2710f03e7b676f84a7dbe92c8143a1bd.ppt
- Количество слайдов: 155

Protecting Human Subjects -b Carleton College October 10, 2002 What Everyone Pw. C Needs to Know Tom Puglisi, Ph. D

The Protection of Human Subjects in Research Medical Research Summit Michele Russell-Einhorn, JD Tom Puglisi, Ph. D May 5, 2003 Pw. C © 2002 Pricewaterhouse. Coopers refers to the individual member firms of the world-wide Pricewaterhouse. Coopers organisation. All rights reserved.

Pricewaterhouse. Coopers

Pricewaterhouse. Coopers

Pricewaterhouse. Coopers

Government Shutdowns § Massachusetts Eye and Ear Infirmary § UCLA § VA Health Sys. Greater Los Angeles § Rush Presbyterian St Luke’s Med Ctr. § University of Illinois Chicago § Duke University Med Ctr. § Univ. Texas Medical Branch Galveston § University of Oklahoma Tulsa § Johns Hopkins University Pricewaterhouse. Coopers

Research Involving Human Subjects § Human Research Halted at Major Institutions – Deficient Informed Consent – Inadequate Initial and Continuing IRB Review – Multiple Areas of Concern § Death in Gene Transfer Research – Conflicts of Interest – Unreported Deaths and Injuries § Media Attention – Congressional Hearings – Distrust Pricewaterhouse. Coopers

Research Involving Human Subjects “What’s at Stake is the Integrity of Research and Public Confidence in Research” -- HHS Secretary, Donna Shalala, May 2000 Pricewaterhouse. Coopers

Historical Overview Pw. C

Historical Overview § Nazi Doctor Trials – Nuremberg Code – 1947 – Informed Consent § Declaration of Helsinki – World Medical Association – Ethical Principles for Medical Research Involving Human Subjects 1964 (revised 2000) Pricewaterhouse. Coopers

Historical Overview § Public Health Service (PHS) Policy – Prior Review of Research by “Institutional Associates” (PPO 129, February 8, 1966) § United States Public Health Service – Syphilis Study at Tuskegee (1932 -1972) Pricewaterhouse. Coopers

Historical Overview § Dr. Henry Beecher’s Review of Medical Literature § Radiation Experiments § Cancer Cell Injections § “Tea Room Trade” Study § Kansas City “Jury Deliberations” Research § Social Psychology Research – Conformity / Authority Pricewaterhouse. Coopers

Historical Overview -- 1974 § Congressional Hearings – Senator Walter Mondale – Senator Edward Kennedy § HHS Regulations § National Research Act – National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research, July 12, 1974 Pricewaterhouse. Coopers

Historical Overview: The Belmont Report – April 18, 1979 Ethical Principles and Guidelines for the Protection of Human Subjects of Research Respect for Persons Beneficence Justice §Informed Consent §Capacity to Consent §Do no Harm §Maximize Benefit §Equitable Selection of Subjects §Equitable Burdens and Benefits Pricewaterhouse. Coopers

Roles and Responsibilities § Institutional Review Boards (IRBs) § Research Investigators § Sponsors § Data Safety Monitoring Boards (DSMBs) Pricewaterhouse. Coopers

Roles and Responsibilities: Institutional Responsibility § Institutional Commitment and Infrastructure § Authorized Institutional Official § IRB Chair, IRB Members, IRB Staff § Other Institutional Committees § Research Investigators and Co-Investigators § Study Coordinators and Research Staff § Everyone Else Involved in the Research Enterprise Pricewaterhouse. Coopers

Roles and Responsibilities: Authorized Institutional Official § Legal Signatory for Institution (e. g. , Assurance) § Overall Organizational Responsibility § Ensure Adequate placement of IRB within Institutional Structure § Ensure Adequate resources for IRB (staff, computers, office space, etc. ) § Inspire and Enforce Institutional Culture of Respect and Compliance (e. g. , Oversight and Monitoring of Research) Pricewaterhouse. Coopers

Roles and Responsibilities: Institutional Review Board (IRB) § Review and Approve Proposed Research – Risks Minimized through Sound Research Design – Risks Reasonable Relative to Benefits – Subject Selection Equitable – Informed Consent Obtained and Documented – Privacy and Confidentiality Protections Adequate – Safety Monitoring is Adequate – Protections for Vulnerable Subjects are Adequate § Exercise Continuing Oversight of Research Pricewaterhouse. Coopers

Roles and Responsibilities: Principal Investigators General Responsibilities of Principal Investigators: § Accept and exercise responsibility for all aspects of the research § Ensure adequate training for entire research team § Ensure adequate supervision of entire research team § Know and ensure compliance with all regulatory requirements, IRB requirements, and protocol requirements § Ensure adherence to enrollment criteria § Monitor and report unanticipated problems and adverse events to sponsor and IRB Pricewaterhouse. Coopers

Federal Oversight of Human Subject Research § HHS Regulations – Revised 1981 § FDA Regulations – Revised 1981 § Federal Policy for the Protection of Human Subjects (Common Rule) – Adopted 1991 Pricewaterhouse. Coopers

DHHS Regulations: 45 CFR Part 46 § Subpart A – Basic Protections (“Common Rule”) – IRB Review – Informed Consent – Institutional “Federalwide Assurance” (FWA) § Subpart B - Protections for Pregnant Women, Fetuses, and Neonates § Subpart C - Protections for Prisoners § Subpart D - Protections for Children Pricewaterhouse. Coopers

Federal Policy (Common Rule) for the Protection of Human Subjects § 17 Federal Agencies Adopted HHS Subpart A § Some Agencies Required Additional Protections – VA requires compensation for research – related injuries § Some Agencies Never Adopted the Federal Policy – Department of Labor - Miners and Coal Dust – Appalachian Regional Commission – Telemedicine – Department of Transportation - Sleepy Truck Drivers § No Federal Regulation for Research Not Covered Under The Common Rule or FDA Regulations Pricewaterhouse. Coopers

DHHS Federalwide Assurance (FWA) § For Federally-Supported Research – Common Rule Protections of HHS Subpart A – IRB Review & Informed Consent § For HHS-Supported Research – Protections of HHS Subparts A, B, C, D § MPA-FWA Institutions – Voluntary application of all HHS Subparts to all research, regardless of funding source Pricewaterhouse. Coopers

Applying the Regulations to Research Involving Human Subjects Pw. C

Definition of Research: 45 CFR 46. 102(d) § Research means: – a systematic investigation – designed to develop or contribute to generalizable knowledge § Research includes: – research development, testing, evaluation, i. e. , pilot studies Pricewaterhouse. Coopers

Definition of Human Subject: 45 CFR 46. 102(f) § “Human Subject” means: – a living individual – about whom an investigator… conducting research obtains: 1. data through intervention or interaction with the individual, or 2. identifiable private information Pricewaterhouse. Coopers

Definition of Human Subject: 45 CFR 46. 102(f) § “Private Information” means: – Information about behavior in a context in which an individual can reasonably expect that no observation or recording is taking place – Information, provided for specific purposes, that the individual can reasonably expect will not be made public (e. g. , a medical record) Pricewaterhouse. Coopers

Definition of Minimal Risk: 45 CFR 46. 102(i) § “Minimal Risk” means: – The probability and magnitude of harm or discomfort; – Are not greater than those ordinarily encountered in daily life; or – During the performance of routine physical or psychological examinations or tests. Pricewaterhouse. Coopers

IRB Requirements and Procedures Pw. C

Institutional Review Board (IRB) § Mission => To protect the rights and welfare of individuals participating in research involving human subjects § Duties => To approve, disapprove, modify, suspend research as necessary to ensure protections for human subjects in research § Authority => To exercise final authority within the institution for ensuring adequate protections for subjects. Officials of the institution may not approve research if it has not been approved by an IRB. Pricewaterhouse. Coopers

Institutional Review Board (IRB): Composition § Minimum of 5 members § Diverse in gender and racial background § Sufficiently qualified in experience and expertise § One scientific member § Non-scientific member § One member not otherwise affiliated with the institution § Expertise in vulnerable populations for regular review of such research Pricewaterhouse. Coopers

IRB Approval Includes Findings That. . . § Review, Approve (§ 46. 111), Exercise Continuing Oversight: 1. Risks are minimized through sound research design 2. Risks are reasonable relative to anticipated benefits 3. Selection of subjects is equitable 4. Informed consent will be obtained 5. Informed consent will be documented 6. Privacy and Confidentiality provisions are adequate 7. Data safety monitoring is adequate 8. Appropriate safeguards are included for vulnerable subjects Pricewaterhouse. Coopers

IRB Oversight Includes. . . § Continued ethical evaluation of the research § Monitoring of the informed consent process § Analysis (as received) of new information, adverse events, and unanticipated problems involving risks to subjects and others § Formal Continuing Review at intervals appropriate to the degree of risk and no less than annually Pricewaterhouse. Coopers

Oversight Issues for IRBs § Special oversight mechanisms: – Data & Safety Monitoring Boards (DSMBs) – Consent Monitors – Random Audits of Research – Continuing Education Pricewaterhouse. Coopers

Types of IRB Review • Verification of Exemption • Expedited Review • Convened (Full) Review • Continuing Review NOTE: Initial and Continuing Review Require Vote of the Convened IRB, Meeting All Quorum Requirements, Unless Specific Conditions for Use of Expedited Review are Satisfied Pricewaterhouse. Coopers

Convened (Full Board) Review • Majority of Total Membership Must Be Present • Non-Scientist Member Must Be Present • Approval Requires a Majority of Those Members Present • Vote Must Be Documented • Same Requirements for Initial and Continuing Review Pricewaterhouse. Coopers

Expedited Review: Initial or Continuing Review § Conducted by Chair or IRB member designated by Chair § Only minimal risk research § Must fit into a category on November 1998 list § All other provisions and requirements apply § Can only approve research -- Cannot disapprove § Must be reported to full IRB Pricewaterhouse. Coopers

Expedited Review: Minor Changes to Approved Research § MINOR changes in previously approved research § During the established approval period § Conducted by Chair or IRB member designed by Chair § Must be reported to full IRB Pricewaterhouse. Coopers

Expedited Review 45 CFR 46. 110 Minimal Risk Research in the Following Categories: (1) Clinical studies of drugs and medical devices where an IND (drugs) or IDE (devices) is not required. (2) Collection of blood samples by finger stick, heel stick, ear stick, or venipuncture: (a) from healthy, non-pregnant adults weighing at least 100 lbs: 550 ml in 8 -wk period, limited to 2 collections per week; (b) from other adults and children, not more than 50 ml or 3 ml per kg in 8 -wk period, limited to 2 collections per week. Pricewaterhouse. Coopers

Expedited Review 45 CFR 46. 110 Minimal Risk Research in the Following Categories: (3) Prospective collection of biological specimens by noninvasive means. (4) Collection of data through noninvasive procedures (not involving general anesthesia or sedation) employed in clinical practice, excluding procedures involving x-rays or microwaves. Where medical devices are employed, they must be cleared/approved for marketing. (Studies intended to evaluate the safety and effectiveness of the medical device are no generally eligible for expedited review, including studies of cleared medical devices for new indications. ) Pricewaterhouse. Coopers

Expedited Review 45 CFR 46. 110 Minimal Risk Research in the Following Categories: (5) Research involving materials (data, documents, records, or specimens) that: -- have been collected -- will be collected for non-research purposes (6) Collection of data from voice, video, digital, or image recordings made for research purposes. (7) Research on individual or group behavior or characteristics -- cognition, motivation, identity, language, communication, cultural beliefs/practices, social behavior; survey, interview, oral history, focus group, program evaluation, human factor, quality assurance methodologies. Pricewaterhouse. Coopers

Expedited Review 45 CFR 46. 110 Minimal Risk Research in the Following Categories: (8) Continuing review of research previously approved by the convened IRB where (a) the research is permanently closed to new enrollments, all subjects have completed all research-related interventions, and research remains active only for long-term follow-up of subjects; or (b) no subjects have been enrolled and no additional risks have been identified; or (c) remaining research activities are limited to data analysis. Pricewaterhouse. Coopers

Expedited Review 45 CFR 46. 110 Minimal Risk Research in the Following Categories: (9) Continuing review of research. . . where. . . the IRB has determined and documented at a convened meeting that the research involves no greater than minimal risk, and no additional risks have been identified. Pricewaterhouse. Coopers

Expedited Review: Compliance Problems § Inappropriate use of expedited review – greater than minimal risk – no appropriate category – failure to document category and determination § Greater than minor changes to approved research § Inappropriate use for Continuing Review Pricewaterhouse. Coopers

Continuing Review 45 CFR 46. 109(e) § Required to occur within one year (no grace period) § IRB must review all relevant materials § Continuing review is opportunity to see what has happened once the research started. (NOTE: At initial review the research had not yet begun) § More than status reports should be reviewed -- review must be substantive and meaningful Pricewaterhouse. Coopers

IRB Meetings and Record Keeping • All members receive complete set of materials • Adequate time to review materials • Minutes of meetings must be comprehensive • Attendance and votes should be recorded • OHRP recent approval of teleconferencing if each participating member (i) has received all pertinent material prior to the meeting; and (ii) can actively and equally participate in the discussion of all protocols Pricewaterhouse. Coopers

Exempt Research Pw. C

Six Exemptions: 45 CFR 45. 101(b) (1) Research conducted in: – established or commonly accepted educational settings – involving normal educational practices e. g. instructional strategies effectiveness comparisons Pricewaterhouse. Coopers

Six Exemptions: 45 CFR 45. 101(b) (2) Research involving the use of – educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, or observation of public behavior UNLESS – information is recorded in an (directly or indirectly) identifiable manner (NOTE: Coded = identifiable) AND – disclosure would place subject at risk of criminal or civil liability or be damaging to financial standing, employability, or reputation Pricewaterhouse. Coopers

Six Exemptions: 45 CFR 45. 101(b) § Survey and Interview Research Involving Children IS NOT EXEMPT § Passive Observation of Public Behavior Involving Children IS Exempt § Participant Observation of Public Behavior Involving Children IS NOT Exempt Pricewaterhouse. Coopers

Six Exemptions: 45 CFR 45. 101(b) (3) Research involving the use of – educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, or observation of public behavior WHERE – subjects are elected or appointed public officials or candidates for public office or – Federal statutes require confidentiality without exception Pricewaterhouse. Coopers

Six Exemptions: 45 CFR 45. 101(b) (4) Research involving the collection or study of – existing data, documents, records, specimens IF – the sources are publicly available or – the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. NOTE: Even brief recording of identifiers or codes disqualifies the exemption Pricewaterhouse. Coopers

Six Exemptions: 45 CFR 45. 101(b) (5) Research and demonstration programs designed to study, evaluate, or examine (Federal) Public Benefit or Service Programs (6) Taste and food quality evaluation and consumer acceptance studies involving – wholesome foods without additives – additives, chemical, contaminants below safe levels determined by FDA, EPA, USDA Pricewaterhouse. Coopers

Verification of Exemptions § Exemptions must be verified by a trained and qualified institutional official § Exemptions may not be determined by the investigator Pricewaterhouse. Coopers

Informed Consent Pw. C

Informed Consent § Legally effective informed consent – Legally Authorized Representative (LAR) under State law § No coercion or undue influence (recruitment) § Obtained by Investigator/Staff trained and authorized by IRB § Language understandable to the subject § No exculpatory language § Eight required elements § Six additional elements Pricewaterhouse. Coopers

Eight Required Elements (1) Statement that study is research and information on purposes/duration/procedures/experimental procedures (2) Reasonably foreseeable risks or discomforts (3) Benefits which may be reasonably expected (4) Alternative procedures (5) How confidentiality will be maintained Pricewaterhouse. Coopers

Eight Required Elements (cont. ) (6) For more than minimal risk, information on compensation for injuries (7) Contact names -- at least one not associated with the research recommended (8) Statement that participation is voluntary and the subject can withdraw at any time without penalty or loss of benefits to which the subject is otherwise entitled Pricewaterhouse. Coopers

Six Additional Elements § Statement that there may be risks which are unforeseeable § Under what circumstances investigator could terminate subject’s participation § Additional costs to subject § Consequences of subjects withdrawal from research § Statement that will be told of new findings § Approximate number of subjects in study Pricewaterhouse. Coopers

Informed Consent Generally § There is no such thing as “passive consent” – consent is required unless formally waived – documentation is required unless formally waived § There is no such thing as a “secondary subject” – if an investigator obtains “identifiable private information” about a living individual, the individual is a human subject, regardless of the source § Deception Research – Requires a formal waiver of consent Pricewaterhouse. Coopers

Risks to Subjects § A risk or problem is unanticipated if it is not in the protocol or consent document. § Risks discussed in the protocol should usually be included in the consent document § Questions raised as a result of an unanticipated risk: § Does the informed consent form need to be amended? § Do previously enrolled subjects need to be re-consented? § Does a report need to be made to any government office? Pricewaterhouse. Coopers

Waiver of Informed Consent (Not Permitted Under FDA Regulations) § IRB must find and document that 4 criteria met: 1. Minimal risk research 2. Waiver or alteration will not adversely affect the rights and welfare of the subjects 3. Research could not practicably be carried out without the waiver or alteration 4. Subjects will be provided with additional pertinent information Pricewaterhouse. Coopers

Documentation of Informed Consent § Written consent document § In language understandable to the subject or the subject’s LAR § Signed by subject or subject’s LAR § Copy SHALL be given to subject § Opportunity to read before signing Pricewaterhouse. Coopers

Documentation of Informed Consent Short form written consent document requires : (1) oral presentation (2) witness to oral presentation (3) an IRB approved written summary – given to subject – signed by witness – signed by person obtaining consent (4) short form documenting oral presentation – signed by subject or LAR – signed by witness Pricewaterhouse. Coopers

Waiver of Documentation of Informed Consent (Not Permitted by FDA Regs) § The Signed Consent Document Provides the Only Link the Subject’s Identity and Principal Risk is Breach of Confidentiality § The Research Presents No Greater Than Minimal Risk of Harm to Subjects and Involves No Procedures Requiring Consent in a Non-Research Context § IRB May Require a Subject Information Sheet Pricewaterhouse. Coopers

Advanced IRB Issues Pw. C

FDA Regulations § Informed Consent - 21 CFR 50 § IRB Review - 21 CFR 56 § Investigational Drugs - 21 CFR 312 – Marketing Approval - 21 CFR 314 § Biologics - 21 CFR 600 – Biologics Licensing – 21 CFR 601 § Investigational Devices - 21 CFR 812 – Pre. Market Approval – 21 CFR 814 § Financial Disclosure – 21 CFR 54 § Electronic Records – 21 CFR 11 Pricewaterhouse. Coopers
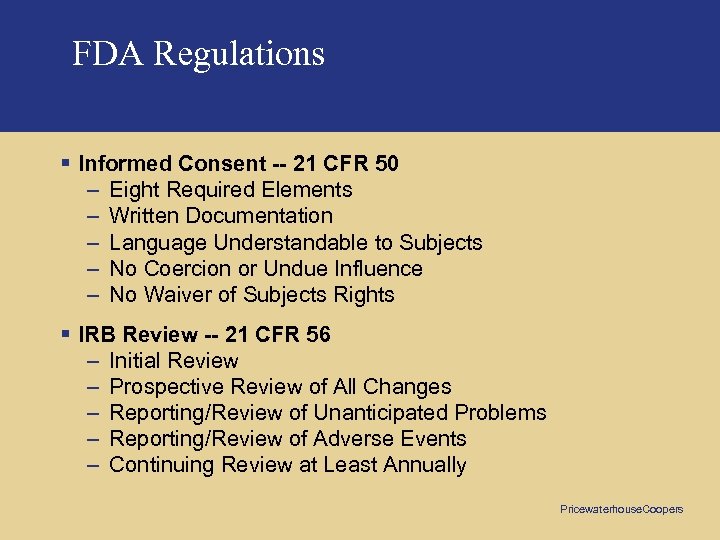
FDA Regulations § Informed Consent -- 21 CFR 50 – Eight Required Elements – Written Documentation – Language Understandable to Subjects – No Coercion or Undue Influence – No Waiver of Subjects Rights § IRB Review -- 21 CFR 56 – Initial Review – Prospective Review of All Changes – Reporting/Review of Unanticipated Problems – Reporting/Review of Adverse Events – Continuing Review at Least Annually Pricewaterhouse. Coopers

FDA Regulations § Drugs and Biologics – Investigational New Drug Application (IND) – 21 CFR Part 312 § Devices – Investigational Device Exemption (IDE) – 21 CFR Part 812 Pricewaterhouse. Coopers

FDA Regulations: Responsibilities of Sponsors § Maintaining the IND § Obtaining Qualified Investigators and Monitors § Providing Necessary Information / Training for Investigators § Monitoring the Investigation § Controlling the Investigational Agent § Reporting Significant Adverse Events to FDA and to Investigators § Maintaining and Retaining Accurate Records Pricewaterhouse. Coopers

FDA Regulations: Responsibilities of Investigators Specific Responsibilities: § Ensuring Conduct of the Research per the Investigator Agreement, Investigational Plan, and All Applicable Regulations § Protecting the Rights, Safety, and Welfare of the Research Subjects § Controlling access to and use of the test article (drug / biologic / device) § Monitoring and Reporting Adverse Events § Maintaining and Retaining Accurate Records Pricewaterhouse. Coopers

FDA Reporting Requirements: Investigational New Drug Application (IND) Adverse Event Reporting • Investigator must report promptly (immediately if alarming) to the Sponsor any adverse effect that may reasonably be regarded as caused by the drug (21 CFR 312. 64) • Sponsor must notify FDA of any adverse experience associated with the drug that is both serious and unexpected – Serious Adverse Drug Experience = death, life-threatening, hospitalization, persistent / significant disability / incapacity, congenital anomaly / birth defect (21 CFR 312. 32) – Unexpected Drug Experience = any adverse drug experience, the specificity or severity of which is not consistent with the current investigator brochure or IND application (21 CFR 312. 32) Pricewaterhouse. Coopers

FDA Reporting Requirements: Investigational Device Exemption (IDE) Adverse Event Reporting § Investigator must report any unanticipated adverse device effect to Sponsor and the IRB as soon as possible and within 10 working days [21 CFR 812. 150] § Sponsor must report any unanticipated adverse device effect to FDA, all reviewing IRBs, and investigators [21 CFR 812. 150] § Unanticipated Adverse Device Effect = any serious adverse effect on health or safety, or any life-threatening problem or death, caused by or associated with a device if not previously identified in nature, severity, or degree of incidence in the investigational plan or application [21 CFR 812. 3] Pricewaterhouse. Coopers

Requirements for Reporting to the IRB § Required by HHS Human Subject Regs. [45 CFR 46. 103(b)(5)] and FDA IRB Regs [21 CFR 56. 108(b), 312. 66] – Unanticipated problems involving risks to subjects or others – Serious or continuing noncompliance with Regs. or IRB § Adverse Events (required by FDA for devices only) – Local IRB Policy Determines Requirements 1) Any serious adverse events experienced by subjects 2) Any adverse events reported to the study sponsor Pricewaterhouse. Coopers

FDA Regulation Exceptions and Exemptions: Emergency Use of a Test Article § Without Informed Consent – 21 CFR 50. 23(a) – Life Threatening Situation Necessitating the Use – Inability to Communicate with Subject for Legal Consent – Insufficient Time to Obtain Consent from Legally Authorized Representative (LAR) – No Alternative Therapy Available – Certification in Writing from Investigator and an other Nonparticipating Physician of the Above – Report to IRB Within 5 Working Days § IRB Review – 21 CFR 56. 104 (c) – Life Threatening Situation Necessitating the Use – No Alternative Available – Insufficient time for IRB review – Report to IRB Within 5 Working Days – Subsequent Use Requires IRB Review Pricewaterhouse. Coopers

FDA Regulation Brain Teasers: SR Devices vs. NSR Devices IRB Must Make a Specific Determination • Significant Risk Device = Investigational device that presents a potential for serious risk to the health, safety, or welfare of subjects, including implants • Non-Significant Risk Device = Investigational devices that does NOT present the potential for serious risk to the health, safety, or welfare of subjects – Non-Significant Risk is NOT the same as Minimal Risk • Once IRB-approves the research as not involving a Significant Risk Device, the research is considered to have an approved IDE, unless the FDA has notified the sponsor otherwise. Pricewaterhouse. Coopers

FDA Regulation Brain Teasers: “Off-Label Use” § FDA-approved products (i. e. , marketed products) may be used by physicians outside of labeled indications FOR THE PRACTICE OF MEDICINE § Such use in RESEARCH (i. e. , as part of a systematic investigation designed to develop or contribute to generalizable knowledge) requires IRB REVIEW § Such use intended to support a CHANGE in labeling requires IRB REVIEW and an IND / IDE Pricewaterhouse. Coopers

FDA Regulations Brain Teasers: “Standard of Care” Comparisons § Systematic comparison of FDA-approved products (i. e. , marketed products) used for approved indications constitutes RESEARCH and requires IRB REVIEW § Systematic comparison of competing STANDARDS OF CARE constitutes RESEARCH and requires IRB REVIEW Pricewaterhouse. Coopers

FDA Regulation Brain Teasers: “Compassionate Use” § Does not Appear in IND Regulations or Guidance § Often Confused With: – Emergency Use of a Test Article (without IRB Review and/or without Informed Consent) – Treatment Use of Investigational Drugs (with or without a Treatment IND) Requires IRB Review and Informed Consent – Use of Orphan Drugs (disease affects < 200, 000 Americans) Requires IRB Review & Informed Consent – Parallel Track Use Requires IRB Review & Informed Consent Pricewaterhouse. Coopers

FDA Regulation Brain Teasers: “Compassionate Use” § Compassionate Use of an Unapproved Device may be approved by FDA when it is the only option for a patient with a serious condition § Requires as many of the following as possible: – Informed Consent – Institutional Approval – Concurrence of IRB Chair (but NOT IRB APPROVAL) – Independent Assessment of Uninvolved Physician – Authorization of the Sponsor Pricewaterhouse. Coopers

FDA Regulation Brain Teasers: Humanitarian Device Exemption (HDE) § Humanitarian Use Device (HUD) – Device tested but not profitable for marketing § Requires: – IRB Review (Limited) and Approval – No Research Informed Consent Pricewaterhouse. Coopers

FDA Regulation Brain Teasers: Planned “Emergency” Research § Ordinarily Requires IRB Review and Informed Consent of Subject or Subject’s Legally Authorized Representative (as determined by State Law for Research Contexts) § Exception from Informed Consent Requirement Involves Many Specific IRB Determinations and Approval by FDA or OHRP Pricewaterhouse. Coopers

Vulnerable Research Subjects § Pregnant Women, Human Fetuses, and Neonates § Prisoners § Children Pricewaterhouse. Coopers

HHS Subpart B: Research Involving Pregnant Women, Human Fetuses, and Neonates Subpart B -- Revised December 2001 § Research involving pregnant women § Research involving fetuses § Research involving neonates of uncertain viability, nonviable neonates, or viable neonates Pricewaterhouse. Coopers

HHS Subpart B: 45 CFR 46. 204 Research Involving Pregnant Women or Fetuses Pregnant women or fetuses may be involved in research if all of the following conditions are met: a) Where appropriate, preclinical studies on pregnant animals and clinical studies on nonpregnant women have provided data to assess potential risks b) Risk to the fetus is caused solely by procedures holding out the prospect of direct benefit for the woman or the fetus or the risk to the fetus is no greater than minimal and the purpose is to develop important biomedical knowledge that cannot be obtained by other means Pricewaterhouse. Coopers

HHS Subpart B: 45 CFR 46. 204 Research Involving Pregnant Women or Fetuses c) The risk is the least possible for achieving the objectives of the research d) If the research holds out the prospect of direct benefit to the pregnant woman or to both the pregnant woman and fetus or the research holds out no prospect of direct benefit but present no more than minimal risk to the fetus as in (b) above, the informed consent of the pregnant woman is obtained Pricewaterhouse. Coopers

HHS Subpart B: 45 CFR 46. 204 Research Involving Pregnant Women or Fetuses e) If the research hold out the prospect of direct benefit solely for the fetus, the informed consent of the pregnant woman and the father is obtained, except the father’s consent need not be obtained if he us unable to consent because of unavailability, incompetence, or temporary incapacity, or if the pregnancy resulted from rape or incest f) Consenting individuals are fully informed regarding the reasonably foreseeable impact of the research on the fetus or neonate Pricewaterhouse. Coopers

HHS Subpart B: 45 CFR 46. 204 Research Involving Pregnant Women or Fetuses g) For children who are pregnant, assent of the pregnant child and permission of the child’s parents are obtain in accordance with Subpart D h) No inducements, monetary or otherwise, are offered to terminate a pregnancy i) Individual engaged in the research have no part in any decisions as to the timing, method, or procedures to terminate a pregnancy j) Individual engaged in the research have no part in determining viability of a neonate Pricewaterhouse. Coopers
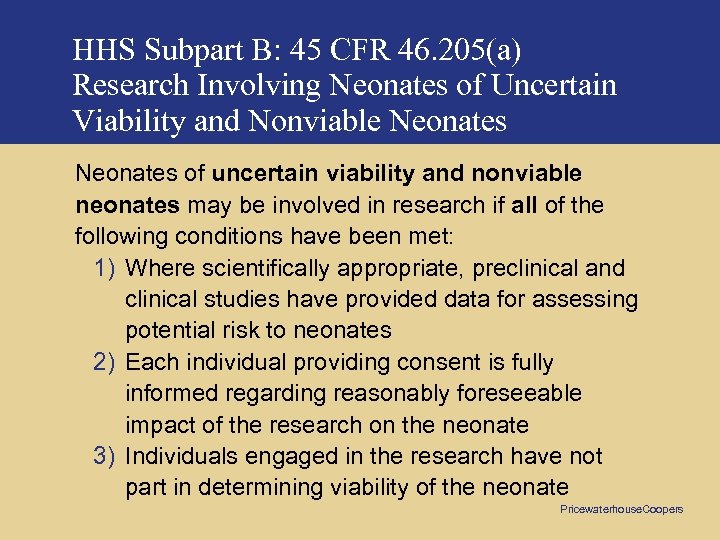
HHS Subpart B: 45 CFR 46. 205(a) Research Involving Neonates of Uncertain Viability and Nonviable Neonates of uncertain viability and nonviable neonates may be involved in research if all of the following conditions have been met: 1) Where scientifically appropriate, preclinical and clinical studies have provided data for assessing potential risk to neonates 2) Each individual providing consent is fully informed regarding reasonably foreseeable impact of the research on the neonate 3) Individuals engaged in the research have not part in determining viability of the neonate Pricewaterhouse. Coopers

HHS Subpart B: 45 CFR 46. 205(b) Research Involving Neonates of Uncertain Viability Until viability is ascertained, neonates of uncertain viability man not be involved in research unless the additional conditions are met: 1. The IRB determines that (i) the research holds out the prospect of enhancing the survival of the neonate to viability and any risk is the least possible for achieving that objective or (ii) the purpose of the research is the development of important biomedical knowledge which cannot be obtained by other means and there will be no added risk to the neonate from the research Pricewaterhouse. Coopers

HHS Subpart B: 45 CFR 46. 205(b) Research Involving Neonates of Uncertain Viability 2) The legally effective informed consent of either parent of the neonate ð If neither parent is able to consent because of unavailability, incompetence, or temporary incapacity, the legally effective informed consent of either parent’s legally authorized representative is obtained ð Consent of the father or the father’s legally authorized need not be obtained if the pregnancy resulted from rape or incest Pricewaterhouse. Coopers

HHS Subpart B: 45 CFR 46. 205(c) Research Involving Nonviable Neonates After delivery, nonviable neonates may not be involved in research unless all of the following conditions are met: 1) Vital function of the neonate will not be artificially maintained 2) The research will not terminate heartbeat or respiration of the neonate 3) There will be no added risk to the neonate from the research 4) The purpose of the research is to develop important biomedical knowledge that cannot be obtained by other means Pricewaterhouse. Coopers
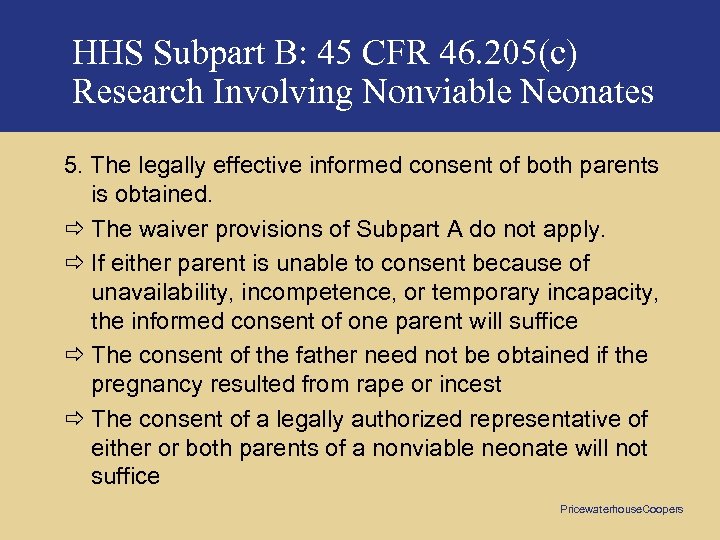
HHS Subpart B: 45 CFR 46. 205(c) Research Involving Nonviable Neonates 5. The legally effective informed consent of both parents is obtained. ð The waiver provisions of Subpart A do not apply. ð If either parent is unable to consent because of unavailability, incompetence, or temporary incapacity, the informed consent of one parent will suffice ð The consent of the father need not be obtained if the pregnancy resulted from rape or incest ð The consent of a legally authorized representative of either or both parents of a nonviable neonate will not suffice Pricewaterhouse. Coopers

HHS Subpart B: 45 CFR 46. 205(d) Research Involving Viable Neonates After delivery, a neonate that has been determined to be viable is a child and may only be included in research to the extent permitted by Subpart D. Pricewaterhouse. Coopers

HHS Subpart C: 45 CFR 46. 303(c) Research Involving Prisoners Definition of Prisoner: § Any individual involuntarily confined or detained in a penal institution under a criminal or civil statute § Individuals detained in other facilities as an alternative to criminal prosecution or incarceration in a penal institution § Individuals detained pending arraignment, trial, or sentencing Pricewaterhouse. Coopers
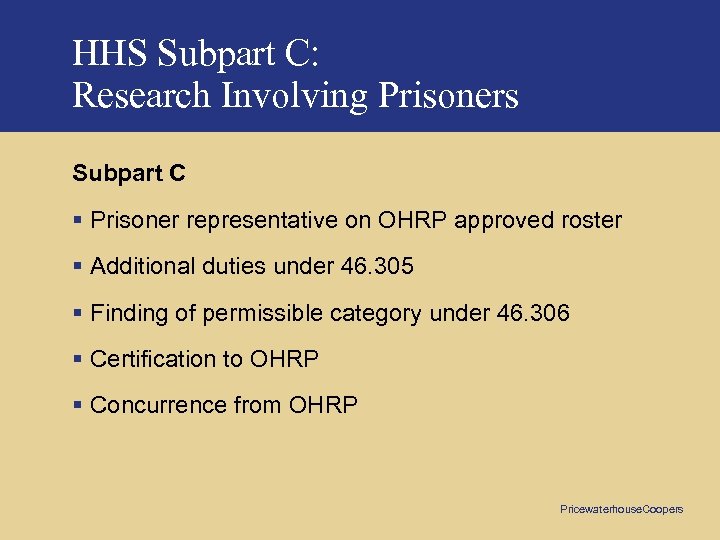
HHS Subpart C: Research Involving Prisoners Subpart C § Prisoner representative on OHRP approved roster § Additional duties under 46. 305 § Finding of permissible category under 46. 306 § Certification to OHRP § Concurrence from OHRP Pricewaterhouse. Coopers

Additional IRB Duties HHS Subpart C: 45 CFR 46. 305 § Research is permissible within a 46. 306 category § Advantages of participating are not coercive given the limited choice prison environment § Risks commensurate with those accepted by nonprisoner volunteers § Selection procedures are fair § Language is understandable to the subject population § Participation has no affect on parole, and prisoners are so informed § Adequate provisions will be made if there may be a need for follow-up care, and given varying lengths of sentences, and prisoners are so informed § If HHS-supported, institution (IRB) certifies to OHRP that it has carried out its duties under these sections Pricewaterhouse. Coopers

Categories of Permissible Research HHS Subpart C: 45 CFR 46. 306(b) § (i) The study of the possible causes, effects, and processes of incarceration, and of criminal behavior, provided that the study presents no more than minimal risk and no more than inconvenience to the subjects. § (ii) The study of prisons as institutional structures or of prisoners as incarcerated persons, provided that the study presents no more than minimal risk and no more than inconvenience to the subjects. Pricewaterhouse. Coopers

Categories of Permissible Research HHS Subpart C: 45 CFR 46. 306(b) § (iii) Research on conditions particularly affecting prisoners as a class - ONLY AFTER DHHS CONSULTATION WITH EXPERTS AND PUBLICATION OF A NOTICE TO APPROVE IN THE FEDERAL REGISTER. § (iv) Research on practices, both innovative and accepted, which have the intent and reasonable probability of involving the health or wellbeing of the subject. IF CONTROL GROUP WITH NO BENEFIT, CONSULTATION WITH EXPERTS AND FEDERAL REGISTER IS NOTICE REQUIRED. Pricewaterhouse. Coopers

Lawsuit Involving Prisoners § DOJ funded research in Pennsylvania prison: – mandatory drug testing (urine vs. hair) – no consent – solitary confinement for refusal to be tested – facts of case not contested § Acres of Skin – Dow, U Pennsylvania, City of Philadelphia – Prisoners told experiments were harmless Pricewaterhouse. Coopers
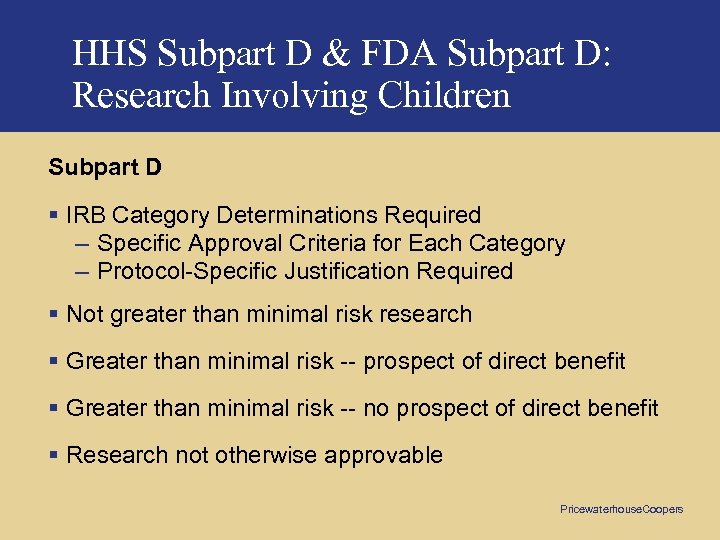
HHS Subpart D & FDA Subpart D: Research Involving Children Subpart D § IRB Category Determinations Required – Specific Approval Criteria for Each Category – Protocol-Specific Justification Required § Not greater than minimal risk research § Greater than minimal risk -- prospect of direct benefit § Greater than minimal risk -- no prospect of direct benefit § Research not otherwise approvable Pricewaterhouse. Coopers

Subpart D: 45 CFR 46. 404 & 21 CFR 50. 51: Research involving no greater than minimal risk Children may be involved in research where the IRB finds that: § The research presents no greater than minimal risk to the child § Adequate provision are made for obtaining § The assent of the child § The permission of the child’s parents or guardians Pricewaterhouse. Coopers

Subpart D: 45 CFR 46. 405 & 21 CFR 50. 52: Greater than minimal risk but presenting the prospect of direct benefit to the individual subjects. Children may be involved in research where the IRB finds that more than minimal risk to children is presented by (i) an intervention or procedure that holds out the prospect of direct benefit for the individual subject, or (ii) a monitoring procedure that is likely to contribute to the subject’s wellbeing if: a) The risk is justified by anticipated benefit to subjects; b) The relation of anticipated benefit to risk is at least as favorable as available alternatives; c) Assent of child and permission of parents are sought. Pricewaterhouse. Coopers
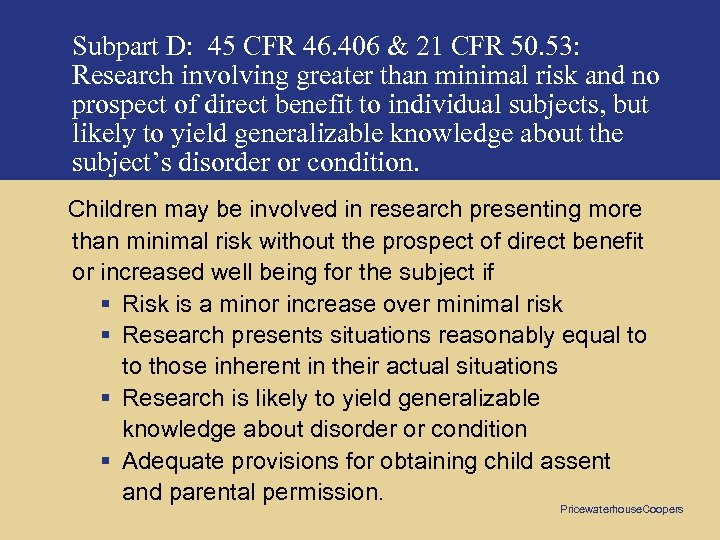
Subpart D: 45 CFR 46. 406 & 21 CFR 50. 53: Research involving greater than minimal risk and no prospect of direct benefit to individual subjects, but likely to yield generalizable knowledge about the subject’s disorder or condition. Children may be involved in research presenting more than minimal risk without the prospect of direct benefit or increased well being for the subject if § Risk is a minor increase over minimal risk § Research presents situations reasonably equal to to those inherent in their actual situations § Research is likely to yield generalizable knowledge about disorder or condition § Adequate provisions for obtaining child assent and parental permission. Pricewaterhouse. Coopers

Subpart D: 45 CFR 46. 407 & 21 CFR 50. 54: Research not otherwise approvable but presenting an opportunity to understand, prevent, or alleviate a serious problem affecting the health or welfare of children § IRB finds the research presents opportunity as above § HHS Secretary / FDA Commissioner, after consultation with panel of experts and following public review and comment, determines § The research presents reasonable opportunity as above § The research will be conducted in accordance with sound ethical principles § Adequate provisions are made for obtaining child assent and parental permission Pricewaterhouse. Coopers

Research involving Children Subpart D: Parental Permission Required 45 CFR 46. 408 & 21 CFR 50. 55 • Both Parents’ Permission Required If Greater than Minimal Risk • Standard HHS Waiver Not Contained in FDA Regulations • HHS Waiver in Interest of Child Not Contained in FDA Regulations Pricewaterhouse. Coopers

Research involving Children Subpart D: Assent of Child Required 45 CFR 46. 408 & 21 CFR 50. 55 • Developmentally Appropriate as Determined by the IRB • Documentation as Determined by the IRB • Unless the Research Holds the Prospect of • Direct Benefit Available Only in the Research or Unless Waived by the IRB per criteria at 45 CFR 46. 116(d) Pricewaterhouse. Coopers

Research involving Children Subpart D: Ward of the State 45 CFR 46. 409 & 21 CFR 56 1. Where greater than minimal risk and providing no direct benefit to the child, research with wards is permitted only if: 1. Related to status as wards or – Conducted in settings where majority of children are not wards • Child advocate required who is not otherwise associated with the research Pricewaterhouse. Coopers

Social and Behavioral Research: Recurring Controversy about Regulation • Applicability to Social and Behavioral Research • Compatibility with Social and Behavioral Research • Applicability by Discipline? • Review of Exempt Research • Student Research Pricewaterhouse. Coopers

Definition of Research: 45 CFR 46. 102(d) • Research means: – a systematic investigation – designed to develop or contribute to generalizable knowledge • Research includes: – research development, testing, evaluation, – i. e. , pilot studies Pricewaterhouse. Coopers

Definition of Human Subject: 45 CFR 46. 102(f) § “Human Subject” means: – a living individual – about whom an investigator… conducting research obtains: 1. data through intervention or interaction with the individual, or 2. identifiable private information Pricewaterhouse. Coopers

Definition of Human Subject: 45 CFR 46. 102(f) § “Private Information” means: – Information about behavior in a context in which an individual can reasonably expect that no observation or recording is taking place – Information, provided for specific purposes, that the individual can reasonably expect will not be made public (e. g. , a medical record) Pricewaterhouse. Coopers

Social and Behavioral Issues – Institutional Evaluations / Quality Assurance • • • Is the activity “Research” ? Research means: – A Systematic Investigation – Designed to develop or contribute to generalizable knowledge What does “Generalizable” mean : – – Beyond the immediate situation Beyond the institution Pricewaterhouse. Coopers

Social and Behavioral Issues – What if the Purpose / Intent Changes ? • When did the purpose change ? – After the investigation was completed – IRB review of existing data – Consent / Waiver of Consent – During the investigation – IRB review for collected data – IRB review for prospective data – Consent / Waiver of Consent Pricewaterhouse. Coopers

Social and Behavioral Issues – “Secondary subjects” • • • “Target” subjects vs “Secondary” subjects Who is a subject? A subject is a living individual about whom an investigator conducting research obtains: – Identifiable Private Information Pricewaterhouse. Coopers

Definition of Human Subject: 45 CFR 46. 102(f) § “Private Information” means: – Information about behavior in a context in which an individual can reasonably expect that no observation or recording is taking place – Information, provided for specific purposes, that the individual can reasonably expect will not be made public (e. g. , a medical record) Pricewaterhouse. Coopers

Social and Behavioral Issues – Existing Data Sets • Data sets containing only non-coded, non • • identifiable information do not involve human subjects Data sets “anonymized” before transfer to the investigator do not involve human subjects “Anonymized” means – No codes or links of any kind, available to anyone, anywhere – But note possible OHRP exception Pricewaterhouse. Coopers

Social and Behavioral Issues – Existing Data Sets • “Existing” means: – All data has been collected (i. e. , on – – the shelf) prior to the research For a purpose other than the proposed research Includes data (or specimens) collected in research and nonresearch activities. Pricewaterhouse. Coopers

Social and Behavioral Issues – Existing Data Sets • “Publicly available” data sets are exempt • “Publicly available” means: – Generally available to anyone – Commercially available – ? • Access to data sets with identifiable private information is exempt if: – Investigator does not record identifiers Pricewaterhouse. Coopers

Social and Behavioral Issues – Existing Data Sets 1. Other use of data sets containing identifiable private information requires IRB review 2. IRB may determine that: 1. Additional informed consent is needed 2. Original informed consent covers the new research 3. Informed consent requirements can be waived under 45 CFR 46. 116(d) Pricewaterhouse. Coopers
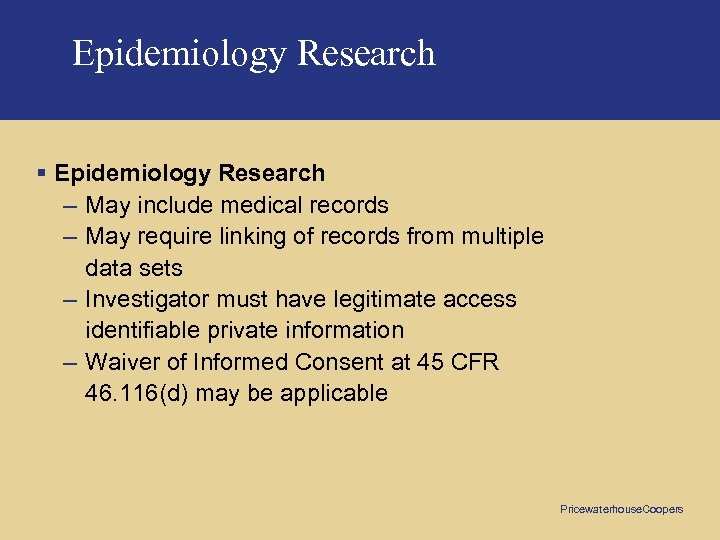
Epidemiology Research § Epidemiology Research – May include medical records – May require linking of records from multiple data sets – Investigator must have legitimate access identifiable private information – Waiver of Informed Consent at 45 CFR 46. 116(d) may be applicable Pricewaterhouse. Coopers

Social and Behavioral Issues – Research Involving Deception • Requires waiver of informed consent requirement under 45 CFR 46. 116(d) • IRB must find and document that 4 criteria are met: – – No greater than minimal risk to subjects Waiver or alteration will not adversely affect the rights and welfare of the subjects Research could not practicably be carried out without the waiver or alteration Subjects will be provided with additional pertinent information Pricewaterhouse. Coopers

Social and Behavioral Research: My IRB is Unreasonable ! ! § Academic freedom gives me the right to conduct my research § This research is harmless and shouldn’t need IRB review § Requiring informed consent will lower participation rates § Having to sign a consent form will scare subjects away § Individual consent is meaningless among my subjects § College students are adults § “Subject Pool” requirements are an educational prerogative § “Passive Consent” is traditional in my field § Mom, dad, and sibs aren’t subjects my research § My use of these data sets shouldn’t need IRB review Pricewaterhouse. Coopers

Dealing with the “Unreasonable” IRB § Regulations Require Professional Competence (§ 46. 107): – IRBs must have the Scientific and Professional Competence to understand the research they review (including Social and Behavioral research) – IRBs must understand regulatory options so as to apply them appropriately § Possible Solutions – Increase Academic Diversity of Existing IRB – Create Social and Behavioral Sciences IRB – Educate IRB Members (and investigators) Pricewaterhouse. Coopers

OHRP Compliance Determinations Analysis of Common Concerns Pw. C

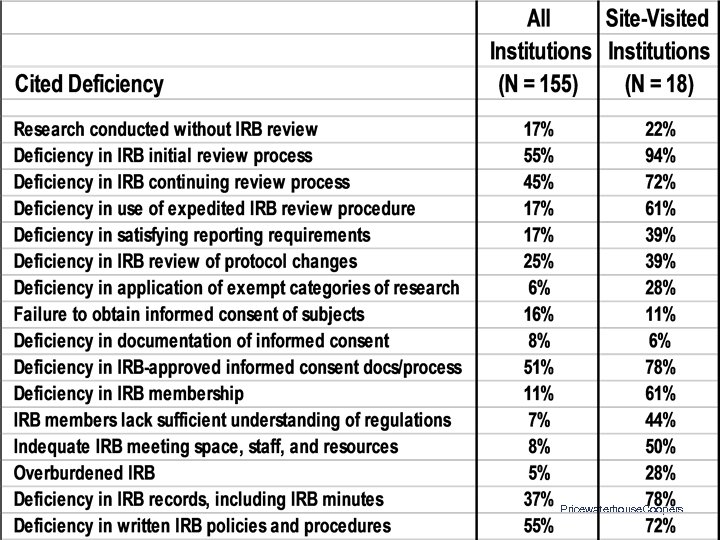
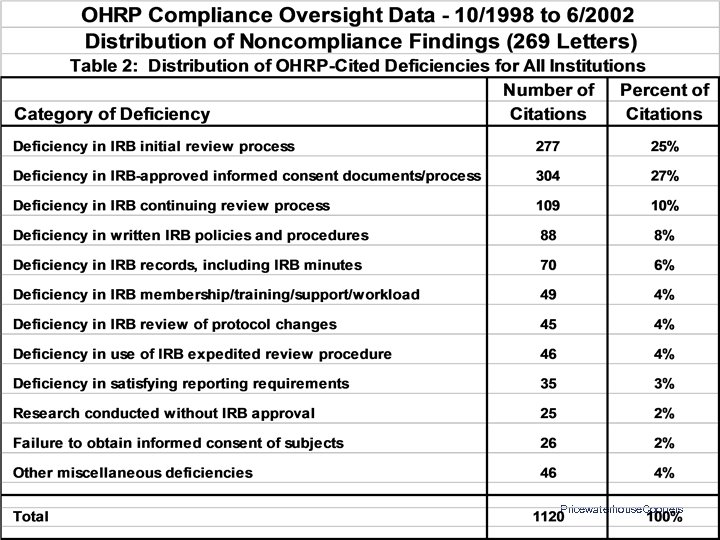
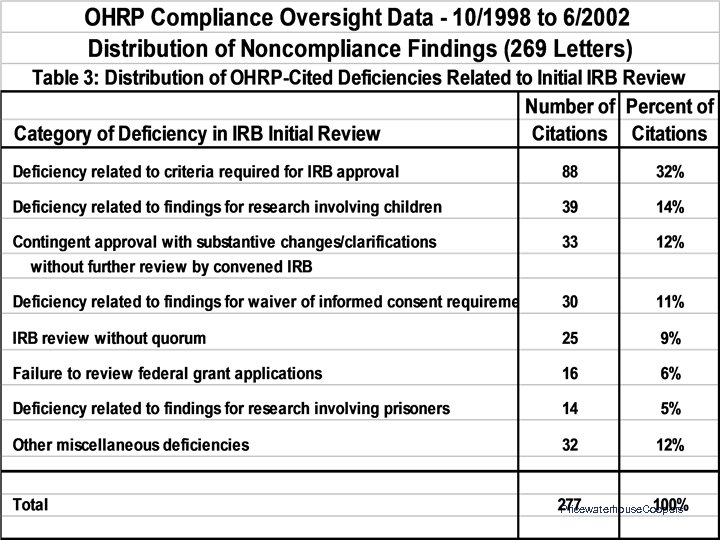
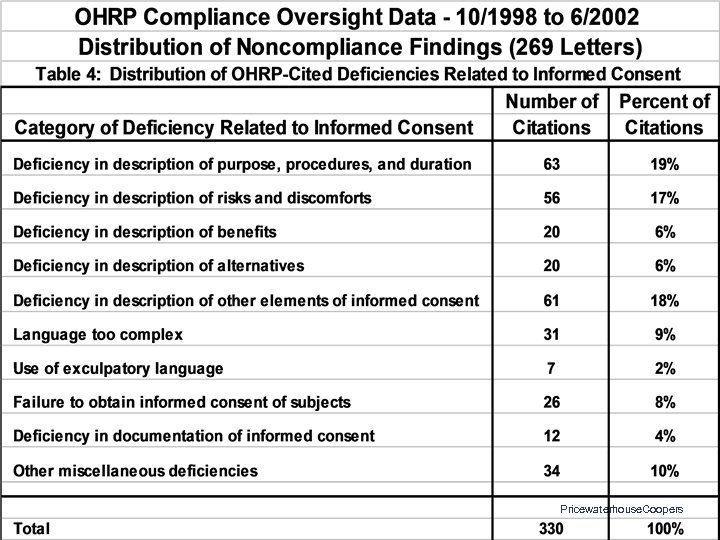
OHRP Compliance Determinations: Letters Issued 10/01/1998 to 06/30/2002 • • 269 Letters to 155 Institutions 18 Institutions Site-Visited 1, 120 Citations of Noncompliance or Deficiency 142 Institutions (92%) Had at Least One Citation – Median = 4 – Range: 0 - 53 Pricewaterhouse. Coopers

OHRP Compliance Findings: Common Areas of Noncompliance • • • Initial and continuing IRB review • Documentation of IRB procedures, activities, and findings Expedited IRB review procedures Reporting of unanticipated problems IRB review of protocol changes Informed consent IRB membership, expertise, staff, support, and workload Pricewaterhouse. Coopers
Pricewaterhouse. Coopers
Pricewaterhouse. Coopers
Pricewaterhouse. Coopers
Pricewaterhouse. Coopers

Managing Complaints and Site Visits Managing internal complaints – Types of complaints – Managing investigation of complaints – Reporting to regulatory authorities § Managing a government site visit – Corrective action plan – IRB operations assessment Pricewaterhouse. Coopers

Related Issues Pw. C

Health Insurance Portability and Accountability Act (HIPAA) • HIPAA Passed by Congress and Signed by President in 1996 • Required HHS to Issue a Rule or Congress would legislate. • History of Privacy Rule: – – December 28, 2000: Final Rule – August 14, 2002 Regulations Amended March, 2001: Rule Opened for Additional comment • Compliance Deadline: April 14, 2003 Pricewaterhouse. Coopers

HIPAA Privacy Rule In General -HIPAA covers the Use and Disclosure of “Protected Health Information” by “Covered Entities” • • Protected Health Information (PHI) is: – Individually identifiable health information A “Covered Entity” is: – Health Care Provider, Plan, or Clearinghouse Consent is not required to use or disclose PHI for treatment, payment, or health care operations Other use or disclosure of PHI, including Research, generally requires “Authorization” Pricewaterhouse. Coopers

HIPAA Privacy Rule In General -Use or Disclosure of “Protected Health Information” for research purposes requires either: • • • A written Authorization from the subject or Verification that the research involves: => De-Identified Information => Limited Data Sets => Reviews Preparatory to Research => Decedents’ Information or A Waiver approved by the Privacy Board / IRB Pricewaterhouse. Coopers

HIPAA Privacy Rule In General - • The Privacy Rule permits Use and Disclosure of Protected Health Information as required under applicable statute or regulation • The Privacy Rule provides individuals with: => The right to Access Protected Health Information about themselves => The right to obtain an Accounting of Disclosures of their Protected Health Information • Does not supercede more stringent State requirements Pricewaterhouse. Coopers

Authorization Generally, uses and disclosures of protected health information in research must be conducted pursuant to a valid Authorization…. • 6 Core elements • 3 Required statements • 2 Additional requirements Pricewaterhouse. Coopers

Authorization = Most Advantageous Route to Research • • No Representations (Assurances) Required No Privacy Board Review Required No Accounting of Disclosures Required No “Minimum Necessary” Limitations Pricewaterhouse. Coopers

Conflict of Interest in Research: Current Requirements and Recent Guidance Conflict of Interest: • Any situation in which financial, professional, or personal obligations • May compromise or appear to compromise • Professional judgment in designing, conducting, analyzing, reporting, or supporting research. Pricewaterhouse. Coopers

Types of Conflict of Interest • Individual – Clinical investigators – Study coordinators – Research technicians – Research officials – IRB members • Institutional – Financial holdings of the – institution Decisions regarding research funding or allocation of resources for research • Financial – Consulting fees – Stock ownership – Honoraria – Salary – Intellectual property rights – Enrollment bonuses – Spouse / dependent finances • Professional – Pressure to publish – Professional rivalries – Career advancement Pricewaterhouse. Coopers

What Prompted the Recent Focus on Financial COIs in Research? • Researchers and Institution held equity interest in research technology significantly above FDA requirements • Two prominent research institutions involved – Gene-transfer research – Cancer research center • Results – NIH Conference to examine the issue of financial COIs – Development of recent guidance, including draft interim recommendations from DHHS – Authorship and sponsorship of research (statement by the International Council of Medical Journal Editors) Pricewaterhouse. Coopers
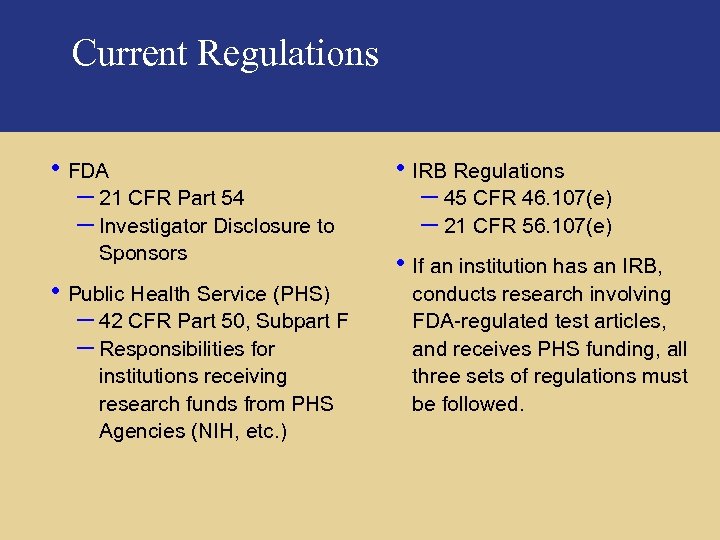
Current Regulations • FDA – 21 CFR Part 54 – Investigator Disclosure to Sponsors • Public Health Service (PHS) – 42 CFR Part 50, Subpart F – Responsibilities for institutions receiving research funds from PHS Agencies (NIH, etc. ) • IRB Regulations – 45 CFR 46. 107(e) – 21 CFR 56. 107(e) • If an institution has an IRB, conducts research involving FDA-regulated test articles, and receives PHS funding, all three sets of regulations must be followed. Pricewaterhouse. Coopers

FDA Regulations • Require: – Disclosure of potentially conflicting financial interests of investigator (including spouse and dependent children) • When to disclose? – Compensation depends on the outcome of the clinical study – Investigator has significant equity interest in the sponsor (in excess – – of $50, 000 during study and for 1 year after completion) When investigator has a proprietary interest in the test product (including patent, trademark, copyright, or licensing agreement) When investigator has received significant payments of other sorts (defined as a value of more than $25, 000, exclusive of the costs of conducting the study, during the study and for 1 year after completion) Pricewaterhouse. Coopers

PHS Regulations: Investigator Requirements • Disclosure of “significant financial interests” • Anything of monetary value • Salary and other payments for services • Equity interests • Intellectual property interests Pricewaterhouse. Coopers

PHS Regulations: Investigator Need Not Disclose • • Salary, royalties, or other remuneration from the institution Income from seminars, lectures, or teaching engagements sponsored by public or non-profit entities Equity interests (including spouse and dependent children) which do not exceed $10, 000 and does not represent more than 5% ownership in any single entity Salary, royalties, and other payments, which when aggregated for the investigator, spouse, and dependent children, are not expected to exceed $10, 000 over the next 12 months Pricewaterhouse. Coopers

PHS Regulations: Institutional Requirements • Develop policies and procedures per PHS regulations • Inform investigators of their responsibilities • Designate an institutional official(s) to solicit and review financial disclosure statements • Issue guidelines to identify, manage, reduce, or eliminate conflicts • Allocate space for maintaining records for 3 years • Establish enforcement mechanisms/sanction • Certify in each PHS funding application that a written and enforced administrative process for financial COIs exists • Assure PHS that any financial COIs will be managed, reduced, or eliminated before funds spent (or at least on an interim basis, for 60 Pricewaterhouse. Coopers days after initial report)

IRB Requirements: 45 CFR 46. 107(e) & 21 CFR 56. 107(e): • “No IRB member may participate in initial or • • continuing review of research in which they have a conflicting interest except to provide information requested by the IRB” Includes professional, personal, and financial conflicts. IRB members must recuse themselves Pricewaterhouse. Coopers

IRB “Best Practice” Standards • IRB policies defines what might constitute a • • conflicting interest or has procedures for determining conflicting interest Conflicted IRB member absents self from meeting during IRB deliberation and vote IRB minutes document recusal and absence from deliberation and vote Pricewaterhouse. Coopers
Reporting of Conflicts of Interest to Regulatory Bodies • FDA (=Investigator) – Investigator conducting research on FDA-regulated test article discloses to research sponsor – Disclosure applies to investigator, spouse, and dependent children – If potential conflict, report steps taken to minimize bias using Form FDA 3455 – No financial interests, use Form FDA 3454 • PHS (=Institution) – Investigator supported by funds from any PHS Agency discloses significant financial interest to the institution – Disclosure applies to investigator, spouse, and dependent children – Institution must certify to PHS Agency that significant financial interests have been eliminated, reduced, or managed Pricewaterhouse. Coopers

Recent Guidance • Department of Health and Human Services (DHHS) – Draft Interim Guidance (January 10, 2001) • American Association of Medical Colleges (AAMC) – Task Force on Financial Conflicts of Interest in Clinical Research (December 2001) • General Accounting Office – Report GAO-02 -89, November 2001) • Association of American Universities – Task Force on Research Accountability: Individual and Institutional Financial Conflict of Interest (October 2001) • American Medical Association – Council on Ethical and Judicial Affairs Report 3 -I-00, Managing Conflicts of Interest in the Conduct of Clinical Trials (December 2000) Pricewaterhouse. Coopers

Management Decisions for Conflicts of Interest Policy Development • • Who’s covered by the Policy? • Examples of management plans: – Institutions may wish to cover other – Public disclosure of financial individuals involved in research, interests research decisions, research – Divestiture of the interest oversight, and the institution’s – Monitoring of the research by financial holdings. independent reviewers – Modification of research plan Disclosure thresholds – Disclosure in informed consent – FDA and PHS policies have documents, manuscripts, oral different disclosure thresholds. presentations based on the – For consistency and simplicity, research institutions may chose to adopt a single disclosure threshold. Pricewaterhouse. Coopers

Conflict of Interest Recommendations Distilled from Recent Guidance • • • Conflict of interest committee – More representation from lay public Administrative positioning of the IRB high within the organization. – IRB should not be the sole locus of responsibility for conflicts of interest Design educational programs for all research staff, IRB, institutional officials with research and finance decision-making responsibilities • Make policies, procedures, and documents available on-line • Expand scope to include all research, regardless of funding for the sake of fairness and consistency • Establish “firewall” between offices responsible for financial and research decisions • “Rebuttable presumption” and “compelling circumstances” as conflict standards Pricewaterhouse. Coopers

Billing Compliance Institutional Responsibility Patient Care vs. Research National Coverage Decision Pricewaterhouse. Coopers

Pwc Pw. C
2710f03e7b676f84a7dbe92c8143a1bd.ppt