9a56c12511c3144124b113fd05d2d8ee.ppt
- Количество слайдов: 26
 Purdue University School of Pharmacy and Pharmaceutical Sciences Department of Industrial and Physical Pharmacy Graduate Certificate and Masters Degree Programs in… Regulatory and Quality Compliance 1 Department of Industrial and Physical Pharmacy Purdue University 2006
Purdue University School of Pharmacy and Pharmaceutical Sciences Department of Industrial and Physical Pharmacy Graduate Certificate and Masters Degree Programs in… Regulatory and Quality Compliance 1 Department of Industrial and Physical Pharmacy Purdue University 2006
 Educating Regulatory, Quality and Compliance Professionals 2 Department of Industrial and Physical Pharmacy Purdue University 2006
Educating Regulatory, Quality and Compliance Professionals 2 Department of Industrial and Physical Pharmacy Purdue University 2006
 Unique Features of Purdue’s Program Design • • Comprehensive program Clearly defined criteria Consistency in content and delivery Student accountability 3 Department of Industrial and Physical Pharmacy Purdue University 2006
Unique Features of Purdue’s Program Design • • Comprehensive program Clearly defined criteria Consistency in content and delivery Student accountability 3 Department of Industrial and Physical Pharmacy Purdue University 2006
 Our Purpose… To grow your professional capabilities in the areas of regulatory affairs and quality and compliance practices. To make you more insightful and perceptive, and a better problem seeker and solver. To make you a more effective team member, and a learned mentor to others. To prepare you to help your organization avoid costly and time consuming problems in new product development and registrations. To develop you to grow professionally and to contribute significant value to your company. Department of Industrial and Physical Pharmacy Purdue University 2006 4
Our Purpose… To grow your professional capabilities in the areas of regulatory affairs and quality and compliance practices. To make you more insightful and perceptive, and a better problem seeker and solver. To make you a more effective team member, and a learned mentor to others. To prepare you to help your organization avoid costly and time consuming problems in new product development and registrations. To develop you to grow professionally and to contribute significant value to your company. Department of Industrial and Physical Pharmacy Purdue University 2006 4
 Learning Environment • Instructors – University professors – Industry experts • Experience based discussion – Peer to peer learning – Instructor expertise • University setting 5 Department of Industrial and Physical Pharmacy Purdue University 2006
Learning Environment • Instructors – University professors – Industry experts • Experience based discussion – Peer to peer learning – Instructor expertise • University setting 5 Department of Industrial and Physical Pharmacy Purdue University 2006
 Benefits for Student • Format compatible for working professionals • Focused on relevant and current content • Broad basic understanding across discovery, development, registration, and marketing • Opportunity for career acceleration and professional development • Graduate Certificate Degree or Masters Degree 6 Department of Industrial and Physical Pharmacy Purdue University 2006
Benefits for Student • Format compatible for working professionals • Focused on relevant and current content • Broad basic understanding across discovery, development, registration, and marketing • Opportunity for career acceleration and professional development • Graduate Certificate Degree or Masters Degree 6 Department of Industrial and Physical Pharmacy Purdue University 2006
 Business Benefits • Exposure to pharmaceutical and medical device regulations • FDA and ex-US regulations • Masters’ project pertinent to business situations • Focuses on value of continuous improvement • Professional growth for employee 7 Department of Industrial and Physical Pharmacy Purdue University 2006
Business Benefits • Exposure to pharmaceutical and medical device regulations • FDA and ex-US regulations • Masters’ project pertinent to business situations • Focuses on value of continuous improvement • Professional growth for employee 7 Department of Industrial and Physical Pharmacy Purdue University 2006
 History of the Program • Begun in 2001 in the School of Industrial and Physical Pharmacy • Driven by independent input from Todd Chermak (Abbott) and Michael Schmidt ( Lilly, retired) • Established at Purdue by Steve Byrn, Department Head • Grown from – One to seven courses – Graduate certificate plus Masters degree 8 Department of Industrial and Physical Pharmacy Purdue University 2006
History of the Program • Begun in 2001 in the School of Industrial and Physical Pharmacy • Driven by independent input from Todd Chermak (Abbott) and Michael Schmidt ( Lilly, retired) • Established at Purdue by Steve Byrn, Department Head • Grown from – One to seven courses – Graduate certificate plus Masters degree 8 Department of Industrial and Physical Pharmacy Purdue University 2006
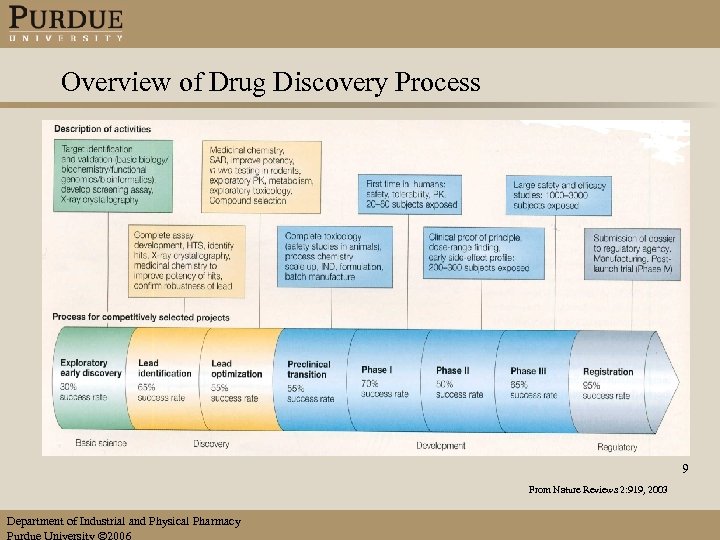 Overview of Drug Discovery Process 9 From Nature Reviews 2: 919, 2003 Department of Industrial and Physical Pharmacy Purdue University 2006
Overview of Drug Discovery Process 9 From Nature Reviews 2: 919, 2003 Department of Industrial and Physical Pharmacy Purdue University 2006
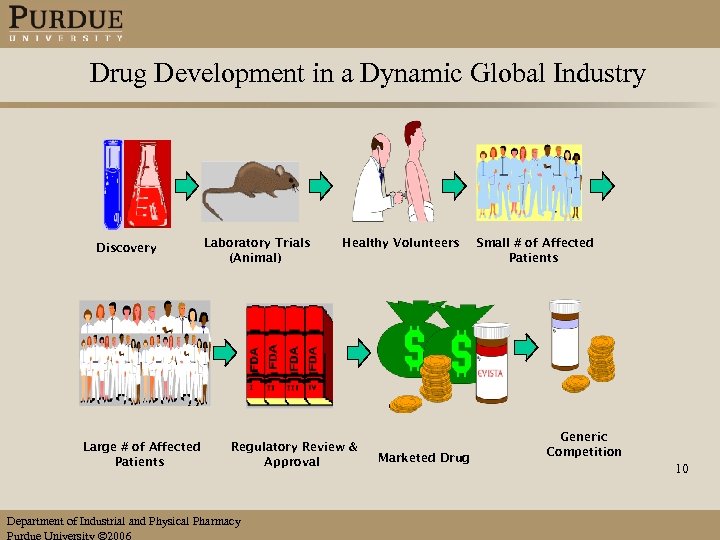 Drug Development in a Dynamic Global Industry Discovery Large # of Affected Patients Laboratory Trials (Animal) Healthy Volunteers Regulatory Review & Approval Department of Industrial and Physical Pharmacy Purdue University 2006 Marketed Drug Small # of Affected Patients Generic Competition 10
Drug Development in a Dynamic Global Industry Discovery Large # of Affected Patients Laboratory Trials (Animal) Healthy Volunteers Regulatory Review & Approval Department of Industrial and Physical Pharmacy Purdue University 2006 Marketed Drug Small # of Affected Patients Generic Competition 10
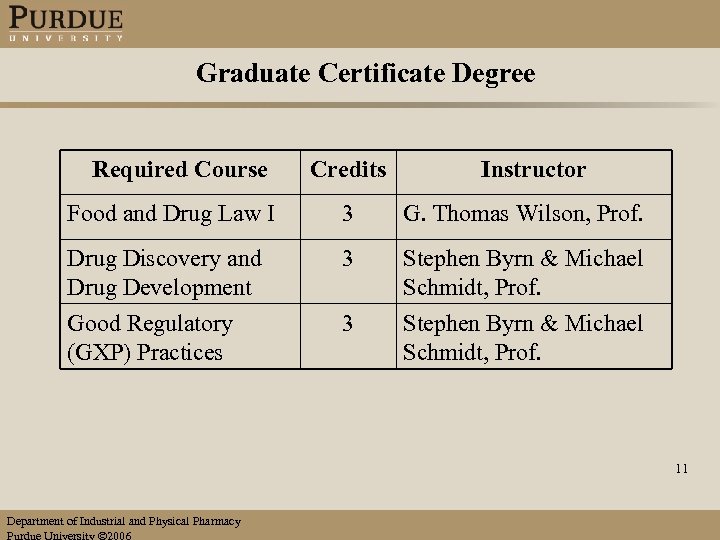 Graduate Certificate Degree Required Course Credits Instructor Food and Drug Law I 3 G. Thomas Wilson, Prof. Drug Discovery and Drug Development 3 Stephen Byrn & Michael Schmidt, Prof. Good Regulatory (GXP) Practices 3 Stephen Byrn & Michael Schmidt, Prof. 11 Department of Industrial and Physical Pharmacy Purdue University 2006
Graduate Certificate Degree Required Course Credits Instructor Food and Drug Law I 3 G. Thomas Wilson, Prof. Drug Discovery and Drug Development 3 Stephen Byrn & Michael Schmidt, Prof. Good Regulatory (GXP) Practices 3 Stephen Byrn & Michael Schmidt, Prof. 11 Department of Industrial and Physical Pharmacy Purdue University 2006
 Graduate Certificate Program • Format – 1 weekend per month – 3 weekends per course – 3 courses per Certificate Program • Note: Credit hours can be applied to the Masters Degree program 12 Department of Industrial and Physical Pharmacy Purdue University 2006
Graduate Certificate Program • Format – 1 weekend per month – 3 weekends per course – 3 courses per Certificate Program • Note: Credit hours can be applied to the Masters Degree program 12 Department of Industrial and Physical Pharmacy Purdue University 2006
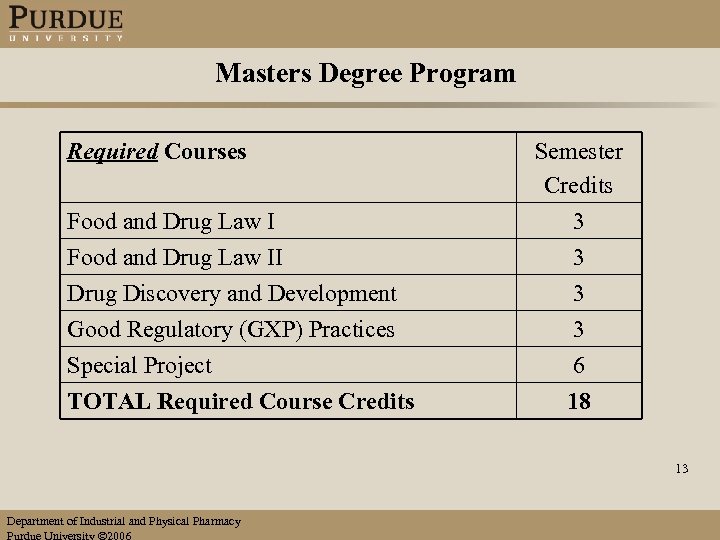 Masters Degree Program Required Courses Food and Drug Law I Semester Credits 3 Food and Drug Law II 3 Drug Discovery and Development Good Regulatory (GXP) Practices Special Project TOTAL Required Course Credits 3 3 6 18 13 Department of Industrial and Physical Pharmacy Purdue University 2006
Masters Degree Program Required Courses Food and Drug Law I Semester Credits 3 Food and Drug Law II 3 Drug Discovery and Development Good Regulatory (GXP) Practices Special Project TOTAL Required Course Credits 3 3 6 18 13 Department of Industrial and Physical Pharmacy Purdue University 2006
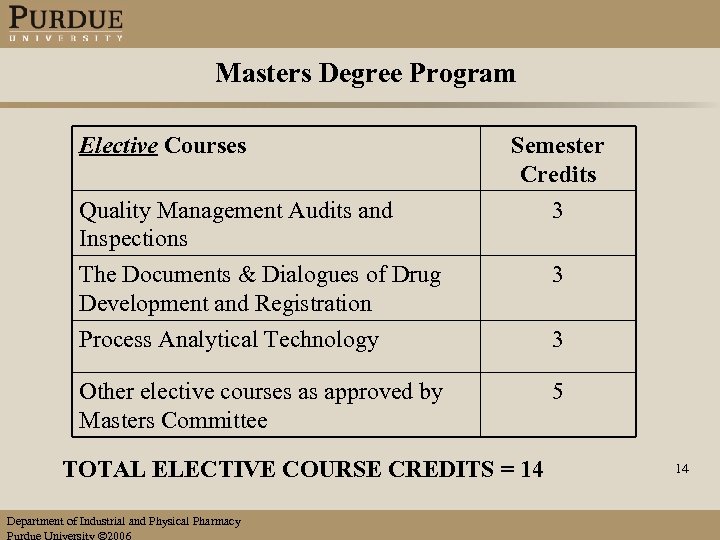 Masters Degree Program Elective Courses Semester Credits Quality Management Audits and Inspections 3 The Documents & Dialogues of Drug Development and Registration Process Analytical Technology 3 Other elective courses as approved by Masters Committee 5 TOTAL ELECTIVE COURSE CREDITS = 14 Department of Industrial and Physical Pharmacy Purdue University 2006 3 14
Masters Degree Program Elective Courses Semester Credits Quality Management Audits and Inspections 3 The Documents & Dialogues of Drug Development and Registration Process Analytical Technology 3 Other elective courses as approved by Masters Committee 5 TOTAL ELECTIVE COURSE CREDITS = 14 Department of Industrial and Physical Pharmacy Purdue University 2006 3 14
 Masters Degree • Requirements – 4 Required Courses – 1 Special Project • Approved by Masters Committee – Elective courses • Total 32 Semester Credits 15 Department of Industrial and Physical Pharmacy Purdue University 2006
Masters Degree • Requirements – 4 Required Courses – 1 Special Project • Approved by Masters Committee – Elective courses • Total 32 Semester Credits 15 Department of Industrial and Physical Pharmacy Purdue University 2006
 Masters Degree Special Project • • • Project Statement presented to Masters Committee Emphasis on process/continuous improvement Add value to organization…integrated into job Approximately 1 year to develop and complete Purdue staff available for counsel End Result: – Written report (30 -40 pages) – Oral presentation for approval by Masters Committee 16 Department of Industrial and Physical Pharmacy Purdue University 2006
Masters Degree Special Project • • • Project Statement presented to Masters Committee Emphasis on process/continuous improvement Add value to organization…integrated into job Approximately 1 year to develop and complete Purdue staff available for counsel End Result: – Written report (30 -40 pages) – Oral presentation for approval by Masters Committee 16 Department of Industrial and Physical Pharmacy Purdue University 2006
 All Special Projects are completed and reviewed under strict confidentiality to protect company intellectual property 17 Department of Industrial and Physical Pharmacy Purdue University 2006
All Special Projects are completed and reviewed under strict confidentiality to protect company intellectual property 17 Department of Industrial and Physical Pharmacy Purdue University 2006
 Program Goal • To provide a meaningful educational experience in regulatory and quality compliance 18 Department of Industrial and Physical Pharmacy Purdue University 2006
Program Goal • To provide a meaningful educational experience in regulatory and quality compliance 18 Department of Industrial and Physical Pharmacy Purdue University 2006
 Program Objectives • To Provide: – A broadened understanding of product development – An understanding of interdisciplinary relationships – Emphasis on strengthening problem solving skills – Framework to educate and mentor colleagues – Interaction between professionals with different experiences and backgrounds 19 Department of Industrial and Physical Pharmacy Purdue University 2006
Program Objectives • To Provide: – A broadened understanding of product development – An understanding of interdisciplinary relationships – Emphasis on strengthening problem solving skills – Framework to educate and mentor colleagues – Interaction between professionals with different experiences and backgrounds 19 Department of Industrial and Physical Pharmacy Purdue University 2006
 This Course Meets These Objectives by: • Presenting and discussing relevant topics • Utilizing experienced professionals to teach and to answer questions • Assigning good reading and reference materials • Requiring homework that renders deep insights and new personal growth • Encouraging and providing time for professional interactions amongst classmates and with instructors • Being academically demanding…highly credible…and widely recognized 20 Department of Industrial and Physical Pharmacy Purdue University 2006
This Course Meets These Objectives by: • Presenting and discussing relevant topics • Utilizing experienced professionals to teach and to answer questions • Assigning good reading and reference materials • Requiring homework that renders deep insights and new personal growth • Encouraging and providing time for professional interactions amongst classmates and with instructors • Being academically demanding…highly credible…and widely recognized 20 Department of Industrial and Physical Pharmacy Purdue University 2006
 Improvement Project Outcomes - Some Students’ Comments Value added…for sure! Won my company’s “Improvement Award” prize. Complex, detailed…a great learning experience. Fit perfectly into my job description…nice. Appreciated, and now being replicated elsewhere. Got to know a lot of new people and areas as a result of doing my project. Great networking! 21 Department of Industrial and Physical Pharmacy Purdue University 2006
Improvement Project Outcomes - Some Students’ Comments Value added…for sure! Won my company’s “Improvement Award” prize. Complex, detailed…a great learning experience. Fit perfectly into my job description…nice. Appreciated, and now being replicated elsewhere. Got to know a lot of new people and areas as a result of doing my project. Great networking! 21 Department of Industrial and Physical Pharmacy Purdue University 2006
 Personal Benefit Outcomes - Some Students’ Comments Got promoted! Out-competed the others for the job, even though I didn’t have as much service credit. Positive comments from my supervision at Performance Management Review time. Moved to a new job. The program gave me insights about this new career path for me. I now can understand track the conversations in team meetings. I don’t “check-out” when toxicology starts talking! Department of Industrial and Physical Pharmacy Purdue University 2006 22
Personal Benefit Outcomes - Some Students’ Comments Got promoted! Out-competed the others for the job, even though I didn’t have as much service credit. Positive comments from my supervision at Performance Management Review time. Moved to a new job. The program gave me insights about this new career path for me. I now can understand track the conversations in team meetings. I don’t “check-out” when toxicology starts talking! Department of Industrial and Physical Pharmacy Purdue University 2006 22
 Now some commentary from and Q & A with our ALUMNI…and your colleagues! 23 Department of Industrial and Physical Pharmacy Purdue University 2006
Now some commentary from and Q & A with our ALUMNI…and your colleagues! 23 Department of Industrial and Physical Pharmacy Purdue University 2006
 Now What…? • • • Come talk to us now Grab information now Follow-up via our web site at www. ipph. purdue. edu Talk to your supervision. Talk to the Continuing Education Department. Call us at (765) 494 -6545 24 Department of Industrial and Physical Pharmacy Purdue University 2006
Now What…? • • • Come talk to us now Grab information now Follow-up via our web site at www. ipph. purdue. edu Talk to your supervision. Talk to the Continuing Education Department. Call us at (765) 494 -6545 24 Department of Industrial and Physical Pharmacy Purdue University 2006
 We would like to thank the many employees of Abbott and Lilly, and the FDA who have contributed to the success of our program. 25 Department of Industrial and Physical Pharmacy Purdue University 2006
We would like to thank the many employees of Abbott and Lilly, and the FDA who have contributed to the success of our program. 25 Department of Industrial and Physical Pharmacy Purdue University 2006
 Purdue University School of Pharmacy and Pharmaceutical Sciences Department of Industrial and Physical Pharmacy Graduate Certificate and Masters Degree Programs in… Regulatory and Quality Compliance “Educating Quality and Compliance Professionals” 26 Department of Industrial and Physical Pharmacy Purdue University 2006
Purdue University School of Pharmacy and Pharmaceutical Sciences Department of Industrial and Physical Pharmacy Graduate Certificate and Masters Degree Programs in… Regulatory and Quality Compliance “Educating Quality and Compliance Professionals” 26 Department of Industrial and Physical Pharmacy Purdue University 2006


