41ae127b5c499bb68d87fa897119aa70.ppt
- Количество слайдов: 27

Properties of Matter Understanding our world and being able to use that knowledge to help us means describing things and understanding how they behave. Ø What are the ways and words we can use to describe “stuff? ” Ø How does “stuff” behave? What does it do when we make changes to it? Ø Is the “stuff” one thing (pure) or more than one thing (mixture)? Ø Once we make changes to stuff what are its new characteristics? Can we change new stuff back into the old stuff? ” Properties are the characteristics and behaviors we use to describe matter!

Properties of Matter - physical state 1 Matter: anything that has mass (weighs something) & takes up space (has volume). Matter has 3 forms or physical states
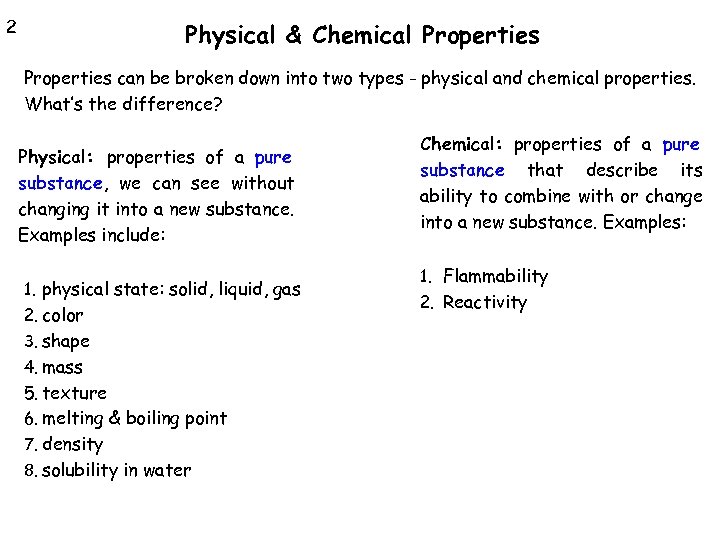
2 Physical & Chemical Properties can be broken down into two types - physical and chemical properties. What’s the difference? Physical: properties of a pure substance, we can see without changing it into a new substance. Examples include: 1. physical state: solid, liquid, gas 2. color 3. shape 4. mass 5. texture 6. melting & boiling point 7. density 8. solubility in water Chemical: properties of a pure substance that describe its ability to combine with or change into a new substance. Examples: 1. Flammability 2. Reactivity

Properties - Pure Substances 3 Matter can either be found in nature as a pure substance or a mixture. What does it mean to be pure? • Pure substance - a substance that contains a single type of matter. When the substance is pure, it has a unique set of properties Ø Example: pure water contains ONLY molecules of water (H 2 O) and NOTHING else!! When water is pure it has the following properites: ü ü no color ü a defined boiling point (100 °C) ü no taste ü • a defined melting point (0 °C) a defined density (1 g/m. L) ü does not burn Pure substances have characteristic properties which we can use to identify the substance… …imagine you had a colorless liquid that boiled at 100° C, melted at 0° C, and had a density of 1 g/m. L, you could say it is most likely water!!

4 • Properties of Matter - Mixtures Mixture - two or more substances mixed together, but not chemically combined. Each component in a mixture keeps its individual properties. Ø Example: salt water contains water molecules (H 2 O) and sodium chloride (Na. Cl) molecules. ü • The mixture will behave differently than the two materials separate. Salt water will have a different boiling point than pure water! Because the parts of a mixture are not chemically combined, the parts can often be separated (purified) into their pure forms by taking advantage of their properties. ü Salt can be separated from water by distilling the water (heating it to boiling), leaving behind salt, collecting water pure as it condenses. ü If you separate salt from water, the two substances will have the same properties as they would before you mixed the two.
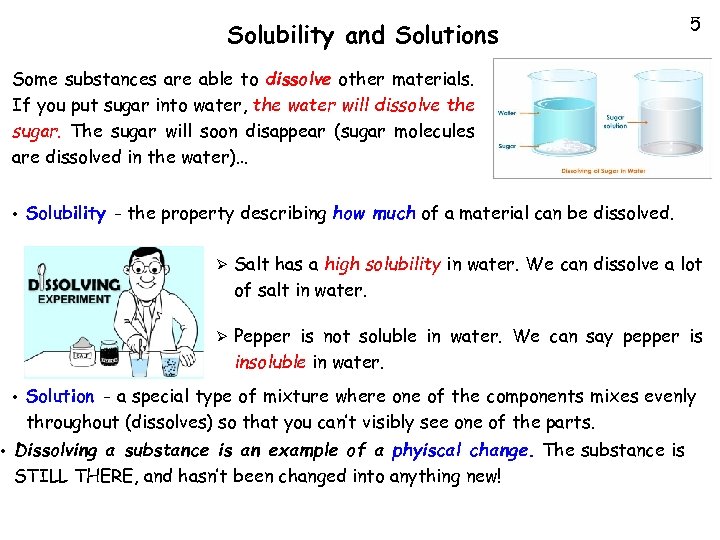
• Solubility and Solutions 5 Some substances are able to dissolve other materials. If you put sugar into water, the water will dissolve the sugar. The sugar will soon disappear (sugar molecules are dissolved in the water)… • Solubility - the property describing how much of a material can be dissolved. Ø Ø • Salt has a high solubility in water. We can dissolve a lot of salt in water. Pepper is not soluble in water. We can say pepper is insoluble in water. Solution - a special type of mixture where one of the components mixes evenly throughout (dissolves) so that you can’t visibly see one of the parts. Dissolving a substance is an example of a phyiscal change. The substance is STILL THERE, and hasn’t been changed into anything new!

4 Mixtures Mixture - If you separate salt from salt water, the water will have the same properties as it would before you mixed the two - BUT… The mixture as a whole can behave differently (salt water will have a different boiling point than pure water) • - A mixture containing stuff not soluble in water could be filtered, leaving behind the stuff that doesn’t dissolve (sand & water) - In mixtures you see more than one Thing (phase) Solution - • Components in mixtures can often be separated (purified) into their pure forms by taking advantage of their properties - Salt can be separated from water by distilling the water (heating it to boiling), leaving behind salt, collecting water pure as it condenses • - In a solution you see only one thing (phase)

Let’s play Solution or Not Solution!! Distilled water? Pure substance! only water - pure The ocean? Mixture! mixture of salts, fish, seaweed… Sprite? Solution Dissolved Ingredients including gas (CO 2) Salt? Pure substance! Sodium Chloride - pure
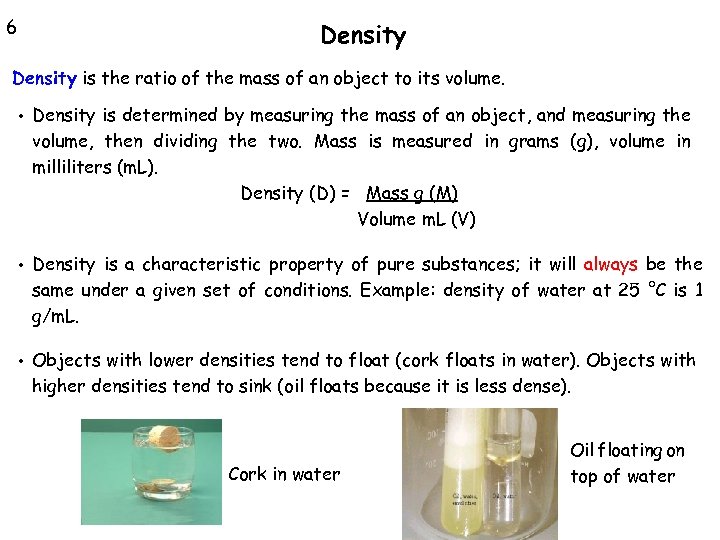
6 Density is the ratio of the mass of an object to its volume. • Density is determined by measuring the mass of an object, and measuring the volume, then dividing the two. Mass is measured in grams (g), volume in milliliters (m. L). Density (D) = Mass g (M) Volume m. L (V) • Density is a characteristic property of pure substances; it will always be the same under a given set of conditions. Example: density of water at 25 °C is 1 g/m. L. • Objects with lower densities tend to float (cork floats in water). Objects with higher densities tend to sink (oil floats because it is less dense). Cork in water Oil floating on top of water

• Lots of things affect density… Density 7 Temperature: cool air sinks, warm air rises Cold water sinks, warm water rises, creating layers of water • Water with salts dissolved in them tend to be more dense than pure water. So, salt water (for example, in the ocean) will sink to the bottom while fresh water will float on top.
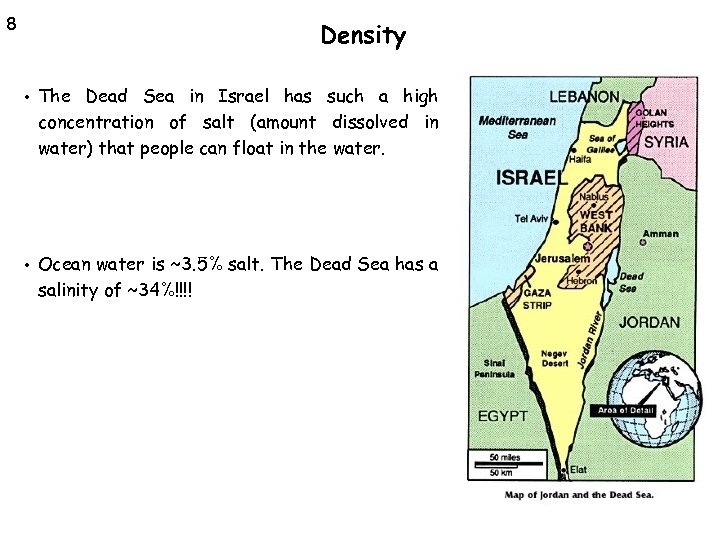
8 Density • The Dead Sea in Israel has such a high concentration of salt (amount dissolved in water) that people can float in the water. • Ocean water is ~3. 5% salt. The Dead Sea has a salinity of ~34%!!!!
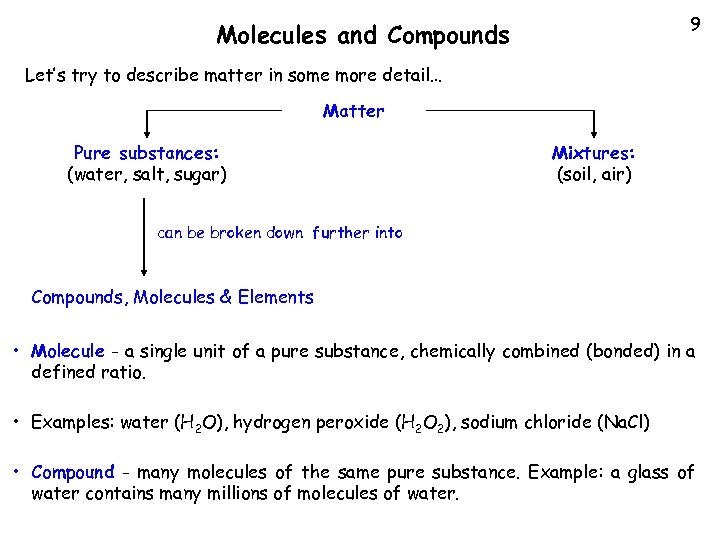
9 Molecules and Compounds Let’s try to describe matter in some more detail… Matter Pure substances: (water, salt, sugar) Mixtures: (soil, air) can be broken down further into Compounds, Molecules & Elements • Molecule - a single unit of a pure substance, chemically combined (bonded) in a defined ratio. • Examples: water (H 2 O), hydrogen peroxide (H 2 O 2), sodium chloride (Na. Cl) • Compound - many molecules of the same pure substance. Example: a glass of water contains many millions of molecules of water.
10 Elements • Element - a pure substance consisting of a single type of atom - each element has distinctive properties. Some are stable, and can be found by themselves in nature. Others are so reactive, they will only be found combined with other elements. - elements can’t be broken down further and still keep their properties - about 100 different elements - elements identified by 1 or 2 letter symbols. Letters often are the first letters of the name of the element (Chlorine -> Cl) - organized in a specific way into the Periodic Table of the Elements.
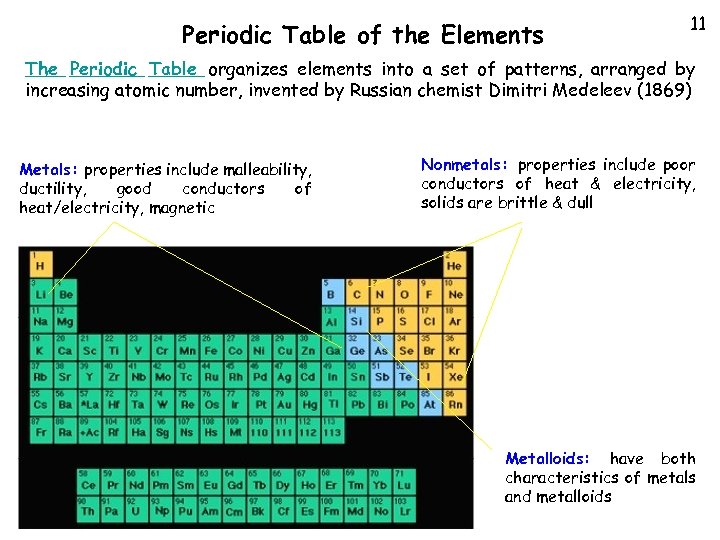
Periodic Table of the Elements 11 The Periodic Table organizes elements into a set of patterns, arranged by increasing atomic number, invented by Russian chemist Dimitri Medeleev (1869) Metals: properties include malleability, ductility, good conductors of heat/electricity, magnetic Nonmetals: properties include poor conductors of heat & electricity, solids are brittle & dull Metalloids: have both characteristics of metals and metalloids
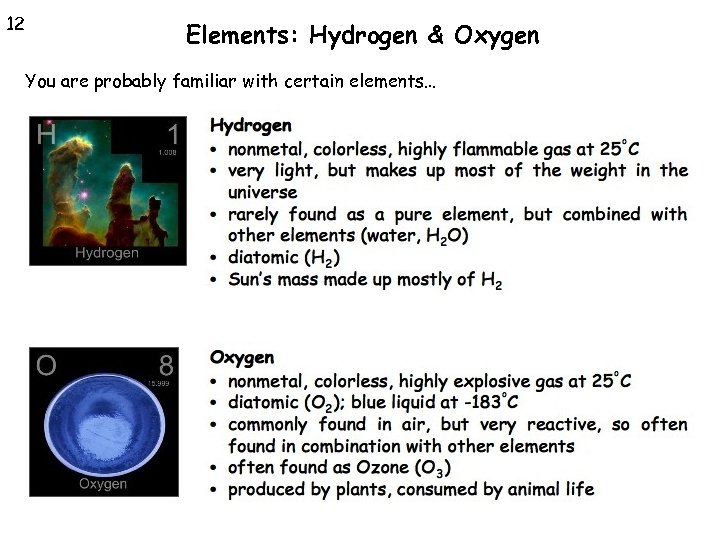
12 Elements: Hydrogen & Oxygen You are probably familiar with certain elements…

Elements: Chlorine & Nitrogen 13
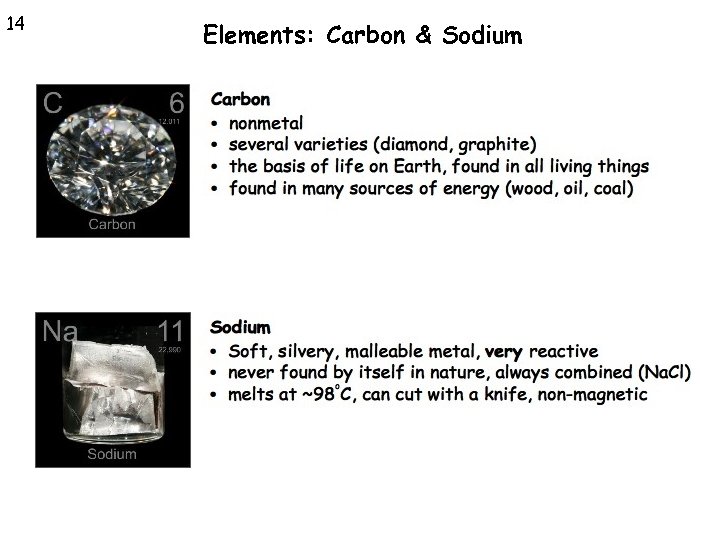
14 Elements: Carbon & Sodium

Elements: Iron & Aluminum Adding other metals (Nickle, Tungsten) to steel gives different properties. 15
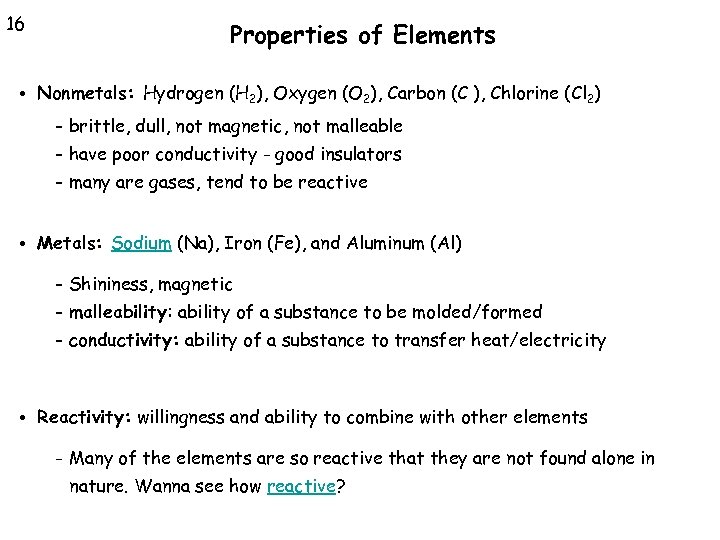
16 Properties of Elements • Nonmetals: Hydrogen (H 2), Oxygen (O 2), Carbon (C ), Chlorine (Cl 2) - brittle, dull, not magnetic, not malleable - have poor conductivity - good insulators - many are gases, tend to be reactive • Metals: Sodium (Na), Iron (Fe), and Aluminum (Al) - Shininess, magnetic - malleability: ability of a substance to be molded/formed - conductivity: ability of a substance to transfer heat/electricity • Reactivity: willingness and ability to combine with other elements - Many of the elements are so reactive that they are not found alone in nature. Wanna see how reactive?
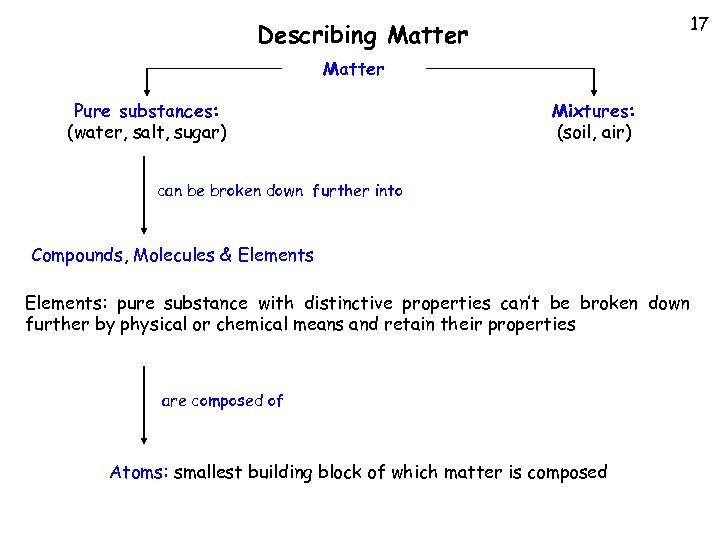
17 Describing Matter Pure substances: (water, salt, sugar) Mixtures: (soil, air) can be broken down further into Compounds, Molecules & Elements: pure substance with distinctive properties can’t be broken down further by physical or chemical means and retain their properties are composed of Atoms: smallest building block of which matter is composed
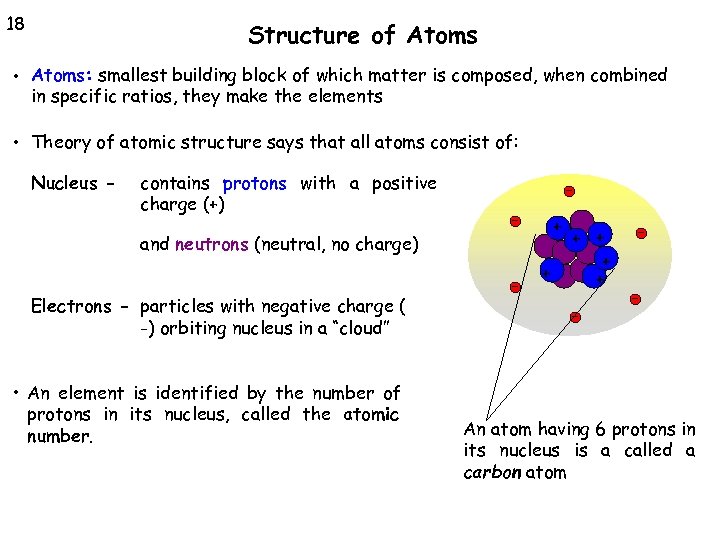
18 Structure of Atoms • Atoms: smallest building block of which matter is composed, when combined in specific ratios, they make the elements • Theory of atomic structure says that all atoms consist of: Nucleus - contains protons with a positive charge (+) - + and neutrons (neutral, no charge) Electrons - particles with negative charge ( -) orbiting nucleus in a “cloud” • An element is identified by the number of protons in its nucleus, called the atomic number. - + + + - - An atom having 6 protons in its nucleus is a called a carbon atom
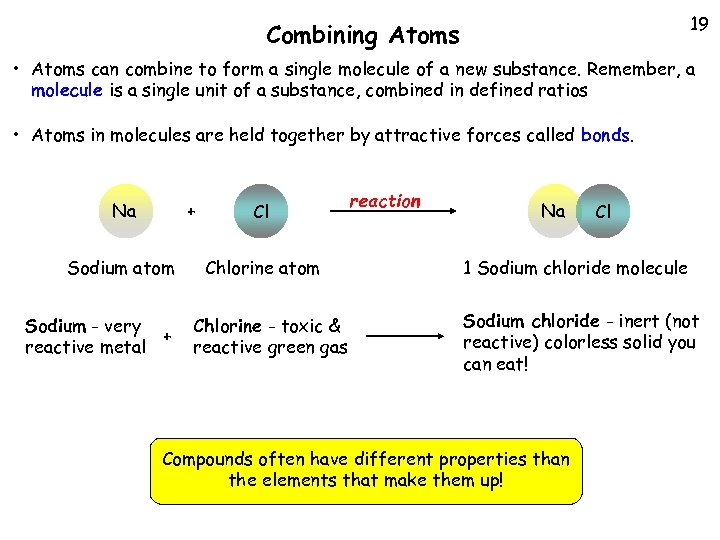
19 Combining Atoms • Atoms can combine to form a single molecule of a new substance. Remember, a molecule is a single unit of a substance, combined in defined ratios • Atoms in molecules are held together by attractive forces called bonds. Na + Sodium atom Sodium - very + reactive metal Cl Chlorine atom Chlorine - toxic & reactive green gas reaction Na Cl 1 Sodium chloride molecule Sodium chloride - inert (not reactive) colorless solid you can eat! Compounds often have different properties than the elements that make them up!
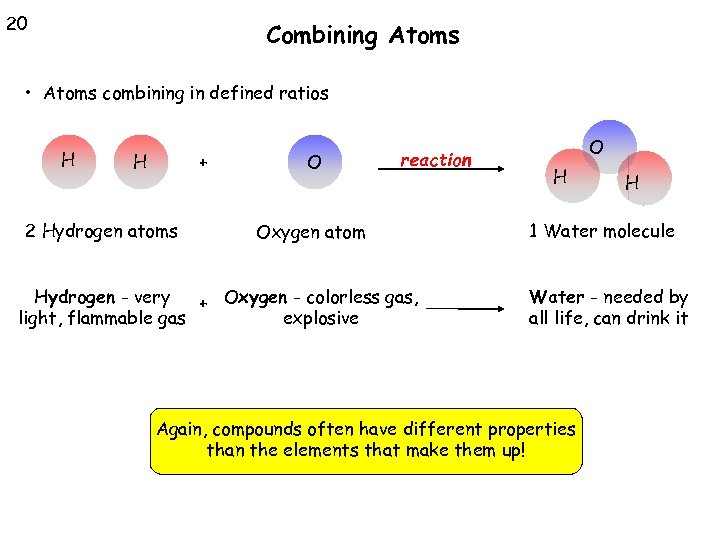
20 Combining Atoms • Atoms combining in defined ratios H H + 2 Hydrogen atoms O reaction Oxygen atom Hydrogen - very + Oxygen - colorless gas, light, flammable gas explosive O H H 1 Water molecule Water - needed by all life, can drink it Again, compounds often have different properties than the elements that make them up!
Changes to Matter 21 Now that we have described matter and put it into different catagories, we can describe how matter changes… • Physical properties: characteristic of a pure substance that can be observed without changing it into another substance. - water always has a density of 1 g/m. L at 25° C - at atmospheric pressure, water always melts at 0°C, and boils at 100°C • Physical changes: changes to a substance that can be observed without changing its identity. Examples… - Change of state, which is easily reversed. For example, water freezes into ice, boils into water vapor, but it’s still water! - Dissolving a substance into another substance. Salt dissolves in water, but they are not chemically combined. They can be separated (distillation) • Physical changes are sometimes hard to notice…
22 Chemical Changes • Chemical properties: a characteristic ability of a substance to change into another substance. - Sodium metal is very reactive, never found alone in nature - Examples of chemical properties: flammablility, reactivity (the desire of a substance to combine with and form new susbtances • Chemical changes: changes to a substance that results in a new substance forming. • Chemical changes are often much more obvious than physical changes 1. Color change 2. Light, heat, or energy released (burning) 3. Gases or solids form where there were none before
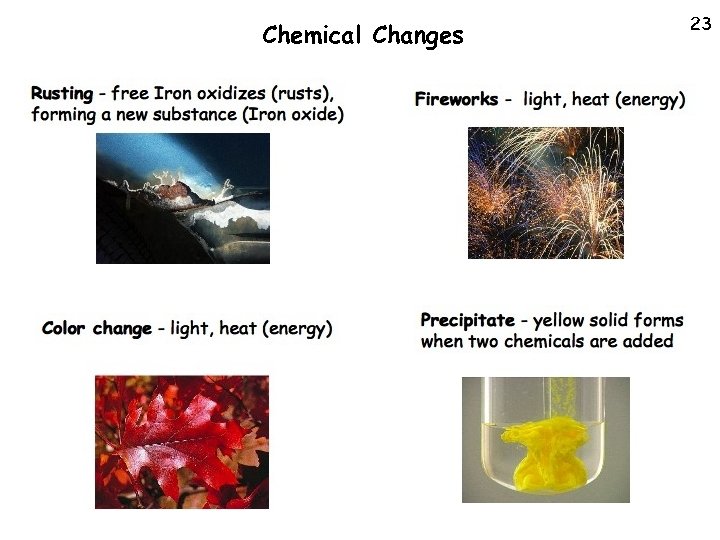
Chemical Changes 23
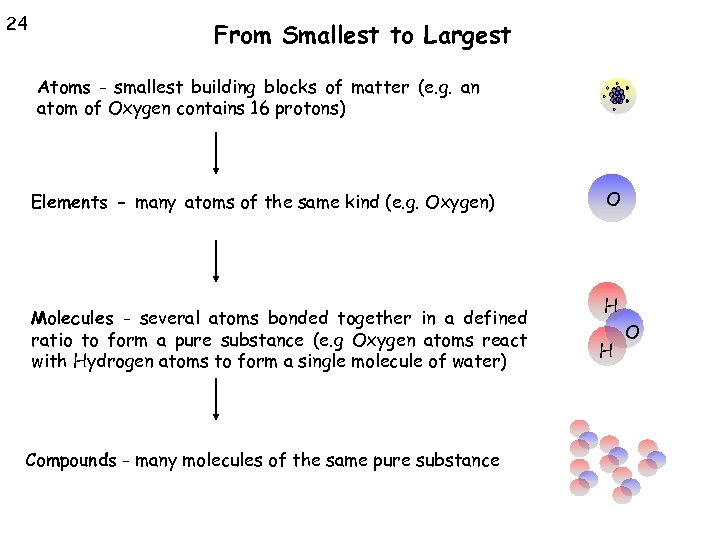
24 From Smallest to Largest Atoms - smallest building blocks of matter (e. g. an atom of Oxygen contains 16 protons) Elements - many atoms of the same kind (e. g. Oxygen) Molecules - several atoms bonded together in a defined ratio to form a pure substance (e. g Oxygen atoms react with Hydrogen atoms to form a single molecule of water) Compounds - many molecules of the same pure substance O H H O
41ae127b5c499bb68d87fa897119aa70.ppt