0d8869b62a802db7d8c0bb20a9426a97.ppt
- Количество слайдов: 36

QU-IRB 31 March, 2015

1. 2. 3. 4. 5. Introduction to Research Ethics Requirements of Research Ethics Institutional Review Board (IRB) Systematic Review of Research Proposal Case reviews QU-IRB 31 March, 2015 Outline

Research Ethics • Support and remind researchers to protect human subjects. • Provide us with a structure for analysis and decisionmaking. • Provide workable definitions of benefits and risks, along with guidelines for evaluating and balancing the benefits and risks of our studies. QU-IRB 31 March, 2015 Concepts of research ethics:

What is Research? Definition of Research: Definition of Human Subject: • A living individual about whom an investigator conducting research obtains Ødata through intervention or interaction with the individual, or Øidentifiable private information (e. g. record review) QU-IRB 31 March, 2015 • a systematic investigation designed to develop or contribute to general knowledge.

Misconduct Ethical Codes and Guidelines QU-IRB 31 March, 2015 Why Research Ethics?

In 2004 and 2005, Dr. Hwang Woo-Suk, published two papers in the journal Science that claimed his team had succeeded in creating human embryonic stem cells through cloning. Allegations later followed from a co-worker that these paper was based on fabricated data. The papers were editorially retracted, Dr. Hwang lost his position at Seoul National University, and the South Korean government ended its financial and legal support of his research. http: //www. nytimes. com/2009/10/27/world/asia/27 clone. html? _r=1&ref=hwangwoosuk QU-IRB 31 March, 2015 Famous Misconduct Cases Stem Cell Case (2005 -2006)
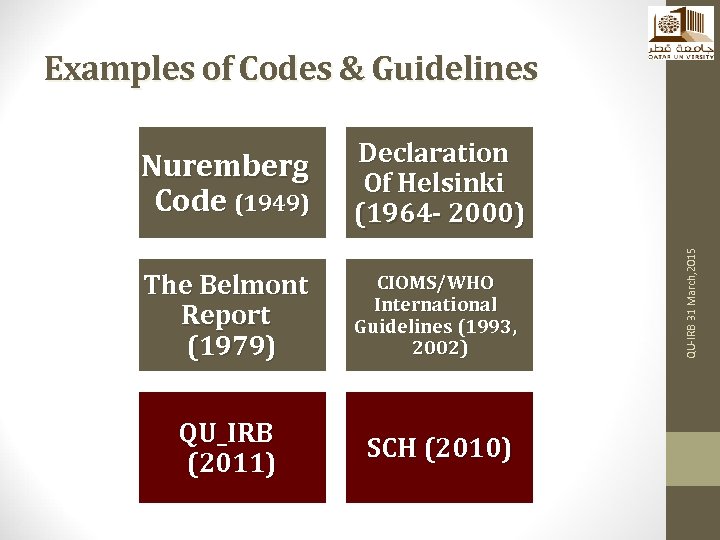
Examples of Codes & Guidelines The Belmont Report (1979) CIOMS/WHO International Guidelines (1993, 2002) QU_IRB (2011) SCH (2010) QU-IRB 31 March, 2015 Nuremberg Code (1949) Declaration Of Helsinki (1964 - 2000)

In 1979 the National Commission published the Belmont Report. The Belmont Report identifies three basic ethical principles that underlie all human subject research. These principles are commonly called the Belmont Principles. The Belmont Principles are: Respect for persons, Beneficence, and Justice. QU-IRB 31 March, 2015 The Belmont Report

The Belmont Principles • Respect for persons include: • Beneficence Include: • The requirement to use the best possible research design to maximize benefits and minimize harms. • The requirements to make sure the researchers are able to perform the procedures and handle the risks. • The prohibition of research that is without a favorable riskbenefit ratio. QU-IRB 31 March, 2015 • The requirement to obtain informed consent. • The requirement to respect the privacy of research subjects.

The Belmont Principles Con. • The principle of justice include: It was the Commission's intention that each of the three principles should have equal moral force. Researchers required to consider each case separately and on its own merits in light of all three principles. QU-IRB 31 March, 2015 • The requirement to select subjects equitably. • The requirement to avoid exploitation of vulnerable populations or populations of convenience.

1. 2. 3. 4. 5. 6. 7. 8. Community Partnership (Respect of culture) Social value (valuable to research subject & community) Scientific validity (use of accepted scientific methods) Fair subject selection (inclusion & exclusion criteria) (vulnerable groups) Favorable risk-benefit ratio (Risk could be physical, psychological, social, economical or legal ) Independent review (To avoid conflicts of interests) Informed consent Respect for human subjects QU-IRB 31 March, 2015 Requirements of Research Ethics
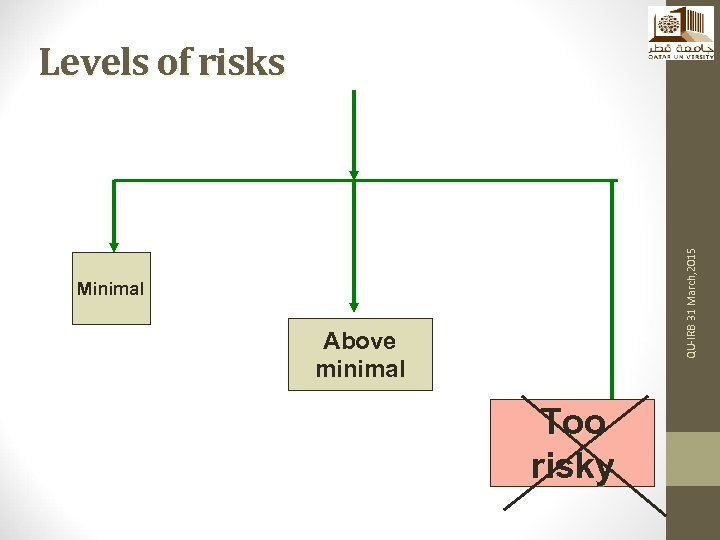
QU-IRB 31 March, 2015 Levels of risks Minimal Above minimal Too risky

Contents: 1. Purpose of the research 2. Procedures (duration) 3. Risks 4. Benefits 5. Alternatives 6. Confidentiality 7. Withdrawal 8. Compensation 9. Who will contact Voluntary Decision No pressure No undueencouragement QU-IRB 31 March, 2015 7 -Informed Consent Process (cont)

QU-IRB 31 March, 2015 Samples

• IRB is a review committee established to help protect the rights and welfare of human research subjects. Regulations require IRB review and approval for research involving human subjects. Most research institutions, professional organizations, and scholarly journals apply the same requirements to all human research. Although federal regulations refer to IRBs, an institution may have chosen a different name for the committee (Ethics Review Committee). QU-IRB 31 March, 2015 Institutional Review Board (IRB)

IRB protects: 1 - The research subjects 2 - The researchers 3 - The institutes 4 -The community QU-IRB 31 March, 2015 • The primary function of an Institutional Review Board (IRB) is protection of human subjects involved in research.

The Composition of the IRB At least five members. Members of both sexes. Members that come from varied professions. At least one member whose primary concerns are in nonscientific areas. • At least one member whose primary concerns are in scientific areas. • At least one member who is not otherwise affiliated with the institution. • • QU-IRB 31 March, 2015 SCH regulations dictate that the IRB membership will include:

SCH regulations stipulate that an IRB can: • Approve research. • Disapprove research. • Modify research. • Conduct continuing reviews. • Observe/verify changes. • Suspend or terminate approval. • Observe the consent process and the research procedures. QU-IRB 31 March, 2015 The Authority of the IRB

Consequences of Not Following IRB Regulations • • • Suspension of research project. Suspension of all of a PI's research projects. Inability to use data or publish results. Notification of sponsors, regulatory agencies and funding agencies of noncompliance. Inability to receive funding from federal grants. Additional monitoring and oversight by the IRB and/or third party monitoring of research activities. Termination of employment. Loss of licenses. Immediate shut-down of ALL research at an organization. QU-IRB 31 March, 2015 If IRB regulations are not followed, consequences could include:

(Qatar University – Institutional Review Board) QU-IRB 31 March, 2015 QU-IRB

• Qatar University’s Institutional Review Board (QU-IRB), under the directives of Supreme Council of Health (SCH) was formed as an independent committee under research compliance in September 2011. All research that is to be conducted on human subjects must be submitted to QU-IRB for ethical acceptability QU-IRB 31 March, 2015 QU-IRB

General Procedures Outline Part 1. (from Applicant) • If both English and Arabic languages are to be used, supporting documents such as surveys, questionnaires, consents/ascents, etc…, should be provided in both languages. • If exemption from Full Ethics Review is requested, it should be specified in the email with reasons for such request. • Exemption criteria is stated in the Research Ethics Detailed Manual which can be accessed through QU-IRB Web Site QU-IRB 31 March, 2015 Filled in forms (Application, Checklist and other supporting documents) should be sent to QU-IRB at the email address QU-IRB@qu. edu. qa. (All forms are available in the Required Forms list)

General Procedures Outline Cont. • Step 2: Once step 1 is complete, the proposals are reviewed by the chairperson for possible exemption or expedited. If ok, the exemption letter is issued without sending the application for a full ethics review. • Step 3: If the application does not meet the exemption requirements, it undergoes a full ethics review. Once approval is granted, approval letter is issued. QU-IRB 31 March, 2015 • Step 1: Application received undergo initial screening to make sure the forms are complete and filled in properly.

Forms Required for QU-IRB: 1. Application 2. Checklist 4. Consents / Ascents (if applicable) 5. Supervisor Letter for student graduate projects 6. QU-IBC approval (if applicable) 6. Collaborating Institute’s IRB Approval (if applicable) 7. Any other relevant supporting documents (Dept. Approval) In case of a renewal request, only a renewal request is needed. QU-IRB 31 March, 2015 3. Surveys / Questionnaires
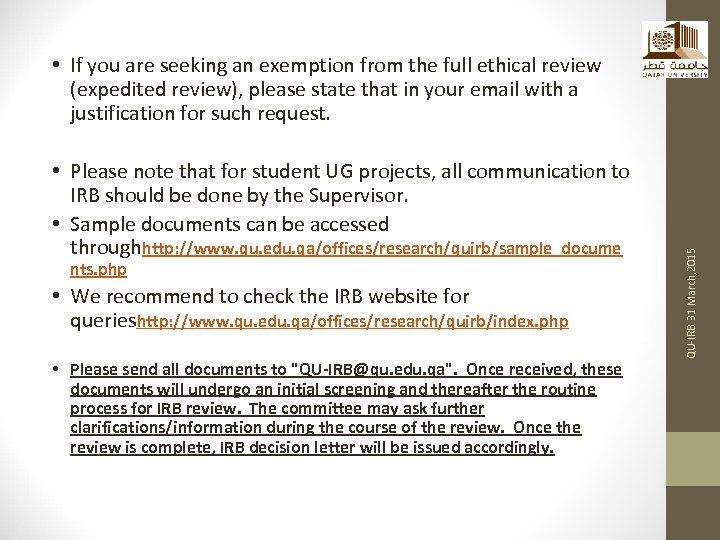
• Please note that for student UG projects, all communication to IRB should be done by the Supervisor. • Sample documents can be accessed throughhttp: //www. qu. edu. qa/offices/research/quirb/sample_docume nts. php • We recommend to check the IRB website for querieshttp: //www. qu. edu. qa/offices/research/quirb/index. php • Please send all documents to "QU-IRB@qu. edu. qa". Once received, these documents will undergo an initial screening and thereafter the routine process for IRB review. The committee may ask further clarifications/information during the course of the review. Once the review is complete, IRB decision letter will be issued accordingly. QU-IRB 31 March, 2015 • If you are seeking an exemption from the full ethical review (expedited review), please state that in your email with a justification for such request.

What Qualifies as Research Involving Human Subjects? QU-IRB 31 March, 2015 Systematic Review of Research Proposal

Is the project considered research? Does the study involve Human Subjects? QU-IRB 31 March, 2015 First Questions to Ask:
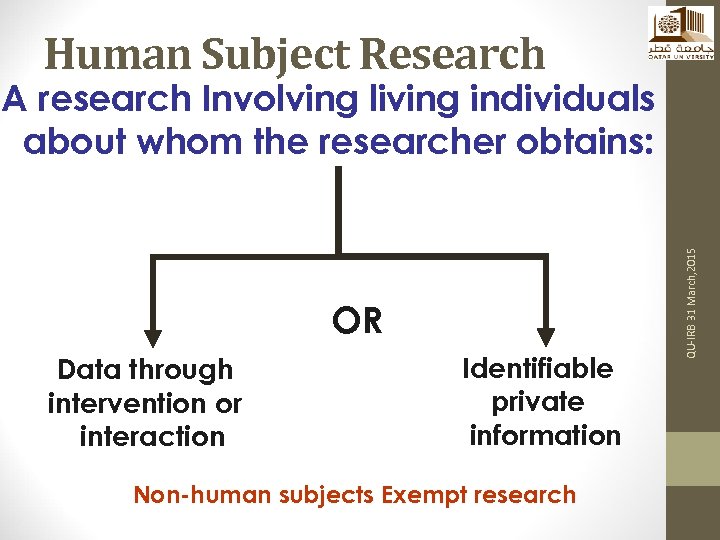
Human Subject Research OR Data through intervention or interaction Identifiable private information Non-human subjects Exempt research QU-IRB 31 March, 2015 A research Involving living individuals about whom the researcher obtains:

Types of IRB Review • Review by IRB chair or his/her designee • Full Board Review QU-IRB 31 March, 2015 • Is it considered research? • Does not involve human subjects? • Can be exempt from Review?
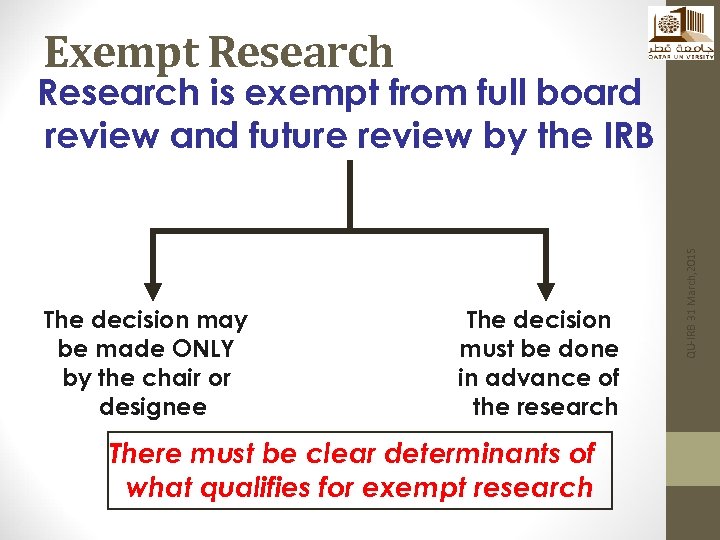
Exempt Research The decision may be made ONLY by the chair or designee The decision must be done in advance of the research There must be clear determinants of what qualifies for exempt research QU-IRB 31 March, 2015 Research is exempt from full board review and future review by the IRB

• Not involving vulnerable subjects • Prisoners • Children • Pregnant women • Not more than minimal risk QU-IRB 31 March, 2015 Limitations for Exempt Research

Categories of Exemption #1 • Research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods. QU-IRB 31 March, 2015 • Research conducted in commonly accepted educational settings, involving normal educational practices, such as: • Research on special education instructional strategies, (Lectures , Seminars, etc. . ).

Categories of Exemption #2 • Research involving the use of Survey Procedures, Interview procedures or Observation of public behavior • information is obtained and recorded by the investigator in such a way that the subject can be identified, AND • disclosure could reasonably place the subject at risk of criminal or civil liability or be damaging to the subject’s financial standing, employability, or reputation. QU-IRB 31 March, 2015 UNLESS

• Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens • If these sources are publicly available OR • The information is recorded in a way that the subject can not be identified directly Or through identifiers linked to the subject QU-IRB 31 March, 2015 Categories of Exemption #3
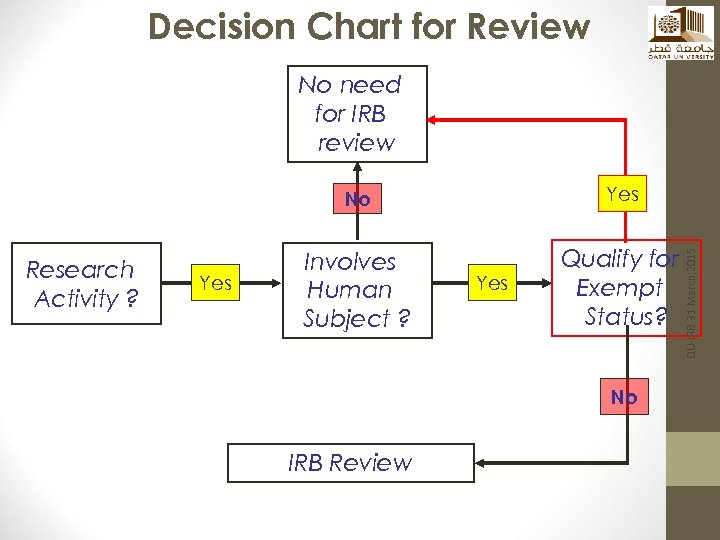
Decision Chart for Review No need for IRB review Research Activity ? Yes Involves Human Subject ? Qualify for Exempt Status? Yes No IRB Review QU-IRB 31 March, 2015 No

QU-IRB 31 March, 2015 Thank You
0d8869b62a802db7d8c0bb20a9426a97.ppt