150213e159e47553c0e221574721f9d8.ppt
- Количество слайдов: 28
 PSI Materials Repository: Goals and Progress Josh La. Baer Director, Harvard Institute of Proteomics Harvard Medical School
PSI Materials Repository: Goals and Progress Josh La. Baer Director, Harvard Institute of Proteomics Harvard Medical School
 Who are we? Harvard Institute of Proteomics • High-throughput methods for biomarker, antigen and interaction discovery; cell-based screens; other functional assays • Clone production—ORF cloning for human genes and several microorganisms • Plasmid repository established 2004 – Currently >80, 000 clones • Plasmid information curated by a Ph. D level scientist prior to import • Plasmid Information Database (Plasm. ID) • Requests daily, distribute world-wide
Who are we? Harvard Institute of Proteomics • High-throughput methods for biomarker, antigen and interaction discovery; cell-based screens; other functional assays • Clone production—ORF cloning for human genes and several microorganisms • Plasmid repository established 2004 – Currently >80, 000 clones • Plasmid information curated by a Ph. D level scientist prior to import • Plasmid Information Database (Plasm. ID) • Requests daily, distribute world-wide
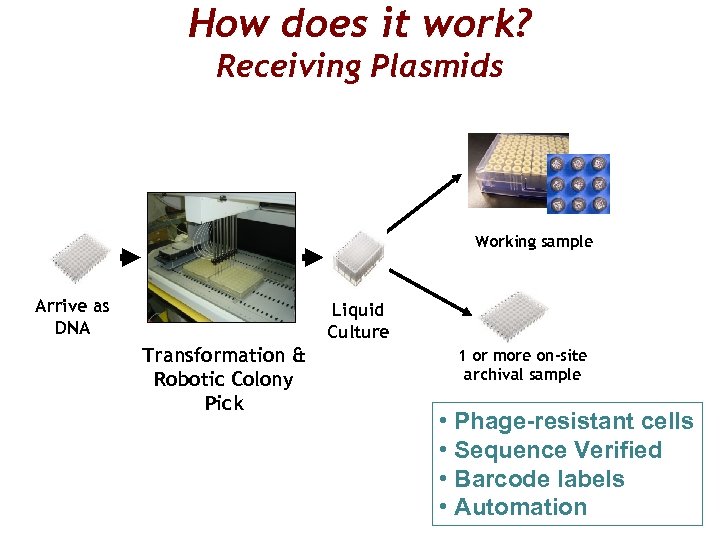 How does it work? Receiving Plasmids Working sample Arrive as DNA Liquid Culture Transformation & Robotic Colony Pick 1 or more on-site archival sample • Phage-resistant cells • Sequence Verified • Barcode labels • Automation
How does it work? Receiving Plasmids Working sample Arrive as DNA Liquid Culture Transformation & Robotic Colony Pick 1 or more on-site archival sample • Phage-resistant cells • Sequence Verified • Barcode labels • Automation
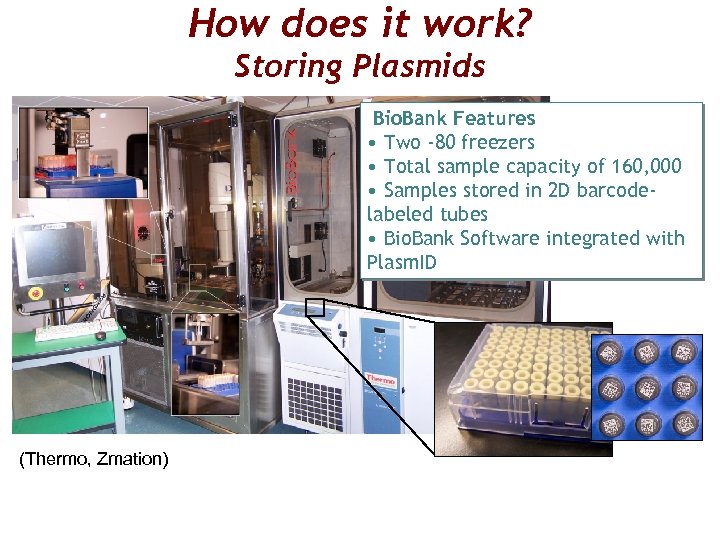 How does it work? Storing Plasmids Bio. Bank Features • Two -80 freezers • Total sample capacity of 160, 000 • Samples stored in 2 D barcodelabeled tubes • Bio. Bank Software integrated with Plasm. ID (Thermo, Zmation)
How does it work? Storing Plasmids Bio. Bank Features • Two -80 freezers • Total sample capacity of 160, 000 • Samples stored in 2 D barcodelabeled tubes • Bio. Bank Software integrated with Plasm. ID (Thermo, Zmation)
 How does it work? Information Handling Plasm. ID: http: //plasmid. hms. harvard. edu
How does it work? Information Handling Plasm. ID: http: //plasmid. hms. harvard. edu
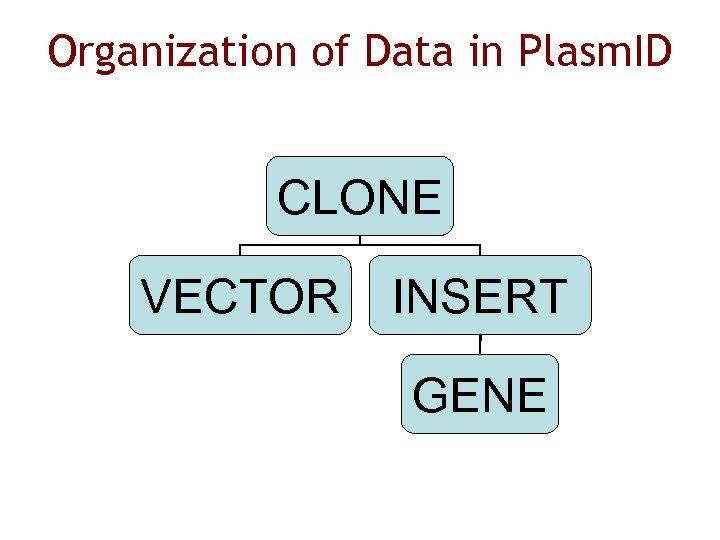 Organization of Data in Plasm. ID CLONE VECTOR INSERT GENE
Organization of Data in Plasm. ID CLONE VECTOR INSERT GENE
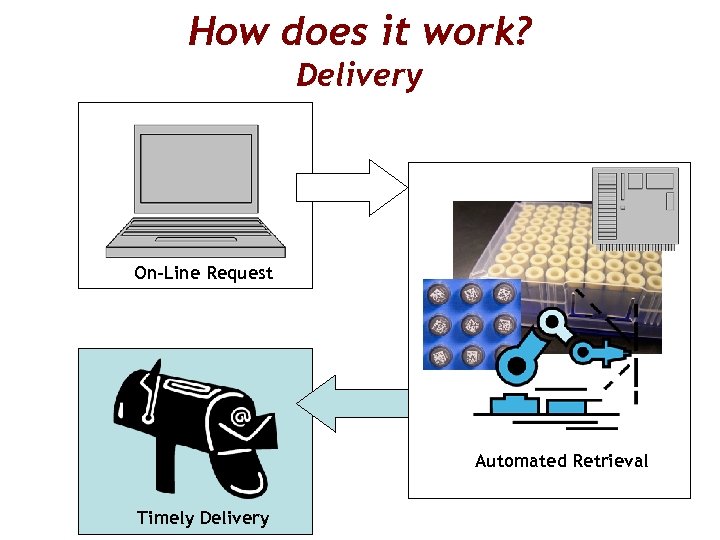 How does it work? Delivery On-Line Request Automated Retrieval Timely Delivery
How does it work? Delivery On-Line Request Automated Retrieval Timely Delivery
 Overall Goals of the PSI-MR • Centralized storage & distribution of information and samples for the >100, 000 plasmids created at PSI sites • Quality analysis and control at every step – – Barcode labels and Information Tracking at All Steps Single Colony Selection & Use of Phage-Resistant Bacterial Host Strains End read Sequencing & Analysis State-of-the-Art Freezer Storage System (Bio. Bank) • Scientifically Curated plasmid Information in Oracle Database (Plasm. ID) – Saves searchable information about all plasmids – Linked to PSI Knowledgebase – Ordering directly from website • Develop terms under which plasmids can be distributed under minimally restrictive terms – Depositors Agreement and Expedited Process MTA
Overall Goals of the PSI-MR • Centralized storage & distribution of information and samples for the >100, 000 plasmids created at PSI sites • Quality analysis and control at every step – – Barcode labels and Information Tracking at All Steps Single Colony Selection & Use of Phage-Resistant Bacterial Host Strains End read Sequencing & Analysis State-of-the-Art Freezer Storage System (Bio. Bank) • Scientifically Curated plasmid Information in Oracle Database (Plasm. ID) – Saves searchable information about all plasmids – Linked to PSI Knowledgebase – Ordering directly from website • Develop terms under which plasmids can be distributed under minimally restrictive terms – Depositors Agreement and Expedited Process MTA
 Early Goals • • • Create points of contact Collect & curate info on vectors Establish Depositor Agreements Establish clone submission format Transfer of information to PSI-MR Update Plasm. ID to reflect connection to PSI • Transfer of samples to PSI-MR • Create PSI-MR website
Early Goals • • • Create points of contact Collect & curate info on vectors Establish Depositor Agreements Establish clone submission format Transfer of information to PSI-MR Update Plasm. ID to reflect connection to PSI • Transfer of samples to PSI-MR • Create PSI-MR website
 Current Goals ü Create points of contact ü Collect & curate info on vectors ü Establish Depositor Agreements • Establish clone submission format • Transfer of information to PSI-MR • Transfer of samples to PSI-MR • Update Plasm. ID to reflect connection to PSI • Create PSI-MR website
Current Goals ü Create points of contact ü Collect & curate info on vectors ü Establish Depositor Agreements • Establish clone submission format • Transfer of information to PSI-MR • Transfer of samples to PSI-MR • Update Plasm. ID to reflect connection to PSI • Create PSI-MR website
 Depositors Agreement • Agreement that sets the terms for distributing plasmids deposited by the PSI-MR sites • Why do we need a DA? – Gives the Materials Repository permission to distribute the property of others • Expressly prohibited by MTAs – Appropriately assigns responsibility for the safety of distribution • MTAs do not address these issues – Universal set of operating conditions – Establishes the MTA that will accompany the clones
Depositors Agreement • Agreement that sets the terms for distributing plasmids deposited by the PSI-MR sites • Why do we need a DA? – Gives the Materials Repository permission to distribute the property of others • Expressly prohibited by MTAs – Appropriately assigns responsibility for the safety of distribution • MTAs do not address these issues – Universal set of operating conditions – Establishes the MTA that will accompany the clones
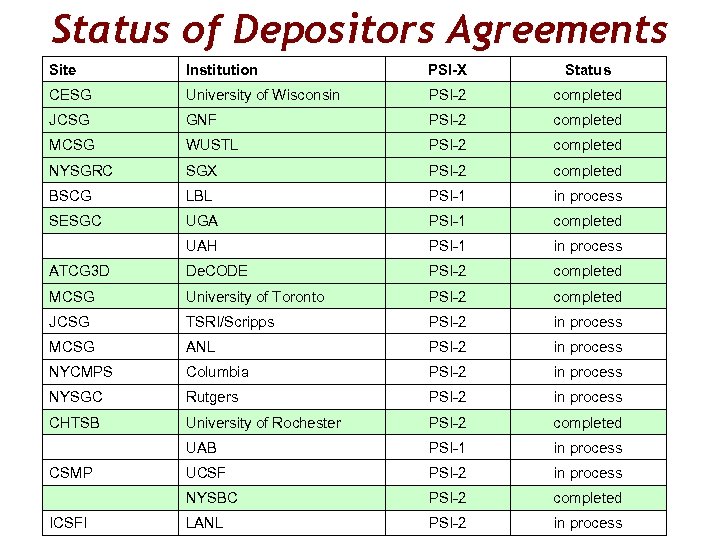 Status of Depositors Agreements Site Institution PSI-X Status CESG University of Wisconsin PSI-2 completed JCSG GNF PSI-2 completed MCSG WUSTL PSI-2 completed NYSGRC SGX PSI-2 completed BSCG LBL PSI-1 in process SESGC UGA PSI-1 completed UAH PSI-1 in process ATCG 3 D De. CODE PSI-2 completed MCSG University of Toronto PSI-2 completed JCSG TSRI/Scripps PSI-2 in process MCSG ANL PSI-2 in process NYCMPS Columbia PSI-2 in process NYSGC Rutgers PSI-2 in process CHTSB University of Rochester PSI-2 completed UAB PSI-1 in process CSMP UCSF PSI-2 in process NYSBC PSI-2 completed ICSFI LANL PSI-2 in process
Status of Depositors Agreements Site Institution PSI-X Status CESG University of Wisconsin PSI-2 completed JCSG GNF PSI-2 completed MCSG WUSTL PSI-2 completed NYSGRC SGX PSI-2 completed BSCG LBL PSI-1 in process SESGC UGA PSI-1 completed UAH PSI-1 in process ATCG 3 D De. CODE PSI-2 completed MCSG University of Toronto PSI-2 completed JCSG TSRI/Scripps PSI-2 in process MCSG ANL PSI-2 in process NYCMPS Columbia PSI-2 in process NYSGC Rutgers PSI-2 in process CHTSB University of Rochester PSI-2 completed UAB PSI-1 in process CSMP UCSF PSI-2 in process NYSBC PSI-2 completed ICSFI LANL PSI-2 in process
 Current Goals ü Create points of contact ü Collect & curate info on vectors ü Establish Depositor Agreements • Continue to work with PSI institutions and PIs to get the Depositors Agreement Signed • • • Establish clone submission format Transfer of information to PSI-MR Transfer of samples to PSI-MR Update Plasm. ID to reflect connection to PSI Create PSI-MR website
Current Goals ü Create points of contact ü Collect & curate info on vectors ü Establish Depositor Agreements • Continue to work with PSI institutions and PIs to get the Depositors Agreement Signed • • • Establish clone submission format Transfer of information to PSI-MR Transfer of samples to PSI-MR Update Plasm. ID to reflect connection to PSI Create PSI-MR website
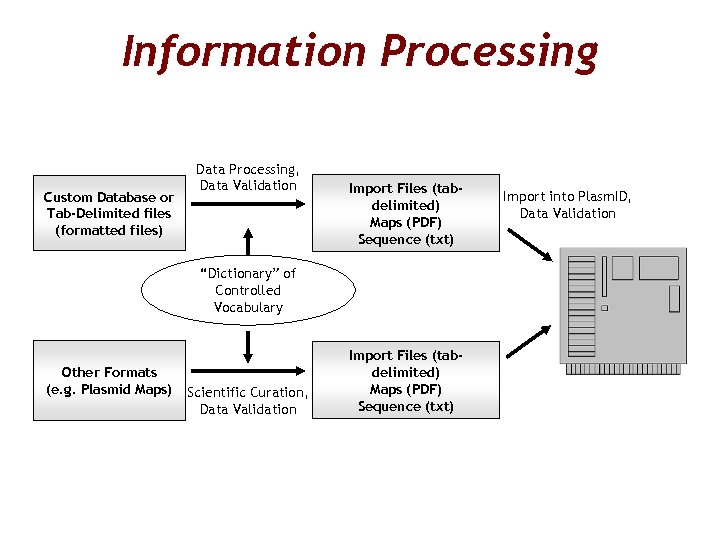 Information Processing Custom Database or Tab-Delimited files (formatted files) Data Processing, Data Validation Import Files (tabdelimited) Maps (PDF) Sequence (txt) “Dictionary” of Controlled Vocabulary Other Formats (e. g. Plasmid Maps) Scientific Curation, Data Validation Import Files (tabdelimited) Maps (PDF) Sequence (txt) Import into Plasm. ID, Data Validation
Information Processing Custom Database or Tab-Delimited files (formatted files) Data Processing, Data Validation Import Files (tabdelimited) Maps (PDF) Sequence (txt) “Dictionary” of Controlled Vocabulary Other Formats (e. g. Plasmid Maps) Scientific Curation, Data Validation Import Files (tabdelimited) Maps (PDF) Sequence (txt) Import into Plasm. ID, Data Validation
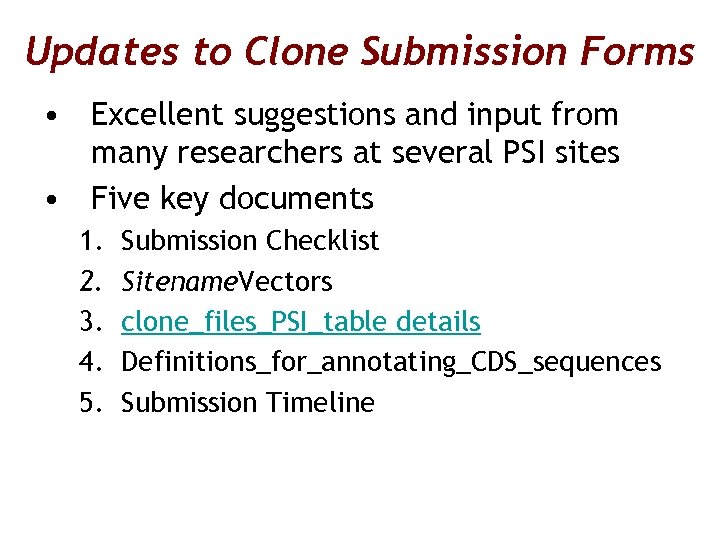 Updates to Clone Submission Forms • Excellent suggestions and input from many researchers at several PSI sites • Five key documents 1. 2. 3. 4. 5. Submission Checklist Sitename. Vectors clone_files_PSI_table details Definitions_for_annotating_CDS_sequences Submission Timeline
Updates to Clone Submission Forms • Excellent suggestions and input from many researchers at several PSI sites • Five key documents 1. 2. 3. 4. 5. Submission Checklist Sitename. Vectors clone_files_PSI_table details Definitions_for_annotating_CDS_sequences Submission Timeline
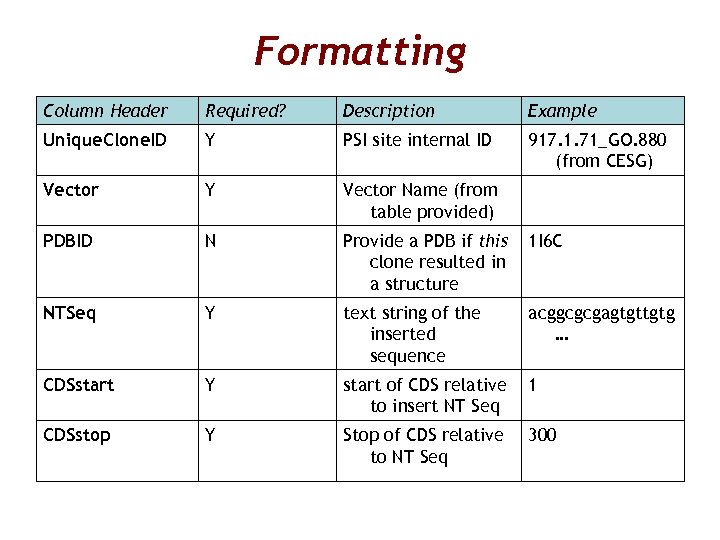 Formatting Column Header Required? Description Example Unique. Clone. ID Y PSI site internal ID 917. 1. 71_GO. 880 (from CESG) Vector Y Vector Name (from table provided) PDBID N Provide a PDB if this clone resulted in a structure 1 I 6 C NTSeq Y text string of the inserted sequence acggcgcgagtgttgtg … CDSstart Y start of CDS relative to insert NT Seq 1 CDSstop Y Stop of CDS relative to NT Seq 300
Formatting Column Header Required? Description Example Unique. Clone. ID Y PSI site internal ID 917. 1. 71_GO. 880 (from CESG) Vector Y Vector Name (from table provided) PDBID N Provide a PDB if this clone resulted in a structure 1 I 6 C NTSeq Y text string of the inserted sequence acggcgcgagtgttgtg … CDSstart Y start of CDS relative to insert NT Seq 1 CDSstop Y Stop of CDS relative to NT Seq 300
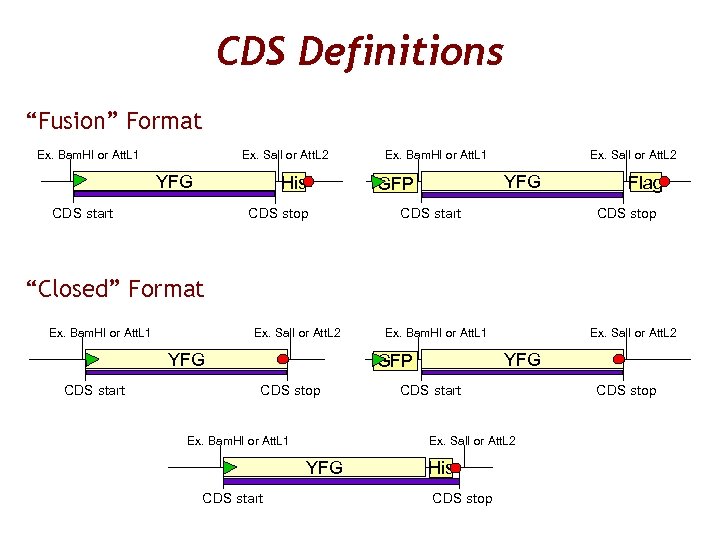 CDS Definitions “Fusion” Format Ex. Bam. HI or Att. L 1 Ex. Sal. I or Att. L 2 YFG His CDS start CDS stop Ex. Bam. HI or Att. L 1 Ex. Sal. I or Att. L 2 YFG GFP CDS start Flag CDS stop “Closed” Format Ex. Bam. HI or Att. L 1 Ex. Sal. I or Att. L 2 YFG CDS start Ex. Bam. HI or Att. L 1 YFG GFP CDS stop Ex. Bam. HI or Att. L 1 CDS start Ex. Sal. I or Att. L 2 YFG CDS start Ex. Sal. I or Att. L 2 His CDS stop
CDS Definitions “Fusion” Format Ex. Bam. HI or Att. L 1 Ex. Sal. I or Att. L 2 YFG His CDS start CDS stop Ex. Bam. HI or Att. L 1 Ex. Sal. I or Att. L 2 YFG GFP CDS start Flag CDS stop “Closed” Format Ex. Bam. HI or Att. L 1 Ex. Sal. I or Att. L 2 YFG CDS start Ex. Bam. HI or Att. L 1 YFG GFP CDS stop Ex. Bam. HI or Att. L 1 CDS start Ex. Sal. I or Att. L 2 YFG CDS start Ex. Sal. I or Att. L 2 His CDS stop
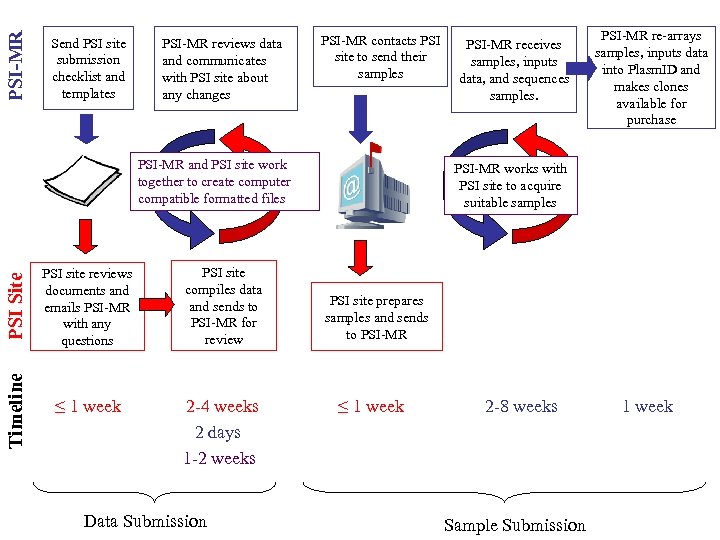 PSI-MR Send PSI site submission checklist and templates PSI-MR reviews data and communicates with PSI site about any changes PSI-MR contacts PSI site to send their samples PSI Site PSI site reviews documents and emails PSI-MR with any questions PSI site compiles data and sends to PSI-MR for review Timeline PSI-MR and PSI site work together to create computer compatible formatted files ≤ 1 week 2 -4 weeks 2 days 1 -2 weeks Data Submission PSI-MR receives samples, inputs data, and sequences samples. PSI-MR re-arrays samples, inputs data into Plasm. ID and makes clones available for purchase PSI-MR works with PSI site to acquire suitable samples PSI site prepares samples and sends to PSI-MR ≤ 1 week 2 -8 weeks Sample Submission 1 week
PSI-MR Send PSI site submission checklist and templates PSI-MR reviews data and communicates with PSI site about any changes PSI-MR contacts PSI site to send their samples PSI Site PSI site reviews documents and emails PSI-MR with any questions PSI site compiles data and sends to PSI-MR for review Timeline PSI-MR and PSI site work together to create computer compatible formatted files ≤ 1 week 2 -4 weeks 2 days 1 -2 weeks Data Submission PSI-MR receives samples, inputs data, and sequences samples. PSI-MR re-arrays samples, inputs data into Plasm. ID and makes clones available for purchase PSI-MR works with PSI site to acquire suitable samples PSI site prepares samples and sends to PSI-MR ≤ 1 week 2 -8 weeks Sample Submission 1 week
 Current Goals ü Create points of contact ü Collect & curate info on vectors ü Establish Depositor Agreements • Continue to work with PSI institutions and PIs to get the Depositors Agreement Signed ü Establish clone submission format • Send these documents to PSI sites • • Transfer of information to PSI-MR Transfer of samples to PSI-MR Update Plasm. ID to reflect connection to PSI Create PSI-MR website
Current Goals ü Create points of contact ü Collect & curate info on vectors ü Establish Depositor Agreements • Continue to work with PSI institutions and PIs to get the Depositors Agreement Signed ü Establish clone submission format • Send these documents to PSI sites • • Transfer of information to PSI-MR Transfer of samples to PSI-MR Update Plasm. ID to reflect connection to PSI Create PSI-MR website
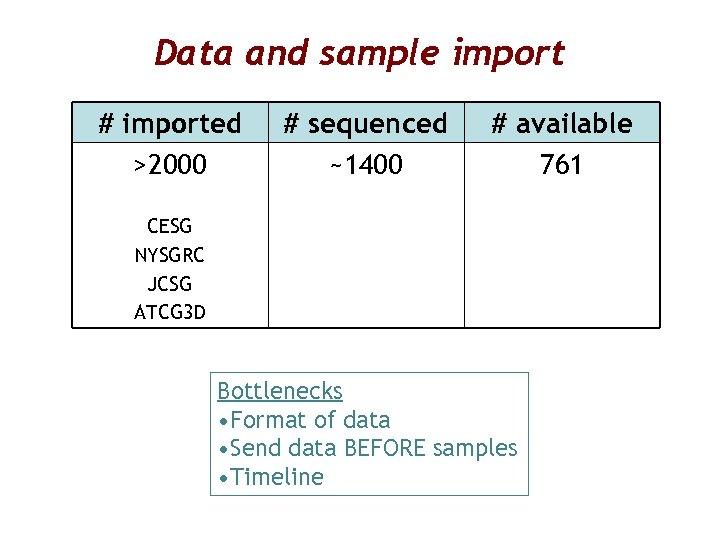 Data and sample import # imported >2000 # sequenced ~1400 # available 761 CESG NYSGRC JCSG ATCG 3 D Bottlenecks • Format of data • Send data BEFORE samples • Timeline
Data and sample import # imported >2000 # sequenced ~1400 # available 761 CESG NYSGRC JCSG ATCG 3 D Bottlenecks • Format of data • Send data BEFORE samples • Timeline
 Current Goals ü Create points of contact ü Collect & curate info on vectors ü Establish Depositor Agreements • Continue to work with PSI institutions and PIs to get the Depositors Agreement Signed ü Establish clone info deposit format • Send these documents to PSI sites • • Transfer of information to PSI-MR Transfer of samples to PSI-MR Update Plasm. ID to reflect connection to PSI Create PSI-MR website
Current Goals ü Create points of contact ü Collect & curate info on vectors ü Establish Depositor Agreements • Continue to work with PSI institutions and PIs to get the Depositors Agreement Signed ü Establish clone info deposit format • Send these documents to PSI sites • • Transfer of information to PSI-MR Transfer of samples to PSI-MR Update Plasm. ID to reflect connection to PSI Create PSI-MR website
 What’s new on Plasm. ID • PSI specific searches • Target. DB/Pepc. DB ID • Protein expression, solubility, or purification • PDB ID • PSI site • Plasmids linked to PSI Structural Genomics Knowledgebase by Target. DB ID • Credit cards now accepted for all plasmid purchases • Pay. Pal account NOT required
What’s new on Plasm. ID • PSI specific searches • Target. DB/Pepc. DB ID • Protein expression, solubility, or purification • PDB ID • PSI site • Plasmids linked to PSI Structural Genomics Knowledgebase by Target. DB ID • Credit cards now accepted for all plasmid purchases • Pay. Pal account NOT required
 Plasm. ID demo http: //plasmid. med. harvard. edu/PLASMID/
Plasm. ID demo http: //plasmid. med. harvard. edu/PLASMID/
 Current Goals ü Create points of contact ü Collect & curate info on vectors ü Establish Depositor Agreements • Continue to work with PSI institutions and PIs to get the Depositors Agreement Signed ü Establish clone info deposit format • Send these documents to PSI sites • • Transfer of information to PSI-MR Update Plasm. ID to reflect connection to PSI Transfer of samples to PSI-MR Create PSI-MR website
Current Goals ü Create points of contact ü Collect & curate info on vectors ü Establish Depositor Agreements • Continue to work with PSI institutions and PIs to get the Depositors Agreement Signed ü Establish clone info deposit format • Send these documents to PSI sites • • Transfer of information to PSI-MR Update Plasm. ID to reflect connection to PSI Transfer of samples to PSI-MR Create PSI-MR website
 PSI-MR Web Portal • Has most up to date clone submission templates • Contains information on how to search for and purchase plasmids on Plasm. ID • Links to PSI-KB and all PSI Modules and Sites • FAQs about PSI-MR, Depositors Agreements and MTAs
PSI-MR Web Portal • Has most up to date clone submission templates • Contains information on how to search for and purchase plasmids on Plasm. ID • Links to PSI-KB and all PSI Modules and Sites • FAQs about PSI-MR, Depositors Agreements and MTAs
 PSI-MR portal demo http: //www. hip. harvard. edu/PSIMR/index. htm
PSI-MR portal demo http: //www. hip. harvard. edu/PSIMR/index. htm
 Future Goals • Have all depositors agreements signed • Expedited Process MTA • Continue to process data and samples from PSI sites • Work with other PSI sites to start the submission process • Vector information updates from all PSI sites – Creating an online vector submission module
Future Goals • Have all depositors agreements signed • Expedited Process MTA • Continue to process data and samples from PSI sites • Work with other PSI sites to start the submission process • Vector information updates from all PSI sites – Creating an online vector submission module
 Acknowledgements NIH/NIGMS Cathy Cormier Janice Williamson Helen Taycher Yanhui Hu Dongmei Zuo Andreas Rolfs Tina Kelley Mike Collins Jason Kramer April Pierce Li Chan Dan Schiwek Jason Xu Stephanie Mohr Jean Chin PSI KB Helen Berman Andrei Kouranov Wendy Tao Raship Shah John Westbrook All PSI Sites Funding:
Acknowledgements NIH/NIGMS Cathy Cormier Janice Williamson Helen Taycher Yanhui Hu Dongmei Zuo Andreas Rolfs Tina Kelley Mike Collins Jason Kramer April Pierce Li Chan Dan Schiwek Jason Xu Stephanie Mohr Jean Chin PSI KB Helen Berman Andrei Kouranov Wendy Tao Raship Shah John Westbrook All PSI Sites Funding:


