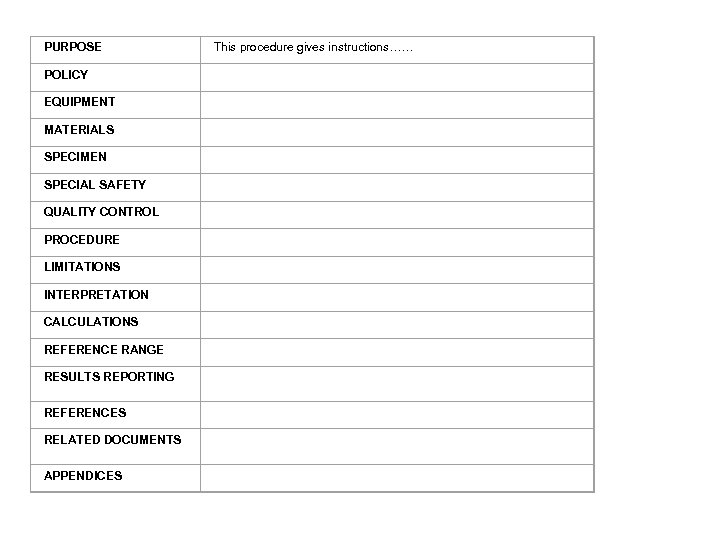 PURPOSE This procedure gives instructions…… POLICY EQUIPMENT MATERIALS SPECIMEN SPECIAL SAFETY QUALITY CONTROL PROCEDURE LIMITATIONS INTERPRETATION CALCULATIONS REFERENCE RANGE RESULTS REPORTING REFERENCES RELATED DOCUMENTS APPENDICES
PURPOSE This procedure gives instructions…… POLICY EQUIPMENT MATERIALS SPECIMEN SPECIAL SAFETY QUALITY CONTROL PROCEDURE LIMITATIONS INTERPRETATION CALCULATIONS REFERENCE RANGE RESULTS REPORTING REFERENCES RELATED DOCUMENTS APPENDICES
 I. Quality Management System (Cont. ) Occurrence Management Quality Control: Corrective Action External and Internal Quality Assurance Turn Around Time (TAT) delay Discrepant Results Corrected Results Specimen Rejection Criteria
I. Quality Management System (Cont. ) Occurrence Management Quality Control: Corrective Action External and Internal Quality Assurance Turn Around Time (TAT) delay Discrepant Results Corrected Results Specimen Rejection Criteria
 II. Organizational Structure, Personnel, and Laboratory Management Medical laboratory services, including appropriate interpretation and advisory services, shall be designed to meet the needs of patients and all clinical personnel responsible for patient care. The laboratory's organizational structure, organizational plan, and policies shall provide the framework to meet the needs of patients and all personnel responsible for patient care. Laboratory management shall consist of those persons (headed by the laboratory director)who manage activities of the laboratory and are accountable to the parent facility. Laboratory management shall ensure that personnel policies (including training, assessment, and continuing education) and job descriptions define the qualifications and duties for all personnel.
II. Organizational Structure, Personnel, and Laboratory Management Medical laboratory services, including appropriate interpretation and advisory services, shall be designed to meet the needs of patients and all clinical personnel responsible for patient care. The laboratory's organizational structure, organizational plan, and policies shall provide the framework to meet the needs of patients and all personnel responsible for patient care. Laboratory management shall consist of those persons (headed by the laboratory director)who manage activities of the laboratory and are accountable to the parent facility. Laboratory management shall ensure that personnel policies (including training, assessment, and continuing education) and job descriptions define the qualifications and duties for all personnel.
 II. Organizational Structure, Personnel, and Laboratory Management (Cont. ) This QSE addresses management’s commitment to quality. As well as developing a mission and vision statement, management must take a leadership role in developing policies and supporting the planning and implementation of each QSE.
II. Organizational Structure, Personnel, and Laboratory Management (Cont. ) This QSE addresses management’s commitment to quality. As well as developing a mission and vision statement, management must take a leadership role in developing policies and supporting the planning and implementation of each QSE.
 II. Organizational Structure, Personnel, and Laboratory Management (Cont. ) Laboratories require: clearly-defined job qualifications; job descriptions; and processes for selection, orientation, training, competency assessment and performance development plans in order to obtain and retain the highly qualified personnel.
II. Organizational Structure, Personnel, and Laboratory Management (Cont. ) Laboratories require: clearly-defined job qualifications; job descriptions; and processes for selection, orientation, training, competency assessment and performance development plans in order to obtain and retain the highly qualified personnel.
 III. Physical Facilities The laboratory's physical facilities shall support its activities. The laboratory space shall be allocated so that its workload can be performed without compromising the quality of work, safety of personnel, and patient care services. Laboratory design shall optimize the comfort of its occupants.
III. Physical Facilities The laboratory's physical facilities shall support its activities. The laboratory space shall be allocated so that its workload can be performed without compromising the quality of work, safety of personnel, and patient care services. Laboratory design shall optimize the comfort of its occupants.
 IV. Equipment, Reagents and Supplies Processes for selection (purchasing), use (inventory control), and records of purchased external services, equipment, and consumable supplies shall be defined as they affect the quality of the service.
IV. Equipment, Reagents and Supplies Processes for selection (purchasing), use (inventory control), and records of purchased external services, equipment, and consumable supplies shall be defined as they affect the quality of the service.
 IV. Equipment, Reagents and Supplies (Cont. ) The laboratory should state the quality expectations for the equipment that is used. In addition to manufacturer’s instructions for calibration, maintenance and use, the laboratory must follow any regulations and accreditation requirements. Specified processes for using and troubleshooting equipment as well as obtaining service and retiring equipment is required.
IV. Equipment, Reagents and Supplies (Cont. ) The laboratory should state the quality expectations for the equipment that is used. In addition to manufacturer’s instructions for calibration, maintenance and use, the laboratory must follow any regulations and accreditation requirements. Specified processes for using and troubleshooting equipment as well as obtaining service and retiring equipment is required.
 IV. Equipment, Reagents and Supplies (Cont. ) Critical supplies and services must be identified and criteria for quality must be established with vendors. These activities are conducted in partnership with the purchasing department. Processes for purchasing, receiving purchased items and for managing inventory must be in place.
IV. Equipment, Reagents and Supplies (Cont. ) Critical supplies and services must be identified and criteria for quality must be established with vendors. These activities are conducted in partnership with the purchasing department. Processes for purchasing, receiving purchased items and for managing inventory must be in place.
 V. Pre-Analytical Process The laboratory shall identify and document preanalytical processes. Each discipline must identify and define all the processes in its operation. For each step in the process, well-written procedures that are understood and used by staff are key to ensuring consistent performance. The Laboratory should use appropriate quality control measures to detect errors and process controls where required to prevent errors.
V. Pre-Analytical Process The laboratory shall identify and document preanalytical processes. Each discipline must identify and define all the processes in its operation. For each step in the process, well-written procedures that are understood and used by staff are key to ensuring consistent performance. The Laboratory should use appropriate quality control measures to detect errors and process controls where required to prevent errors.
 VI. Analytical Process Analytical processes shall be defined to meet the needs of users and shall be documented.
VI. Analytical Process Analytical processes shall be defined to meet the needs of users and shall be documented.
 VII. Quality Assurance Quality assurance (internal quality control and inter-laboratory comparisons such as external quality assessment) shall provide adequate confidence that laboratory examinations fulfill the requirements for quality, and records shall be maintained. Examples are: Quality Indicators Internal & External QA Internal Audits Management Review
VII. Quality Assurance Quality assurance (internal quality control and inter-laboratory comparisons such as external quality assessment) shall provide adequate confidence that laboratory examinations fulfill the requirements for quality, and records shall be maintained. Examples are: Quality Indicators Internal & External QA Internal Audits Management Review
 VIII. Post-Analytical Process Reporting processes shall be defined in accordance with patient care needs and regulations.
VIII. Post-Analytical Process Reporting processes shall be defined in accordance with patient care needs and regulations.
 VIII. Post-Analytical Process (Cont. ) Process Improvement Quality Improvement Activities Occurrence management Problem Solving
VIII. Post-Analytical Process (Cont. ) Process Improvement Quality Improvement Activities Occurrence management Problem Solving
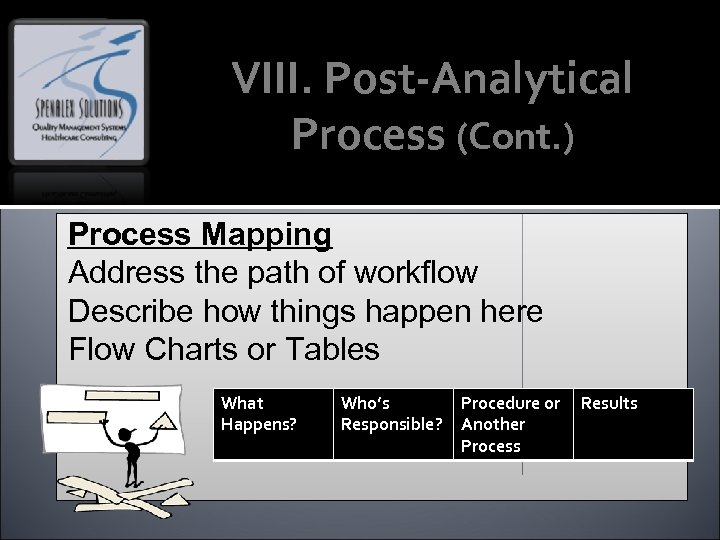 VIII. Post-Analytical Process (Cont. ) Process Mapping Address the path of workflow Describe how things happen here Flow Charts or Tables What Happens? Who’s Responsible? Procedure or Results Another Process
VIII. Post-Analytical Process (Cont. ) Process Mapping Address the path of workflow Describe how things happen here Flow Charts or Tables What Happens? Who’s Responsible? Procedure or Results Another Process
 VIII. Post-Analytical Process (Cont. ) Lean Thinking Techniques “Process Excellence is the proven way to reduce error rates, cut costs, raise productivity, increase capacity, and improve customer satisfaction. It combines the tools and methodologies of: • Six Sigma — measuring and reducing your error and defect rate. • Lean — eliminating waste to speed your workflow and deliver better value. • Design Excellence — designing a process from the outset so that it flows efficiently with minimal opportunities for waste or error” (Orthoclinical. com, 2008).
VIII. Post-Analytical Process (Cont. ) Lean Thinking Techniques “Process Excellence is the proven way to reduce error rates, cut costs, raise productivity, increase capacity, and improve customer satisfaction. It combines the tools and methodologies of: • Six Sigma — measuring and reducing your error and defect rate. • Lean — eliminating waste to speed your workflow and deliver better value. • Design Excellence — designing a process from the outset so that it flows efficiently with minimal opportunities for waste or error” (Orthoclinical. com, 2008).
 IX. Laboratory Information System The laboratory information system (LIS) shall facilitate laboratory services and its processes shall be identified and documented.
IX. Laboratory Information System The laboratory information system (LIS) shall facilitate laboratory services and its processes shall be identified and documented.
 X. Safety The employer shall ensure that the safety and health of employees, patients and visitors are protected. Laboratory management shall assess risk and implement a Safe-working environment in compliance with regulations and good practice. Laboratory management shall be familiar with applicable safety laws and consult reference documents for additional guidance.
X. Safety The employer shall ensure that the safety and health of employees, patients and visitors are protected. Laboratory management shall assess risk and implement a Safe-working environment in compliance with regulations and good practice. Laboratory management shall be familiar with applicable safety laws and consult reference documents for additional guidance.
 XI. Point-of-Care Testing Point-of-care testing (POCT) is testing of samples taken from a patient and performed at or near the bedside with the result leading to possible change in the care of that individual.
XI. Point-of-Care Testing Point-of-care testing (POCT) is testing of samples taken from a patient and performed at or near the bedside with the result leading to possible change in the care of that individual.
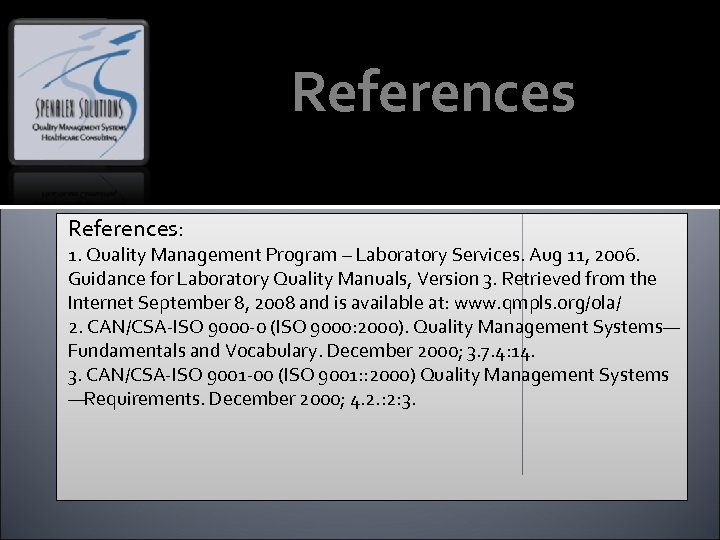 References: 1. Quality Management Program – Laboratory Services. Aug 11, 2006. Guidance for Laboratory Quality Manuals, Version 3. Retrieved from the Internet September 8, 2008 and is available at: www. qmpls. org/ola/ 2. CAN/CSA-ISO 9000 -0 (ISO 9000: 2000). Quality Management Systems— Fundamentals and Vocabulary. December 2000; 3. 7. 4: 14. 3. CAN/CSA-ISO 9001 -00 (ISO 9001: : 2000) Quality Management Systems —Requirements. December 2000; 4. 2. : 2: 3.
References: 1. Quality Management Program – Laboratory Services. Aug 11, 2006. Guidance for Laboratory Quality Manuals, Version 3. Retrieved from the Internet September 8, 2008 and is available at: www. qmpls. org/ola/ 2. CAN/CSA-ISO 9000 -0 (ISO 9000: 2000). Quality Management Systems— Fundamentals and Vocabulary. December 2000; 3. 7. 4: 14. 3. CAN/CSA-ISO 9001 -00 (ISO 9001: : 2000) Quality Management Systems —Requirements. December 2000; 4. 2. : 2: 3.
 Implementation Plan Interview & Listen Preliminary Self-Assessment Critical Needs/GAP analysis Map Processes Write procedures Quality Manual Communicate/Educate/Train Audit Possibly Accreditation
Implementation Plan Interview & Listen Preliminary Self-Assessment Critical Needs/GAP analysis Map Processes Write procedures Quality Manual Communicate/Educate/Train Audit Possibly Accreditation
 Return on Investment & VALUE Several Quantitative ($) and Qualitative ( ) R. O. I. 1. Increasing Staff Productivity ($ ) 2. Reduction in Waste: Time & Energy ($ ) 3. Reduction in Operational Expenses! ($ ) 4. ….
Return on Investment & VALUE Several Quantitative ($) and Qualitative ( ) R. O. I. 1. Increasing Staff Productivity ($ ) 2. Reduction in Waste: Time & Energy ($ ) 3. Reduction in Operational Expenses! ($ ) 4. ….
 Agree on Action Plan for NEXT Steps
Agree on Action Plan for NEXT Steps
 Successful ISO Accreditation Sustainable Quality Management Systems are easily within reach of any Laboratory operation. A Quality Team consistently reviews and audits Quality Indicators, External Quality Assurance, Occurrence Management, etc. to maintain the right path forward. The framework developed via the QMS captures opportunities for additional improvements and allow you to make evidencebased decisions. The data is extractable to reward and recognize your staff as well.
Successful ISO Accreditation Sustainable Quality Management Systems are easily within reach of any Laboratory operation. A Quality Team consistently reviews and audits Quality Indicators, External Quality Assurance, Occurrence Management, etc. to maintain the right path forward. The framework developed via the QMS captures opportunities for additional improvements and allow you to make evidencebased decisions. The data is extractable to reward and recognize your staff as well.
 Peer Assessment During the peer assessment visit, assessors look for conformance to all requirements. A non-conformance is categorized as either major or minor. A minor non-conformance is non-fulfillment of any requirement to a minor degree, or an isolated incident of non-conformance. Minor non conformances are assessed when adherence to requirements and/or the laboratory policies/processes/procedures that address them, is inconsistent. A major non-conformance is non-fulfillment of any requirement to a major degree or consistent/persistent incidence of non-conformance. Major nonconformances are assessed when patient safety is impacted, existing protocols fail to address a requirement, procedures are consistently not followed, and/or there is repeated incidence of non-conformance in the majority of sections of the laboratory.
Peer Assessment During the peer assessment visit, assessors look for conformance to all requirements. A non-conformance is categorized as either major or minor. A minor non-conformance is non-fulfillment of any requirement to a minor degree, or an isolated incident of non-conformance. Minor non conformances are assessed when adherence to requirements and/or the laboratory policies/processes/procedures that address them, is inconsistent. A major non-conformance is non-fulfillment of any requirement to a major degree or consistent/persistent incidence of non-conformance. Major nonconformances are assessed when patient safety is impacted, existing protocols fail to address a requirement, procedures are consistently not followed, and/or there is repeated incidence of non-conformance in the majority of sections of the laboratory.
 Celebrate Success!
Celebrate Success!
 Questions? What are your thoughts? What are some challenges to Implementation? What are some positives you can visualize?
Questions? What are your thoughts? What are some challenges to Implementation? What are some positives you can visualize?


