9a26c7032ca5079056d5633531e69daa.ppt
- Количество слайдов: 21
 Proton conductors Low-temperature systems • water containing systems. e. g. Nafion, heteropolyacids • oxoacids and their salts, which show proton conductivity even in the absence of water due to their self-dissociation, e. g. Cs. HSO 4 (s=10 -3 S cm-1 above 412 K) • blends of organic compounds exhibiting basic sites with acids, e. g. H 3 PO 4 or H 2 SO 4. • Xerogels- amorphous materials obtained by drying of the inorganic gels synthesised using sol-gel route. High temperature systems • oxides, hydroxides and apatites
Proton conductors Low-temperature systems • water containing systems. e. g. Nafion, heteropolyacids • oxoacids and their salts, which show proton conductivity even in the absence of water due to their self-dissociation, e. g. Cs. HSO 4 (s=10 -3 S cm-1 above 412 K) • blends of organic compounds exhibiting basic sites with acids, e. g. H 3 PO 4 or H 2 SO 4. • Xerogels- amorphous materials obtained by drying of the inorganic gels synthesised using sol-gel route. High temperature systems • oxides, hydroxides and apatites
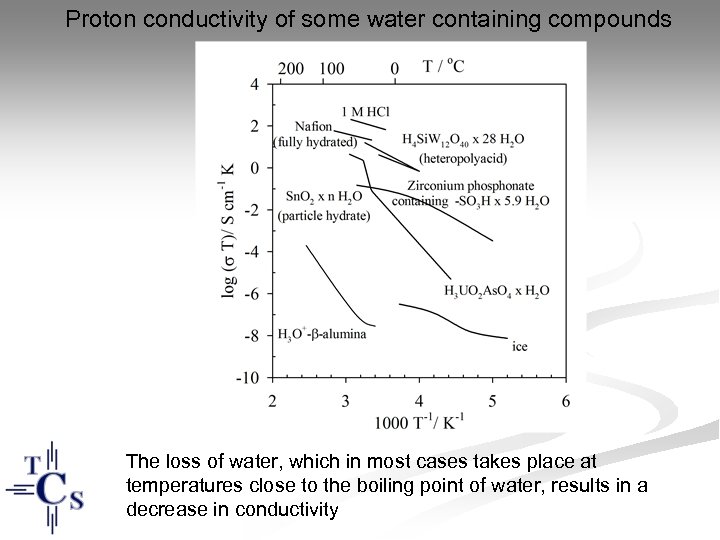 Proton conductivity of some water containing compounds The loss of water, which in most cases takes place at temperatures close to the boiling point of water, results in a decrease in conductivity
Proton conductivity of some water containing compounds The loss of water, which in most cases takes place at temperatures close to the boiling point of water, results in a decrease in conductivity
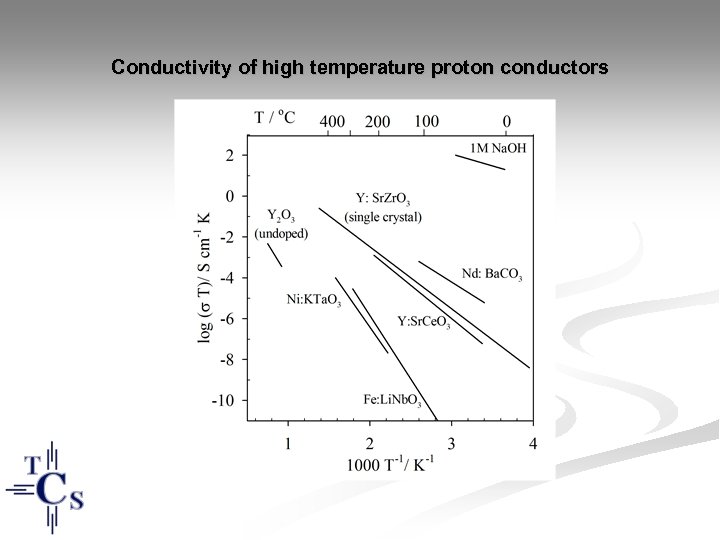 Conductivity of high temperature proton conductors
Conductivity of high temperature proton conductors
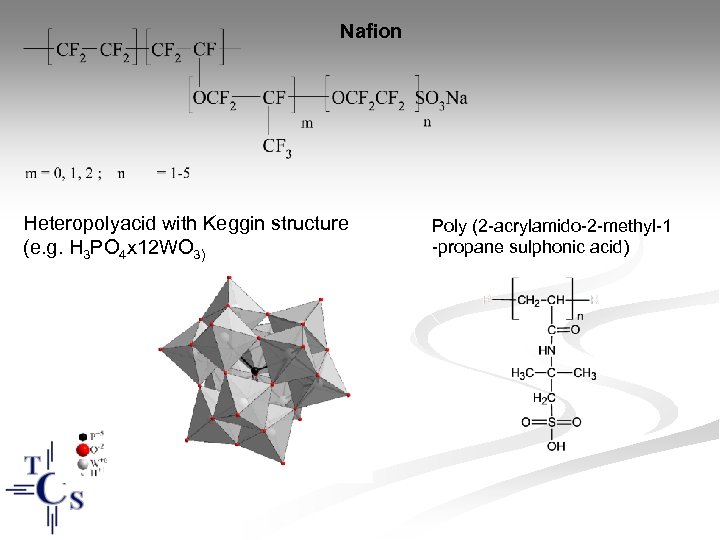 Nafion Heteropolyacid with Keggin structure (e. g. H 3 PO 4 x 12 WO 3) Poly (2 -acrylamido-2 -methyl-1 -propane sulphonic acid)
Nafion Heteropolyacid with Keggin structure (e. g. H 3 PO 4 x 12 WO 3) Poly (2 -acrylamido-2 -methyl-1 -propane sulphonic acid)
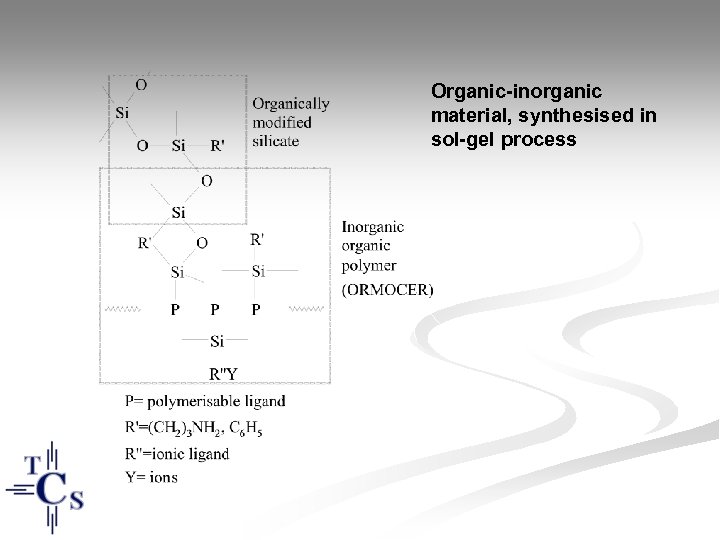 Organic-inorganic material, synthesised in sol-gel process
Organic-inorganic material, synthesised in sol-gel process
 Polymer electrolytes • Acidic groups (-COOH, -SO 3 H) in side or main chain (part of the polymer bachbone), e. g. poly (acrylic acid), PAMPS • Complexes of polymer with salt or acid: polymer with basic sites in a chain is a solvent for the dopant • Polymer gels- three component systems, combining polymer matrix swollen with dopant solution in an an apropriate solvent Polymers which may be applied in proton conducting systems should fulfil some requirements, such as: ‑ chemical and thermodynamic stability ‑ specific protonic conductivity ‑ conductivity range depending on the perspective application, i. e. 10‑ 1‑ 10‑ 3 S cm‑ 1 for fuel cells and 10‑ 5‑ 10‑ 7 S cm‑ 1 for sensors or electrochromic devices ‑ properties independent of the humidity level ‑ thin film configuration. The use in electrochromic devices requires also high transparency of membranes
Polymer electrolytes • Acidic groups (-COOH, -SO 3 H) in side or main chain (part of the polymer bachbone), e. g. poly (acrylic acid), PAMPS • Complexes of polymer with salt or acid: polymer with basic sites in a chain is a solvent for the dopant • Polymer gels- three component systems, combining polymer matrix swollen with dopant solution in an an apropriate solvent Polymers which may be applied in proton conducting systems should fulfil some requirements, such as: ‑ chemical and thermodynamic stability ‑ specific protonic conductivity ‑ conductivity range depending on the perspective application, i. e. 10‑ 1‑ 10‑ 3 S cm‑ 1 for fuel cells and 10‑ 5‑ 10‑ 7 S cm‑ 1 for sensors or electrochromic devices ‑ properties independent of the humidity level ‑ thin film configuration. The use in electrochromic devices requires also high transparency of membranes
 Gel electrolytes Polymers: Acrylic and methacrylic polymers (PMMA, PAN, PGMA, PAAM), poly (vinylidene fluoride), poly (vinyl chloride), PEO Solvents: Propylene carbonate, ethylene carbonate, N, Ndimethylformamide, glymes, N-vinylpyrrolidone Acids: Phosphoric acid and its acidic esters, sulfuric acid, sulphonic acids, phosphonic acids, heteropolyacids
Gel electrolytes Polymers: Acrylic and methacrylic polymers (PMMA, PAN, PGMA, PAAM), poly (vinylidene fluoride), poly (vinyl chloride), PEO Solvents: Propylene carbonate, ethylene carbonate, N, Ndimethylformamide, glymes, N-vinylpyrrolidone Acids: Phosphoric acid and its acidic esters, sulfuric acid, sulphonic acids, phosphonic acids, heteropolyacids
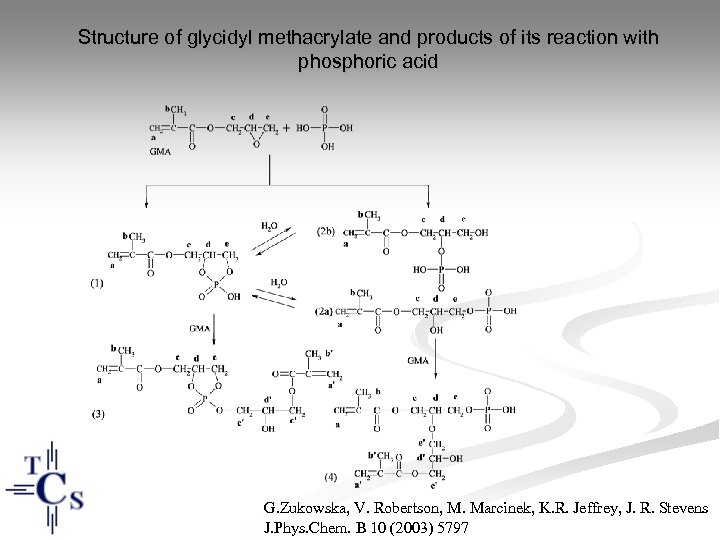 Structure of glycidyl methacrylate and products of its reaction with phosphoric acid G. Zukowska, V. Robertson, M. Marcinek, K. R. Jeffrey, J. R. Stevens J. Phys. Chem. B 10 (2003) 5797
Structure of glycidyl methacrylate and products of its reaction with phosphoric acid G. Zukowska, V. Robertson, M. Marcinek, K. R. Jeffrey, J. R. Stevens J. Phys. Chem. B 10 (2003) 5797
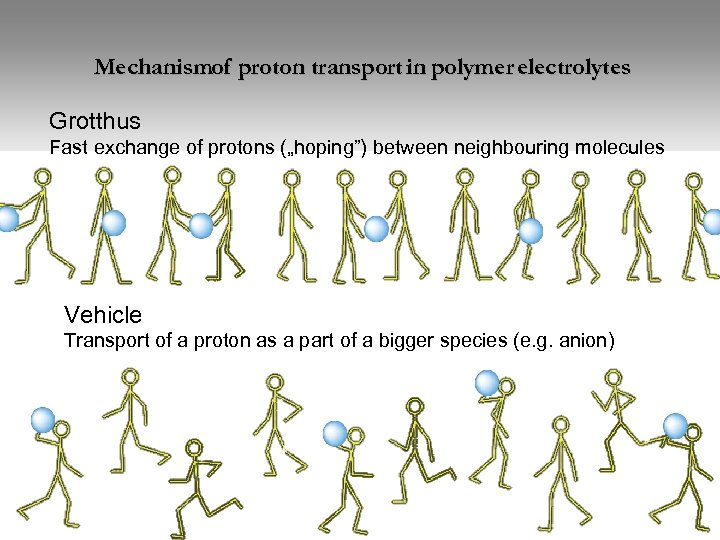 Mechanismof proton transport in polymer electrolytes Grotthus Fast exchange of protons („hoping”) between neighbouring molecules Vehicle Transport of a proton as a part of a bigger species (e. g. anion)
Mechanismof proton transport in polymer electrolytes Grotthus Fast exchange of protons („hoping”) between neighbouring molecules Vehicle Transport of a proton as a part of a bigger species (e. g. anion)
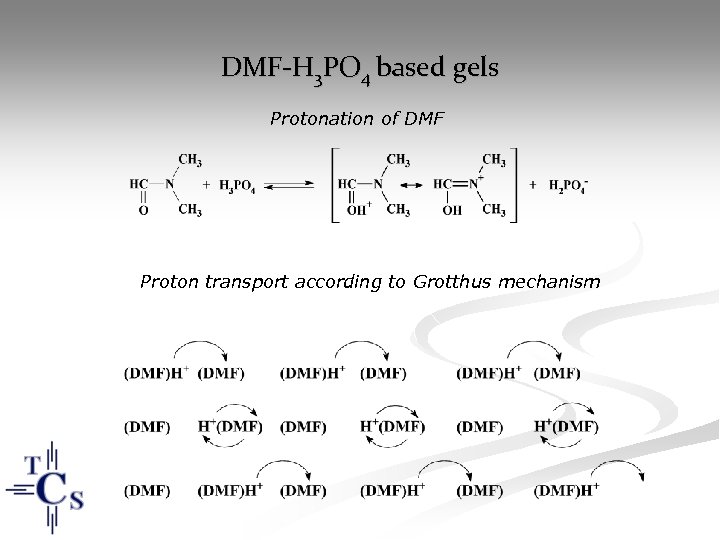 DMF-H 3 PO 4 based gels Protonation of DMF Proton transport according to Grotthus mechanism
DMF-H 3 PO 4 based gels Protonation of DMF Proton transport according to Grotthus mechanism
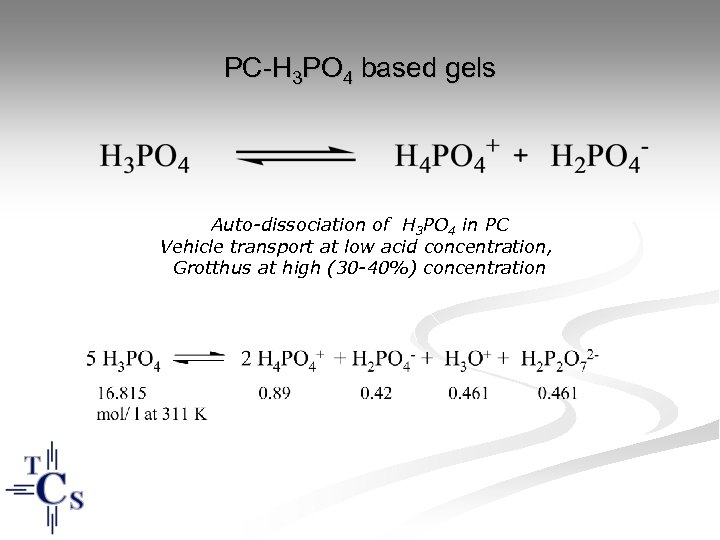 PC-H 3 PO 4 based gels Auto-dissociation of H 3 PO 4 in PC Vehicle transport at low acid concentration, Grotthus at high (30 -40%) concentration
PC-H 3 PO 4 based gels Auto-dissociation of H 3 PO 4 in PC Vehicle transport at low acid concentration, Grotthus at high (30 -40%) concentration
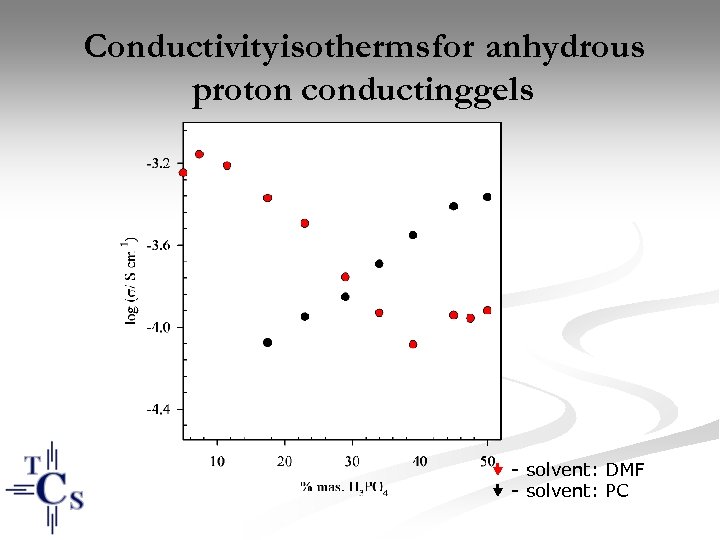 Conductivity isotherms for anhydrous proton conductinggels - solvent: DMF - solvent: PC
Conductivity isotherms for anhydrous proton conductinggels - solvent: DMF - solvent: PC
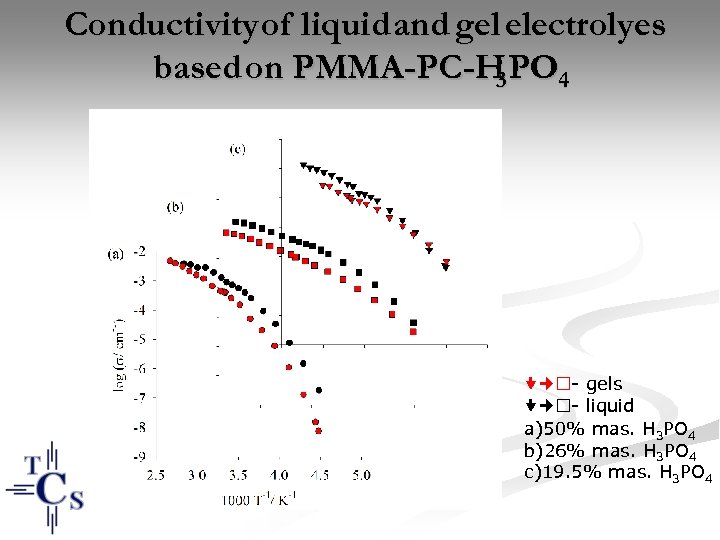 Conductivity of liquid and gel electrolyes based on PMMA-PC-H PO 4 3 - gels - liquid a)50% mas. H 3 PO 4 b)26% mas. H 3 PO 4 c)19. 5% mas. H 3 PO 4
Conductivity of liquid and gel electrolyes based on PMMA-PC-H PO 4 3 - gels - liquid a)50% mas. H 3 PO 4 b)26% mas. H 3 PO 4 c)19. 5% mas. H 3 PO 4
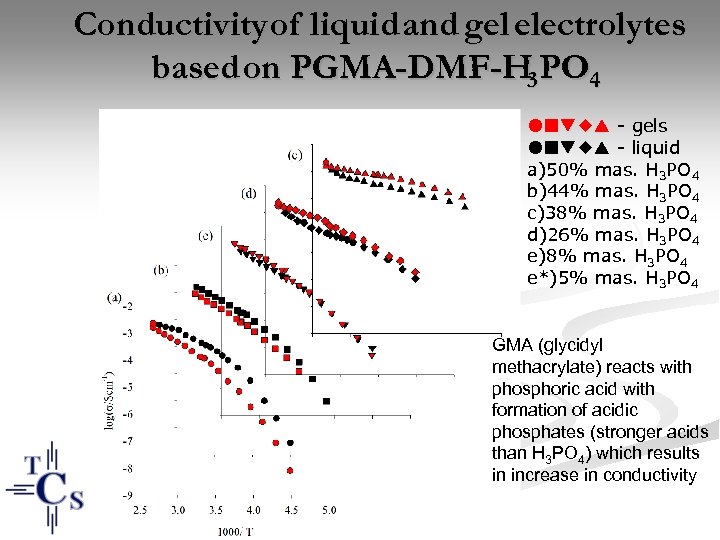 Conductivity of liquid and gel electrolytes based on PGMA-DMF-H 3 PO 4 - gels - liquid a)50% mas. H 3 PO 4 b)44% mas. H 3 PO 4 c)38% mas. H 3 PO 4 d)26% mas. H 3 PO 4 e)8% mas. H 3 PO 4 e*)5% mas. H 3 PO 4 GMA (glycidyl methacrylate) reacts with phosphoric acid with formation of acidic phosphates (stronger acids than H 3 PO 4) which results in increase in conductivity
Conductivity of liquid and gel electrolytes based on PGMA-DMF-H 3 PO 4 - gels - liquid a)50% mas. H 3 PO 4 b)44% mas. H 3 PO 4 c)38% mas. H 3 PO 4 d)26% mas. H 3 PO 4 e)8% mas. H 3 PO 4 e*)5% mas. H 3 PO 4 GMA (glycidyl methacrylate) reacts with phosphoric acid with formation of acidic phosphates (stronger acids than H 3 PO 4) which results in increase in conductivity
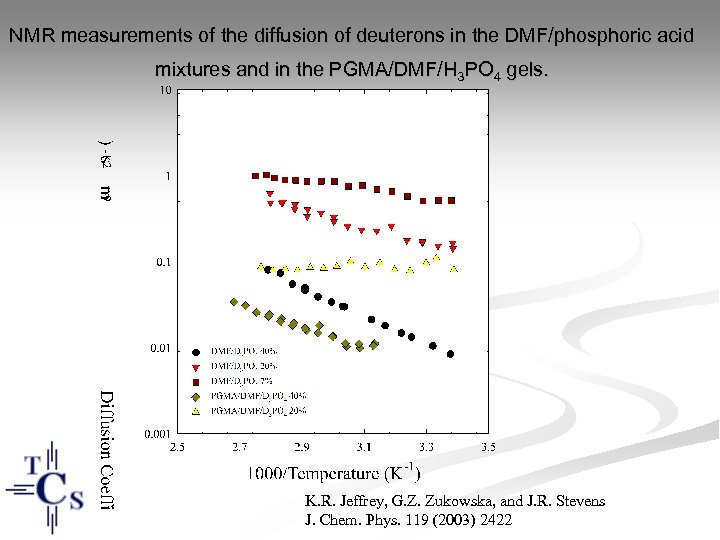 NMR measurements of the diffusion of deuterons in the DMF/phosphoric acid mixtures and in the PGMA/DMF/H 3 PO 4 gels. K. R. Jeffrey, G. Z. Zukowska, and J. R. Stevens J. Chem. Phys. 119 (2003) 2422
NMR measurements of the diffusion of deuterons in the DMF/phosphoric acid mixtures and in the PGMA/DMF/H 3 PO 4 gels. K. R. Jeffrey, G. Z. Zukowska, and J. R. Stevens J. Chem. Phys. 119 (2003) 2422
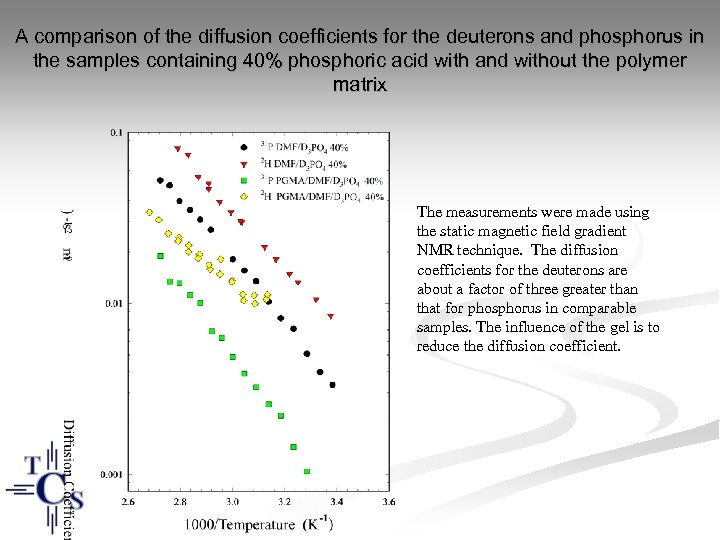 A comparison of the diffusion coefficients for the deuterons and phosphorus in the samples containing 40% phosphoric acid with and without the polymer matrix The measurements were made using the static magnetic field gradient NMR technique. The diffusion coefficients for the deuterons are about a factor of three greater than that for phosphorus in comparable samples. The influence of the gel is to reduce the diffusion coefficient.
A comparison of the diffusion coefficients for the deuterons and phosphorus in the samples containing 40% phosphoric acid with and without the polymer matrix The measurements were made using the static magnetic field gradient NMR technique. The diffusion coefficients for the deuterons are about a factor of three greater than that for phosphorus in comparable samples. The influence of the gel is to reduce the diffusion coefficient.
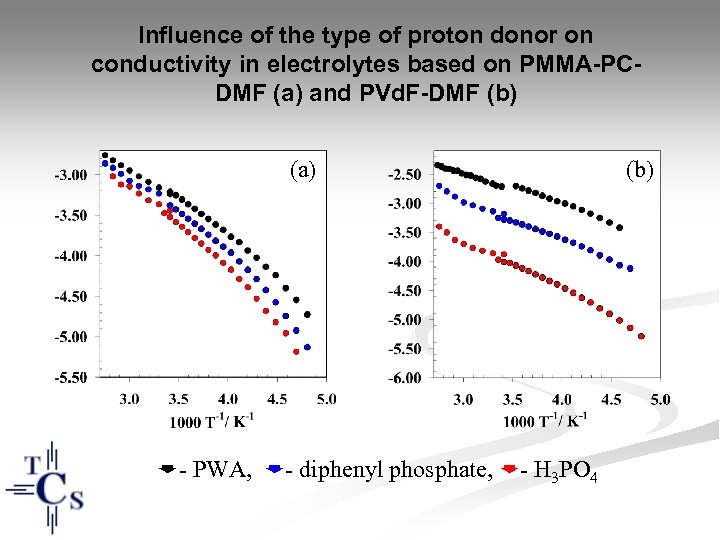 Influence of the type of proton donor on conductivity in electrolytes based on PMMA-PCDMF (a) and PVd. F-DMF (b) (a) - PWA, - diphenyl phosphate, - H 3 PO 4 (b)
Influence of the type of proton donor on conductivity in electrolytes based on PMMA-PCDMF (a) and PVd. F-DMF (b) (a) - PWA, - diphenyl phosphate, - H 3 PO 4 (b)
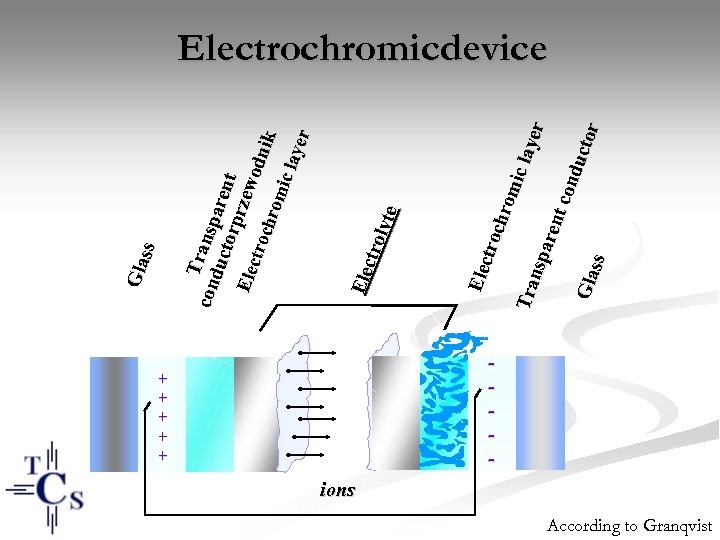 + + + Elec troc hro mic Tra laye nsp r aren t co ndu ctor Gla ss Ele ctro lyte T con ransp duc a torp rent rzew Elec odn troc ik hro mic laye r Gla ss Electrochromicdevice - ions According to Granqvist
+ + + Elec troc hro mic Tra laye nsp r aren t co ndu ctor Gla ss Ele ctro lyte T con ransp duc a torp rent rzew Elec odn troc ik hro mic laye r Gla ss Electrochromicdevice - ions According to Granqvist
 Some applications of electrochromic devices
Some applications of electrochromic devices
 Magicink
Magicink
 Modern house
Modern house


