71a5eb4f3a41bbea31b0067ff0f22dc4.ppt
- Количество слайдов: 31

Public Private Partnerships for Product Development Platforms, Lessons and Challenges for GSPA Robert Ridley Director, TDR WG on GSPA Financing Jan 12 -13, 2009 1

Some Useful References 1. WHO Bulletin Volume 79 (8) 2001 – Special theme issue: Public-Private Partnerships 2. Combating Diseases Associated with Poverty: Financing Strategies for Product Development and the Potential Role of Public Private Partnerships – – A report based on a meeting Principle Authors: Roy Widdus and Katherine White Publisher: Initiative on Public Private Partnerships for Health, 2004 ISBN 2 -940286 -21 -3 3. Upcoming G-FINDER Publication and Report WG on GSPA Financing Jan 12 -13, 2009 2
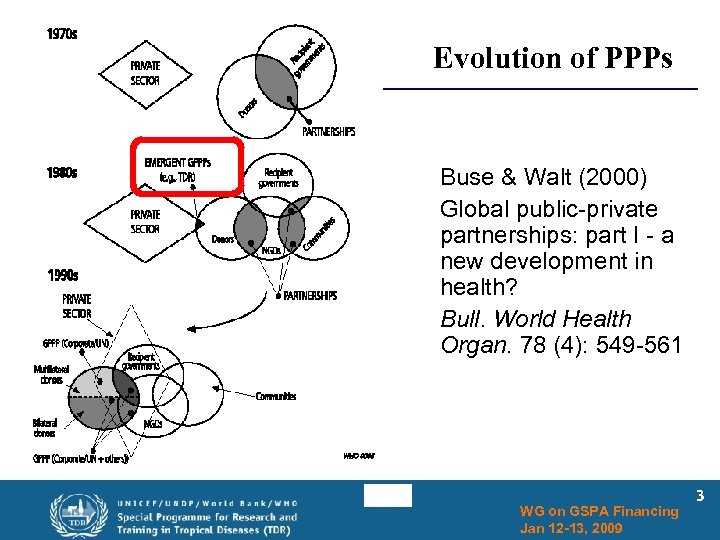
Evolution of PPPs Buse & Walt (2000) Global public-private partnerships: part I - a new development in health? Bull. World Health Organ. 78 (4): 549 -561 WG on GSPA Financing Jan 12 -13, 2009 3

Examples of Success (non-exhaustive) • Mefloquine for malaria (1984) – Roche and Walter Read Army Institute of Research and TDR • Ivermectin for onchocerciasis (1987) – Merck and TDR • Cyclofem monthly injectable contraceptive (1989) – HRP, PATH, Rockefeller Foundation – Concept Foundation • Eflornithine for African Trypanosomiasis (1991) – Marion Merrell Dow and TDR • Miltefosine for Visceral Leishmaniasis (2002) – Zentaris, ICMR and TDR • Paediatric 'dispersible' Coartem (2007) – Novartis and MMV • Amodiaquine - artesunate fixed dose combination (2007) – Sanofi-Aventis and DNDi • Mefloquine – artesunate fixed dose combination (2008) – Farmanguinos and DNDi • Meningococcal Vaccine (2009) – SIL, WHO/IVR and PATH WG on GSPA Financing Jan 12 -13, 2009 4
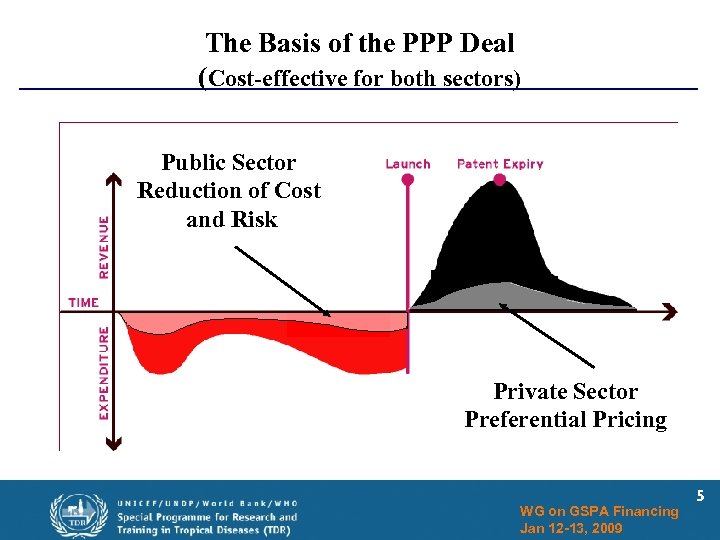
The Basis of the PPP Deal (Cost-effective for both sectors) Public Sector Reduction of Cost and Risk Private Sector Preferential Pricing WG on GSPA Financing Jan 12 -13, 2009 5
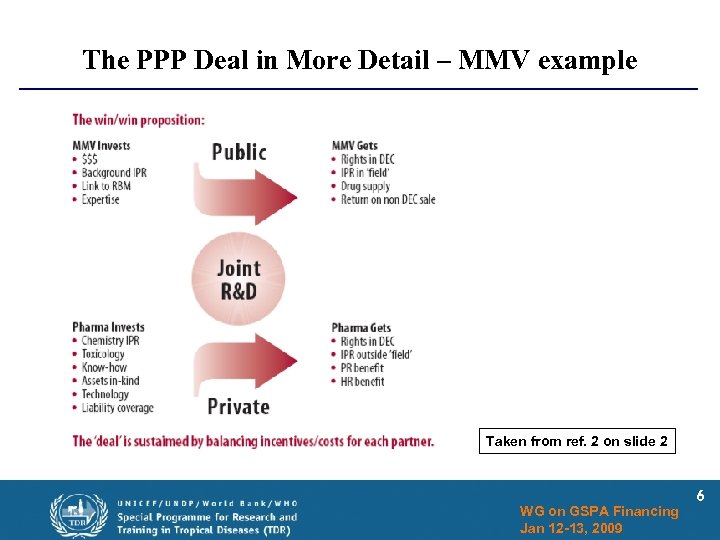
The PPP Deal in More Detail – MMV example Taken from ref. 2 on slide 2 WG on GSPA Financing Jan 12 -13, 2009 6
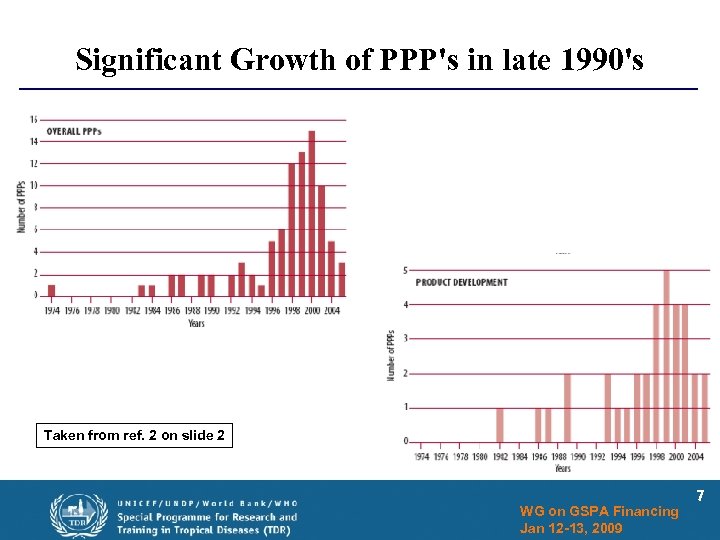
Significant Growth of PPP's in late 1990's Taken from ref. 2 on slide 2 WG on GSPA Financing Jan 12 -13, 2009 7
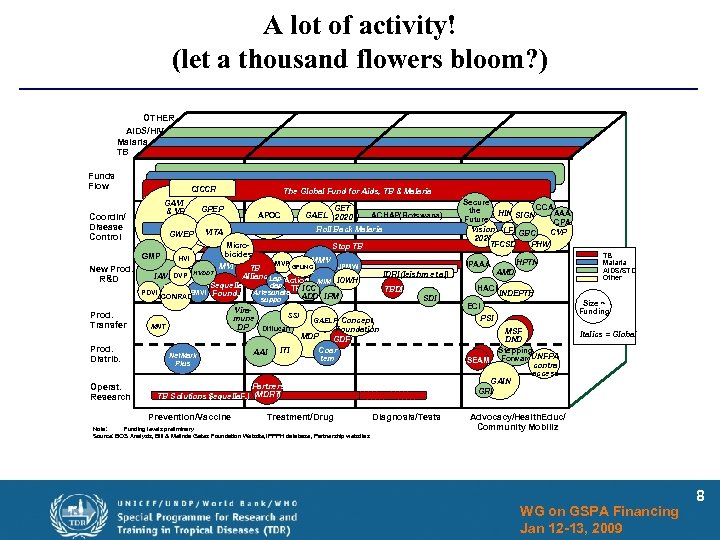
A lot of activity! (let a thousand flowers bloom? ) OTHER AIDS/HIV Malaria TB Funds Flow CICCR GAVI & VF Coordin/ Disease Control GWEP GMP New Prod. R&D Prod. Transfer Prod. Distrib. Operat. Research HVI The Global Fund for Aids, TB & Malaria GPEP GET GAEL 2020 APOC ACHAP(Botswana) Roll Back Malaria VITA Micro bicides Stop TB MMV MVP GFUNCI JPMW MVI IPAAA TB IAVI DVP HVDDT Alliance Lap- Action MIM IOWH Sequella dap TB ICC Artesunate PDVI EMVI Found. ADD IPM CONRAD suppo Viramune DP MNT Net. Mark Plus SSI Diflucan AAI ITI IDRI(leishm et al). SDI Foundation MDP GDF Coar tem Partners ) TB Solutions Sequella. F. ) (MDRT ( Prevention/Vaccine Treatment/Drug HPTN AMD HACI TBDI GAELF Concept Note: Funding levels preliminary Source: BCG Analysis, Bill & Melinda Gates Foundation Website, PPPH database, Partnership websites I Secure CCA the HIN SIGN AAA Future CPA Vision LFI GBC CVP 2020 PHW TFCSD Diagnosis/Tests TB Malaria AIDS/STD Other INDEPTH Size ~ Funding ECI PSI MSF DND Stepping SEAM Forward. UNFPA contra access GAIN GRI Italics = Global Advocacy/Health. Educ/ Community Mobiliz WG on GSPA Financing Jan 12 -13, 2009 8

Some Important Product Development PPP's • Aeras TB vaccine Foundation • CONRAD (Contraceptives R and D) • DNDi (Drugs for Neglected Diseases Initiative) • FIND (Foundation for Innovative Diagnostics) • IAVI (International AIDS Vaccine Initiative) • IOWH (Institute for One World Health) • IPM (International Partnership for Microbicides) • MMV (Medicines for Malaria Venture) • MVI (Malaria Vaccines Initiative) • TB Alliance (for TB drug development) Supporting / Initiating Organizations include: • WHO (TDR, HRP, IVR); Rockefeller Foundation; PATH; Wellcome Trust; Gates Foundation; MSF; various governments; World Bank; others WG on GSPA Financing Jan 12 -13, 2009 9
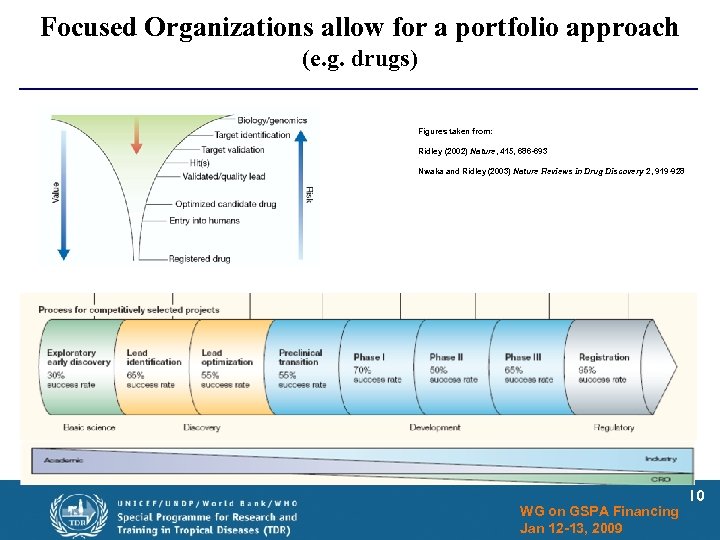
Focused Organizations allow for a portfolio approach (e. g. drugs) Figures taken from: Ridley (2002) Nature, 415, 686 -693 Nwaka and Ridley (2003) Nature Reviews in Drug Discovery 2, 919 -928 WG on GSPA Financing Jan 12 -13, 2009 10
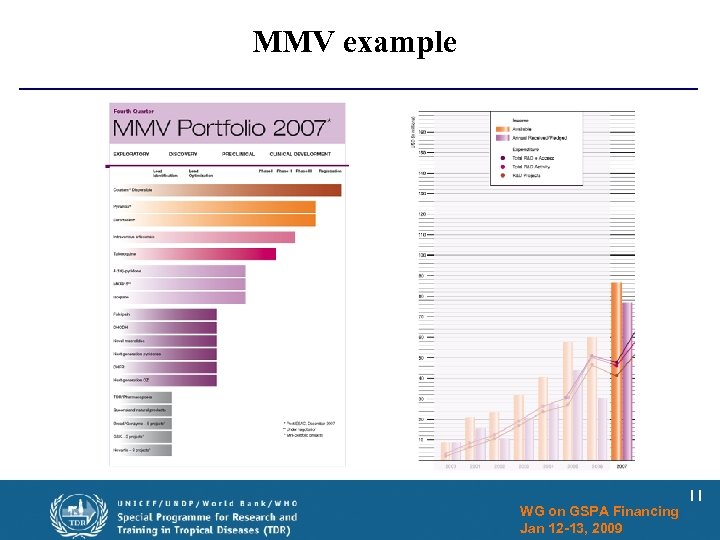
MMV example WG on GSPA Financing Jan 12 -13, 2009 11
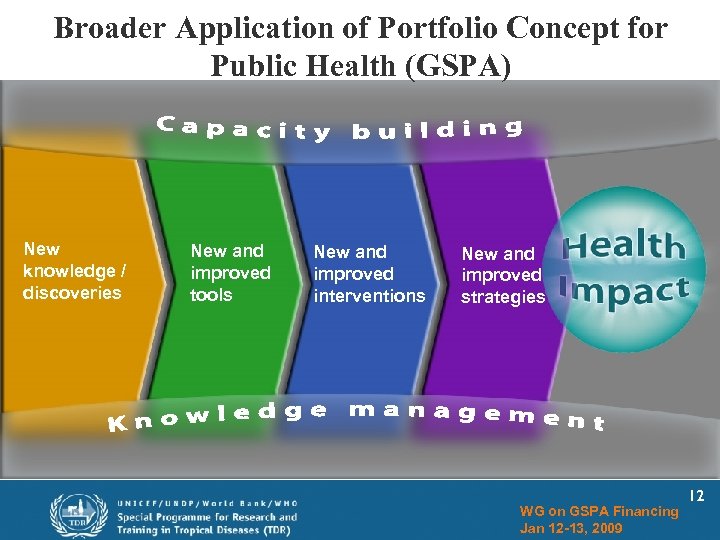
Broader Application of Portfolio Concept for Public Health (GSPA) New knowledge / discoveries New and improved tools New and improved interventions New and improved strategies WG on GSPA Financing Jan 12 -13, 2009 12
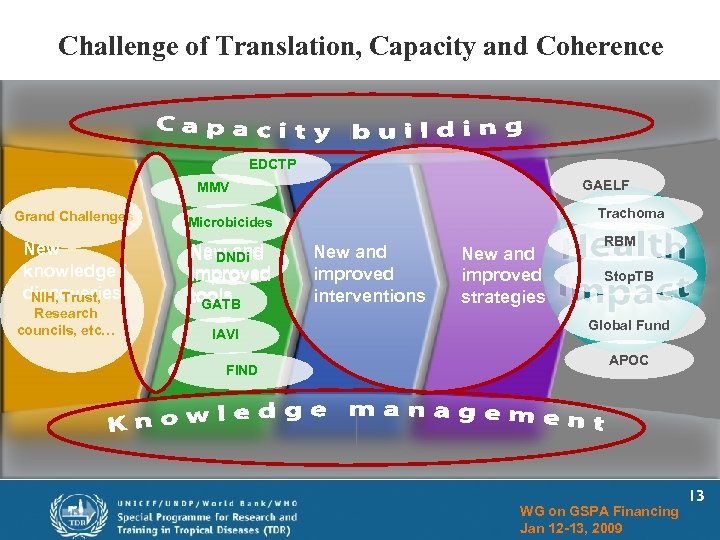
Challenge of Translation, Capacity and Coherence EDCTP GAELF MMV Grand Challenges New knowledge / discoveries NIH, Trust, New and DNDi improved tools GATB Trachoma Microbicides Research councils, etc… IAVI FIND New and improved interventions New and improved strategies RBM Stop. TB Global Fund APOC WG on GSPA Financing Jan 12 -13, 2009 13
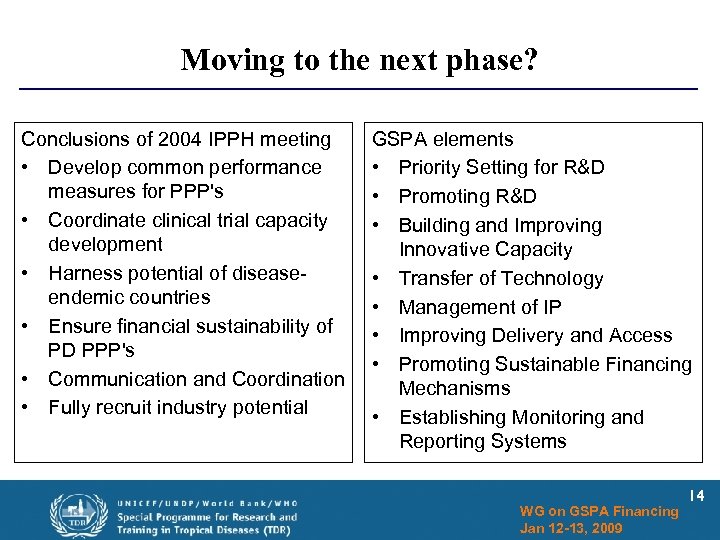
Moving to the next phase? Conclusions of 2004 IPPH meeting • Develop common performance measures for PPP's • Coordinate clinical trial capacity development • Harness potential of diseaseendemic countries • Ensure financial sustainability of PD PPP's • Communication and Coordination • Fully recruit industry potential GSPA elements • Priority Setting for R&D • Promoting R&D • Building and Improving Innovative Capacity • Transfer of Technology • Management of IP • Improving Delivery and Access • Promoting Sustainable Financing Mechanisms • Establishing Monitoring and Reporting Systems WG on GSPA Financing Jan 12 -13, 2009 14
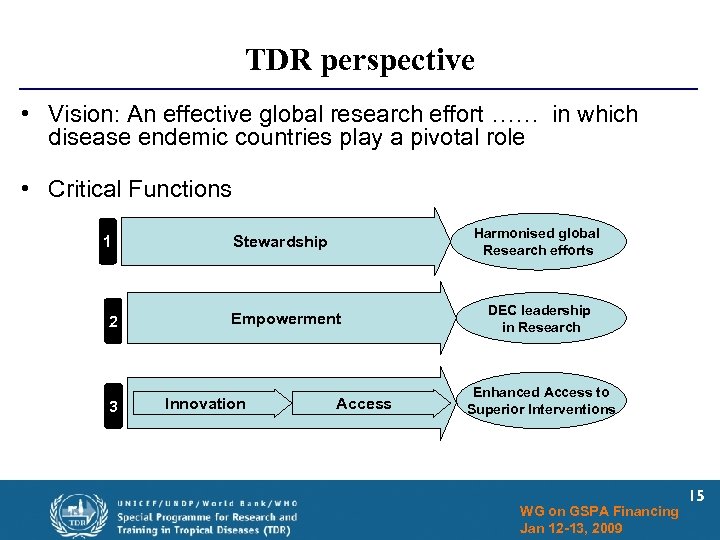
TDR perspective • Vision: An effective global research effort …… in which disease endemic countries play a pivotal role • Critical Functions 1 Stewardship Harmonised global Research efforts 2 Empowerment DEC leadership in Research 3 Innovation Research on Neglected. Access Priorities Enhanced Access to Superior Interventions WG on GSPA Financing Jan 12 -13, 2009 15
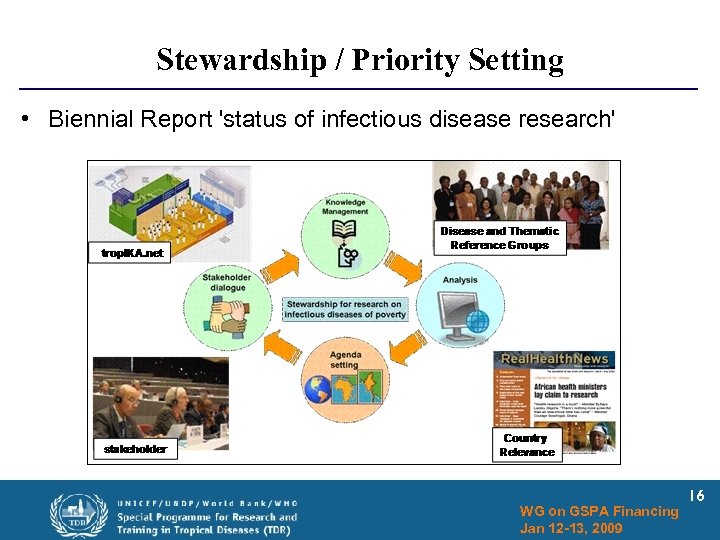
Stewardship / Priority Setting • Biennial Report 'status of infectious disease research' WG on GSPA Financing Jan 12 -13, 2009 16
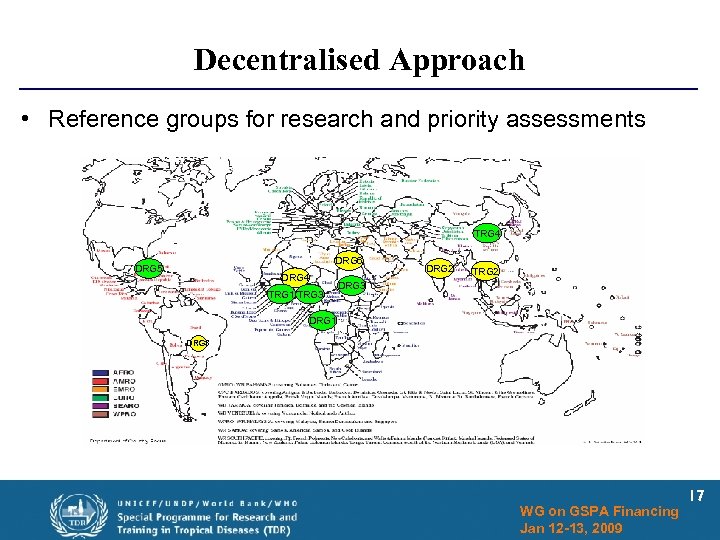
Decentralised Approach • Reference groups for research and priority assessments TRG 4 DRG 6 DRG 5 DRG 4 TRG 1 TRG 3 DRG 2 TRG 2 DRG 3 DRG 1 DRG 3 WG on GSPA Financing Jan 12 -13, 2009 17
Empowerment / Capacity Building • Focus on leadership development WG on GSPA Financing Jan 12 -13, 2009 18

Decentralised Approach • TDR teams managed through Country Institutions • Mobilization of capacity Coordination and Implementation Centres WG on GSPA Financing Jan 12 -13, 2009 19
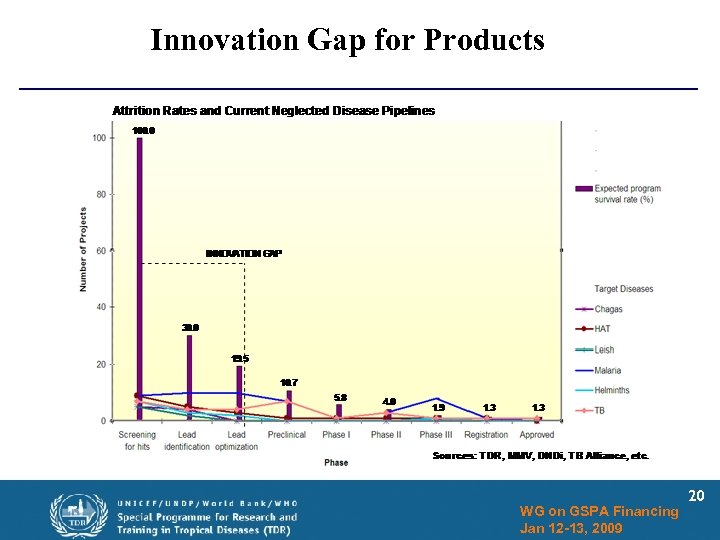
Innovation Gap for Products WG on GSPA Financing Jan 12 -13, 2009 20

Need for Innovative and Inclusive R&D Models • Industry model – Dedicated in house facility – Specific project or mini-portfolio partnership for a disease • Academic model – Compound screening – Dedicated units for Genomics, HTS, Chemistry – Specific PPP projects, and network activities • PPP model involving portfolio management – One or few diseases – Coordinated projects of academia, industry in the north and south. Few dedicated product R&D coordination mechanisms a) within developing countries b) for pre-competitive discovery WG on GSPA Financing Jan 12 -13, 2009 21
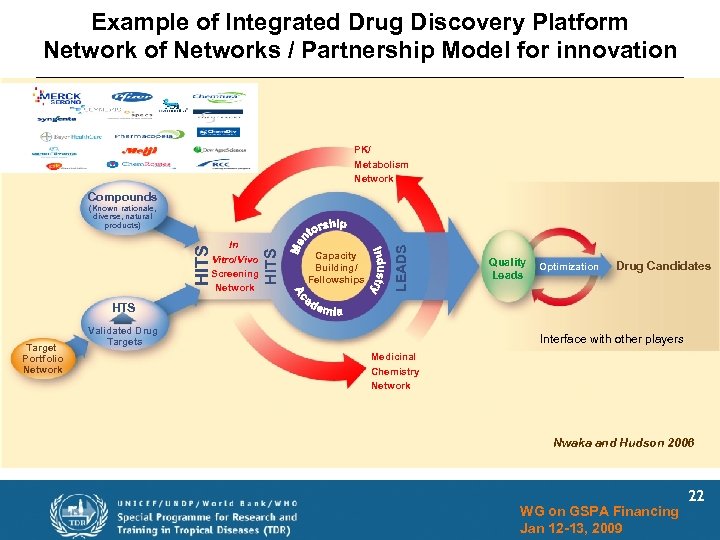
Example of Integrated Drug Discovery Platform Network of Networks / Partnership Model for innovation PK/ Metabolism Network Compounds Capacity Building/ Fellowships LEADS HITS In Vitro/Vivo Screening Network HITS (Known rationale, diverse, natural products) Quality Leads Optimization Drug Candidates HTS Target Portfolio Network Validated Drug Targets Interface with other players Medicinal Chemistry Network Nwaka and Hudson 2006 WG on GSPA Financing Jan 12 -13, 2009 22
Pre-competitive Innovation – Network Approach Discovery and Innovation • Pre-competitive academic and private sector networks for drugs and diagnostics • Agreements established, including IP • New lead compounds discovered • Initiation of an African Network in Abuja, October 2008 – Business plan for African based organization to be developed – Interest from other regions also WG on GSPA Financing Jan 12 -13, 2009 23
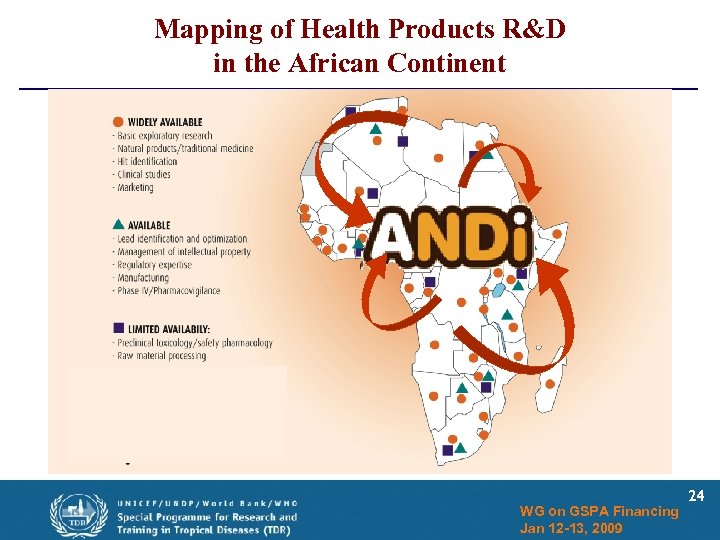
Mapping of Health Products R&D in the African Continent WG on GSPA Financing Jan 12 -13, 2009 24

Power of Networks – from business to social impact WG on GSPA Financing Jan 12 -13, 2009 25

Quality Assured Diagnostics Guidelines and Evaluation Networks Haiti Cuba Puerto Rico Gambia Venezuela Russia China Egypt Nepal Nigeria Benin Cameroon India Central African Ethiopia Republic Thailand Malaysia Kenya Congo Sri Lanka Uganda Rwanda Brazil Philippines Viet Nam Cambodia About the cover Columbia Peru Bangladesh Sudan Tanzania Zambia Madagascar Swaziland South Africa Argentina HAT VL TB SCHISTO DENGUE MALARIA STI WG on GSPA Financing Jan 12 -13, 2009 26

Quality Assured 'Point of Care' Diagnsotics • Define acceptance and evaluation criteria • Access and evaluate marketed diagnostics – Manufacturers agree to publication of data • Acceptable tests go on to WHO procurement list – – – Syphilis tests (6) Visceral Leishmania tests (1) Gonnorea and Chlamydia (0) TB (0) Malaria (40 tests under evaluation) • Country capacity needed for both evaluation and continued testing of batch quality WG on GSPA Financing Jan 12 -13, 2009 27
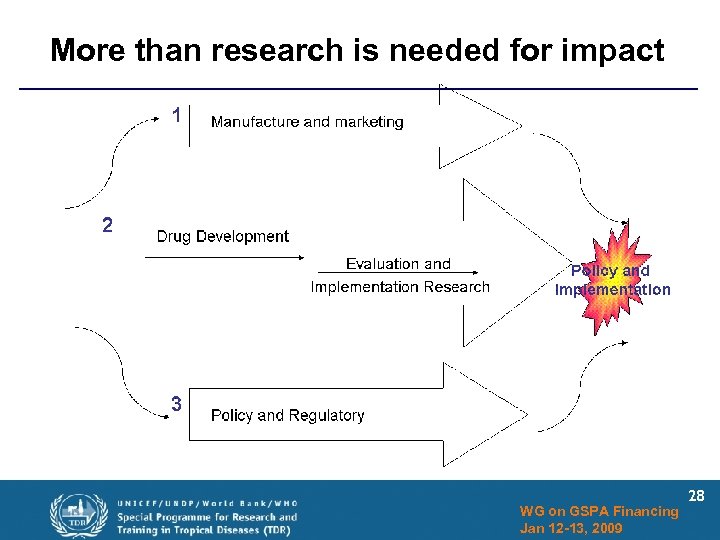
More than research is needed for impact WG on GSPA Financing Jan 12 -13, 2009 28
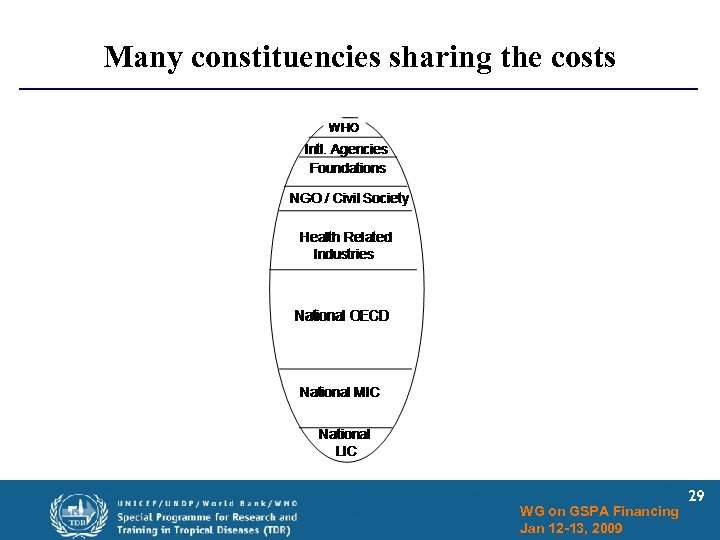
Many constituencies sharing the costs WG on GSPA Financing Jan 12 -13, 2009 29

Impact of Public – Private Partnership and Networks • Cost and time-effective product development (and delivery? ) where limited markets – Impact on health and health equity • Developing innovative capabilities that can feed broadly into health and other sectors – R&D targets are increasingly set as percentage of GDP – EU target 3%; AU target 1% • Linking academia, industry and public policy WG on GSPA Financing Jan 12 -13, 2009 30

Challenges • Sustain (and enhance) gains of last decade • Coherent competition in non-market, environment – Element 1 of GSPA (Priority setting) – Pre-competitive networks • Engaging Developing Countries – As generators of innovation and not just end users and evaluators of innovation • Access, Delivery …. – Importance of policy dimension where markets limited or where public sector drives the market WG on GSPA Financing Jan 12 -13, 2009 31
71a5eb4f3a41bbea31b0067ff0f22dc4.ppt