41f6a2d97589b1145f396a66ef577d20.ppt
- Количество слайдов: 51
 Promoting Integrity in Your Animal Research Program Paul Braunschweiger Ph. D Professor Radiation Oncology Chair, University of Miami IACUC Director, Office of the IACUC Co-Founder, CITI Program. www. citiprogram. org
Promoting Integrity in Your Animal Research Program Paul Braunschweiger Ph. D Professor Radiation Oncology Chair, University of Miami IACUC Director, Office of the IACUC Co-Founder, CITI Program. www. citiprogram. org
 Talking Points • Why does integrity in the Research Enterprise matter? • The Responsible Conduct of Research and the Public Trust – Consequences of violating the Public Trust. • Understanding and managing the conflicts of interest • Promoting Integrity and ethical behavior in your animal research program. – Post approval monitoring – Foundation of ethical behavior is education.
Talking Points • Why does integrity in the Research Enterprise matter? • The Responsible Conduct of Research and the Public Trust – Consequences of violating the Public Trust. • Understanding and managing the conflicts of interest • Promoting Integrity and ethical behavior in your animal research program. – Post approval monitoring – Foundation of ethical behavior is education.
 Integrity “A personal and steadfast commitment to a set of moral or ethical standards defined by your religion, community or professional discipline. ” Miriam Webster Dictionary www. Miriam-Webster. com "Integrity" by Canneto, Columbus, OH
Integrity “A personal and steadfast commitment to a set of moral or ethical standards defined by your religion, community or professional discipline. ” Miriam Webster Dictionary www. Miriam-Webster. com "Integrity" by Canneto, Columbus, OH
 Integrity & Responsible Conduct of Research • Accepted practices for the RCR can vary from discipline to discipline and even from laboratory to laboratory. • Shared values for the RCR that bind all researchers together: – Honesty - conveying information truthfully and honoring commitments. – Accuracy- reporting findings precisely and taking care to avoid errors. – Efficiency -using resources wisely and avoiding waste. – Objectivity - letting the facts speak for themselves and avoiding improper bias. – Compliance with regulatory requirements to document ethical behavior. • Integrity in the Research “Integrity” Fredric Terral 2007
Integrity & Responsible Conduct of Research • Accepted practices for the RCR can vary from discipline to discipline and even from laboratory to laboratory. • Shared values for the RCR that bind all researchers together: – Honesty - conveying information truthfully and honoring commitments. – Accuracy- reporting findings precisely and taking care to avoid errors. – Efficiency -using resources wisely and avoiding waste. – Objectivity - letting the facts speak for themselves and avoiding improper bias. – Compliance with regulatory requirements to document ethical behavior. • Integrity in the Research “Integrity” Fredric Terral 2007
 Why Does Integrity Matter in Research? • • • Puts subjects at risk. Injures careers Wastes Resources Wastes Time Delays acceptance of other research Delays development of affective therapies or advanced technologies. • Undermines the Public Trust.
Why Does Integrity Matter in Research? • • • Puts subjects at risk. Injures careers Wastes Resources Wastes Time Delays acceptance of other research Delays development of affective therapies or advanced technologies. • Undermines the Public Trust.
 The Public Trust • The “Public” supports most of the research in the US. • Sponsored research is a privilege and not an entitlement. • Society Trusts investigators to conduct research ethically and responsibly. • Violation of the public’s trust, brings Regulation. • To preserve the Public’s Trust there must be: – Accountability. – Documentation of ethical conduct. • Federal Regulations (AWA and PHS Policies) specifically require documentation of ethical behavior. • Regulatory requirements to document are designed to: Help investigators conduct research responsibly Preserve the Public Trust in research. RCR 12 -29 -07
The Public Trust • The “Public” supports most of the research in the US. • Sponsored research is a privilege and not an entitlement. • Society Trusts investigators to conduct research ethically and responsibly. • Violation of the public’s trust, brings Regulation. • To preserve the Public’s Trust there must be: – Accountability. – Documentation of ethical conduct. • Federal Regulations (AWA and PHS Policies) specifically require documentation of ethical behavior. • Regulatory requirements to document are designed to: Help investigators conduct research responsibly Preserve the Public Trust in research. RCR 12 -29 -07
 Consequences of betrayal of the Public Trust • • • Puts subjects at risk Federal inquiry / intervention Institutional embarrassment Fines Wasted resources Personal embarrassment Loss of funding, Loss of livelihood Jail Justification and / or vindication for groups with anti-research agenda. A. L. F.
Consequences of betrayal of the Public Trust • • • Puts subjects at risk Federal inquiry / intervention Institutional embarrassment Fines Wasted resources Personal embarrassment Loss of funding, Loss of livelihood Jail Justification and / or vindication for groups with anti-research agenda. A. L. F.
 Ethical Basis for Responsible Conduct of Animal Research • Ethical basis for the Regulations – 569 - 475 BC Pythagoras of Samos – Greek philosopher - mathematics, astronomy, and theory of music. – Believed in Reincarnation – Should not be cruel to animals or eat them. – Vegetarian The Athens School by Raphael
Ethical Basis for Responsible Conduct of Animal Research • Ethical basis for the Regulations – 569 - 475 BC Pythagoras of Samos – Greek philosopher - mathematics, astronomy, and theory of music. – Believed in Reincarnation – Should not be cruel to animals or eat them. – Vegetarian The Athens School by Raphael
 Ethical Basis for Responsible Conduct of Animal Research • Aristotle – – Student of Plato, Teacher to Alexander the Great. Research - Empirical observation and experience. Scientific method. Animals: • Sensation, Passion, memory, understand relationships. • NOT Capable of Thought or reasoning – Aristotle believed that creatures were arranged in a graded scale of perfection. Plato and Aristotle – “The Athens School” by Raphael – Man at a higher state of perfection because Man can reason and reflect. – Animals and plants exist for man’s use, but not abuse.
Ethical Basis for Responsible Conduct of Animal Research • Aristotle – – Student of Plato, Teacher to Alexander the Great. Research - Empirical observation and experience. Scientific method. Animals: • Sensation, Passion, memory, understand relationships. • NOT Capable of Thought or reasoning – Aristotle believed that creatures were arranged in a graded scale of perfection. Plato and Aristotle – “The Athens School” by Raphael – Man at a higher state of perfection because Man can reason and reflect. – Animals and plants exist for man’s use, but not abuse.
 Ethical Basis for Responsible Conduct of Animal Research • Ethical basis for the Regulations – – – 2 nd Century Galen "father of vivisection” 13 th Century St T. Aquinas. Charity to animals. 17 th Century - R. Descartes • Animals are complex automata. • Unconscious beings that can see, hear, touch, anger, fear • Do not think or feel pain. – 17 th Century J. Rousseau. • Animals are sensitive beings, devoid of intellect and freedom • Man has a duty towards animals to not uselessly mistreat them. RCR 12 -29 -07
Ethical Basis for Responsible Conduct of Animal Research • Ethical basis for the Regulations – – – 2 nd Century Galen "father of vivisection” 13 th Century St T. Aquinas. Charity to animals. 17 th Century - R. Descartes • Animals are complex automata. • Unconscious beings that can see, hear, touch, anger, fear • Do not think or feel pain. – 17 th Century J. Rousseau. • Animals are sensitive beings, devoid of intellect and freedom • Man has a duty towards animals to not uselessly mistreat them. RCR 12 -29 -07
 Ethical Basis for Responsible Conduct of Animal Research • Ethical basis for the Regulations – 18 th Century - Jeremy Bentham 1748 - 1832 • • Utilitarianism The moral status of animals Animals had moral status and rights to be treated humanely. “Cruelty towards animals is an incentive to cruelty towards men. ” • In response to Descartes: “The question is not, Can they reason? nor, Can they talk? but, Can they suffer? ”
Ethical Basis for Responsible Conduct of Animal Research • Ethical basis for the Regulations – 18 th Century - Jeremy Bentham 1748 - 1832 • • Utilitarianism The moral status of animals Animals had moral status and rights to be treated humanely. “Cruelty towards animals is an incentive to cruelty towards men. ” • In response to Descartes: “The question is not, Can they reason? nor, Can they talk? but, Can they suffer? ”
 Before his time • • • Marshall Hall by J Holl 1939 Marshall Hall (1790– 1857), a physician and noted physiologist, supported animal research but stated ‘Unhappily… the subjects of animal physiology are sentient, and every experiment is attended by pain and suffering. ’ Hall set out five guiding principles of animal research to stimulate debate in the scientific community: – – – i) the lack of an alternative ii) a clear objective iii) the avoidance of repetition of work iv) the need to minimize suffering v) full and detailed publication of the results. In 1831, he outlined five principles to govern animal experimentation. Of the Principles of Investigation in Physiology. Lancet 1 (1856), pp. 393– 394.
Before his time • • • Marshall Hall by J Holl 1939 Marshall Hall (1790– 1857), a physician and noted physiologist, supported animal research but stated ‘Unhappily… the subjects of animal physiology are sentient, and every experiment is attended by pain and suffering. ’ Hall set out five guiding principles of animal research to stimulate debate in the scientific community: – – – i) the lack of an alternative ii) a clear objective iii) the avoidance of repetition of work iv) the need to minimize suffering v) full and detailed publication of the results. In 1831, he outlined five principles to govern animal experimentation. Of the Principles of Investigation in Physiology. Lancet 1 (1856), pp. 393– 394.
 Ethical Basis for Responsible Conduct of Animal Research • Ethical basis for the Regulations – 20 th Century – Organizations, Codes and Legislation • Nuremberg Code. – Point 3. The experiment should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study that the anticipated results will justify the performance of the experiment. • NIH, Russell WMS and Burch RL (1959); – Reduction, Replacement and Refinement RCR 12 -29 -07
Ethical Basis for Responsible Conduct of Animal Research • Ethical basis for the Regulations – 20 th Century – Organizations, Codes and Legislation • Nuremberg Code. – Point 3. The experiment should be so designed and based on the results of animal experimentation and a knowledge of the natural history of the disease or other problem under study that the anticipated results will justify the performance of the experiment. • NIH, Russell WMS and Burch RL (1959); – Reduction, Replacement and Refinement RCR 12 -29 -07
 Ethical Basis for Responsible Conduct of Animal Research • Ethical basis for the Regulations – 20 th Century – Organizations, Codes and Legislation • The Guide 1963, 1996 • AWA, 1966, amended 1970, 1976, 1985, 1989, 1991, 1993 • HREA 1985 -- IACUC – 21 st Century – • Shift away from animal based models “Alternatives” • Animal welfare vs. animal rights. RCR 12 -29 -07
Ethical Basis for Responsible Conduct of Animal Research • Ethical basis for the Regulations – 20 th Century – Organizations, Codes and Legislation • The Guide 1963, 1996 • AWA, 1966, amended 1970, 1976, 1985, 1989, 1991, 1993 • HREA 1985 -- IACUC – 21 st Century – • Shift away from animal based models “Alternatives” • Animal welfare vs. animal rights. RCR 12 -29 -07
 "Integrity Protecting the Works of Man. " John Quincy Adams Ward, 1903 Integrity in Your Animal Research Program • • • Starts with Institutional Commitment Promoted and nurtured by the IACUC Embraced by the investigators, staff and students as “The right thing to do”
"Integrity Protecting the Works of Man. " John Quincy Adams Ward, 1903 Integrity in Your Animal Research Program • • • Starts with Institutional Commitment Promoted and nurtured by the IACUC Embraced by the investigators, staff and students as “The right thing to do”
 Promoting Integrity and Ethical Behavioral Shared Responsibility • • • Veterinarians Husbandry Technicians Vet Technicians Students Lab technicians. Grad students Post docs Collaborators Principle Investigator Institutional official
Promoting Integrity and Ethical Behavioral Shared Responsibility • • • Veterinarians Husbandry Technicians Vet Technicians Students Lab technicians. Grad students Post docs Collaborators Principle Investigator Institutional official
 Animal Research Enterprise is a Partnership? • • The Public Investigators Veterinarians IACUC Institutional official Institutions Animal subjects Relationships with some common goals and Interest among stakeholders, but, also • Many Conflicting Interests
Animal Research Enterprise is a Partnership? • • The Public Investigators Veterinarians IACUC Institutional official Institutions Animal subjects Relationships with some common goals and Interest among stakeholders, but, also • Many Conflicting Interests
 Conflicts of Interest in the Animal Use Program. • A conflict of interest is a situation in which financial or other personal considerations have the potential to compromise or bias professional judgment and objectivity. – The appearance of a conflict of interest is one in which a reasonable person would think that the professional's judgment is likely to be compromised. – A potential conflict of interest involves a situation that may develop into an actual conflict of interest.
Conflicts of Interest in the Animal Use Program. • A conflict of interest is a situation in which financial or other personal considerations have the potential to compromise or bias professional judgment and objectivity. – The appearance of a conflict of interest is one in which a reasonable person would think that the professional's judgment is likely to be compromised. – A potential conflict of interest involves a situation that may develop into an actual conflict of interest.
 Conflicts of Interest and Commitment • A conflict of interest exists whether or not decisions are affected by a personal interest. • A conflict of interest implies only the potential for bias, not a likelihood. • A conflict of interest is not considered research misconduct. • A conflicts of interest could lead to misconduct, questionable research practices and/or noncompliance. RCR 12 -29 -07
Conflicts of Interest and Commitment • A conflict of interest exists whether or not decisions are affected by a personal interest. • A conflict of interest implies only the potential for bias, not a likelihood. • A conflict of interest is not considered research misconduct. • A conflicts of interest could lead to misconduct, questionable research practices and/or noncompliance. RCR 12 -29 -07
 Conflicts of Interest and Commitment • Tangible Co. I – An institution's researcher holds an executive position with a company that stands to benefit from the animal research he is conducted at the institution. – An institutional official is faced with the suspension of a well funded PI’s protocol for non-compliance. – High profile, big $$ investigator expects preferential treatment from the institution. • Intangible Co. I – Intellectual Bias. • Peer review – Academic conflict of interest. • IACUC reviews • Conflicts of conscience – Religious or Social beliefs might prevent an objective review of a protocol or project (e. g. , animal research; embryonic stem cell research
Conflicts of Interest and Commitment • Tangible Co. I – An institution's researcher holds an executive position with a company that stands to benefit from the animal research he is conducted at the institution. – An institutional official is faced with the suspension of a well funded PI’s protocol for non-compliance. – High profile, big $$ investigator expects preferential treatment from the institution. • Intangible Co. I – Intellectual Bias. • Peer review – Academic conflict of interest. • IACUC reviews • Conflicts of conscience – Religious or Social beliefs might prevent an objective review of a protocol or project (e. g. , animal research; embryonic stem cell research
 Conflicts of Commitment • A researcher is overcommitted. – – – – Clinical duties Academic committees Lab animal research Clinical research Consulting activities Graduate students and fellows Family • Leads to taking short cuts RCR 12 -29 -07
Conflicts of Commitment • A researcher is overcommitted. – – – – Clinical duties Academic committees Lab animal research Clinical research Consulting activities Graduate students and fellows Family • Leads to taking short cuts RCR 12 -29 -07
 Promoting Integrity and Ethical Behavioral Understanding and Managing the Conflict of Interest • The Conflict – Valuable medical advances with animals as test subjects. – Society understands that animals: • Can not understand the research. • Can not consent to participate in what may cause them harm, pain or death. – Society demands that research animals only be used in ethically designed and conducted projects. – Investigators must get the data, publish the papers, get the grants support their labs and get promoted. – Institutions want the grants, patents and indirect costs. – A conflict of interest with respect to institutional welfare, the investigators welfare, the welfare of their animal subjects and the Public Trust
Promoting Integrity and Ethical Behavioral Understanding and Managing the Conflict of Interest • The Conflict – Valuable medical advances with animals as test subjects. – Society understands that animals: • Can not understand the research. • Can not consent to participate in what may cause them harm, pain or death. – Society demands that research animals only be used in ethically designed and conducted projects. – Investigators must get the data, publish the papers, get the grants support their labs and get promoted. – Institutions want the grants, patents and indirect costs. – A conflict of interest with respect to institutional welfare, the investigators welfare, the welfare of their animal subjects and the Public Trust
 Conflict Resolution • Mismanagement of the conflict can lead to noncompliance, questionable research practices, sloppy science, and perhaps misconduct. • The IACUC specifically empowered by the federal regulations to manage the conflicts of interest between: – – Investigators Welfare of Animal Subjects Institutions Society
Conflict Resolution • Mismanagement of the conflict can lead to noncompliance, questionable research practices, sloppy science, and perhaps misconduct. • The IACUC specifically empowered by the federal regulations to manage the conflicts of interest between: – – Investigators Welfare of Animal Subjects Institutions Society
 Promoting Integrity and Ethical Behavioral Managing the conflict of interest • Vast majority of investigators understand the conflicts and the IACUC’s role in managing them. Others require special help: – Case 1. Ends justify the means; • Busy investigator, juggling lab and clinical research • Several big grants, $$$$$ to the institution • Must get the data, – Patients are waiting for new treatments – The editor is waiting. • Lip service to animal welfare. • Follows most of the rules when convenient, but • May take risks because: – Doesn’t have time to submit the amendment. – Chances of getting caught are slim and – Consequences are minor or nonexistent • Ultimately gets in trouble with the IACUC. • Work is suspended • Report to sponsor and regulators
Promoting Integrity and Ethical Behavioral Managing the conflict of interest • Vast majority of investigators understand the conflicts and the IACUC’s role in managing them. Others require special help: – Case 1. Ends justify the means; • Busy investigator, juggling lab and clinical research • Several big grants, $$$$$ to the institution • Must get the data, – Patients are waiting for new treatments – The editor is waiting. • Lip service to animal welfare. • Follows most of the rules when convenient, but • May take risks because: – Doesn’t have time to submit the amendment. – Chances of getting caught are slim and – Consequences are minor or nonexistent • Ultimately gets in trouble with the IACUC. • Work is suspended • Report to sponsor and regulators
 Promoting Integrity and Ethical Behavioral Managing the conflict of interest • Others require special help: – Case 2. Pushing the envelope • • • Senior investigator “I have been doing it this way for 20 years” Wants to “do the right thing”. “Knows the right” thing to do. But abhors any oversight, Sees the IACUC as an impediment to academic freedom. Pushes the administration for less oversight. Reluctantly compliant, but doesn’t buy into the process. Provides poor example to trainees lab staff. Ultimately he or the trainee / staff member gets in trouble with the IACUC. Work may be suspended Report to sponsors and the regulators
Promoting Integrity and Ethical Behavioral Managing the conflict of interest • Others require special help: – Case 2. Pushing the envelope • • • Senior investigator “I have been doing it this way for 20 years” Wants to “do the right thing”. “Knows the right” thing to do. But abhors any oversight, Sees the IACUC as an impediment to academic freedom. Pushes the administration for less oversight. Reluctantly compliant, but doesn’t buy into the process. Provides poor example to trainees lab staff. Ultimately he or the trainee / staff member gets in trouble with the IACUC. Work may be suspended Report to sponsors and the regulators
 Promoting Integrity and Ethical Behavioral Managing the conflict of interest • Others require special help: – Case 3. Ignorance is bliss • • Big $$ program, Corporate and Public funding Thousands of animals. Really wants to do the right thing, but: – – – Poorly organized lab. Personnel Overwhelmed Understaffed, Poorly trained staff Under funded or Too cheap to invest in lab manager. • Inevitably will have problems with the IACUC. • Work will ultimately be suspended. • Report to sponsors and the regulators
Promoting Integrity and Ethical Behavioral Managing the conflict of interest • Others require special help: – Case 3. Ignorance is bliss • • Big $$ program, Corporate and Public funding Thousands of animals. Really wants to do the right thing, but: – – – Poorly organized lab. Personnel Overwhelmed Understaffed, Poorly trained staff Under funded or Too cheap to invest in lab manager. • Inevitably will have problems with the IACUC. • Work will ultimately be suspended. • Report to sponsors and the regulators
 Promoting Integrity and Ethical Behavioral Managing the conflict of interest • Common elements are: – Inevitably will have problems with the IACUC. – Work will ultimately be suspended. – Report to sponsors and the regulators • Investigators are busy, high achievers who are under considerable professional pressures to: – – – – Get the data in print. Get the next grant funded. Keep staff employed Start the company Get promoted. Get the raise
Promoting Integrity and Ethical Behavioral Managing the conflict of interest • Common elements are: – Inevitably will have problems with the IACUC. – Work will ultimately be suspended. – Report to sponsors and the regulators • Investigators are busy, high achievers who are under considerable professional pressures to: – – – – Get the data in print. Get the next grant funded. Keep staff employed Start the company Get promoted. Get the raise
 Promoting Integrity and Ethical Behavioral Managing the conflict of interest • How can the institution help all it’s investigators conduct their work to the highest ethical standards?
Promoting Integrity and Ethical Behavioral Managing the conflict of interest • How can the institution help all it’s investigators conduct their work to the highest ethical standards?
 Promoting Integrity. Institutional level • Institutional Official – Clear understanding of the IO’s role and responsibility • Ethics Education and training • Reporting to the Regulators – Infrastructure & Resources for veterinary staff. – Infrastructure & Resources for the IACUC • Staffing • Training • Oversight Programs – Conflicts of Interest • Institution vs. IACUC vs. Faculty – Commitment to AAALAC accreditation RCR 12 -29 -07
Promoting Integrity. Institutional level • Institutional Official – Clear understanding of the IO’s role and responsibility • Ethics Education and training • Reporting to the Regulators – Infrastructure & Resources for veterinary staff. – Infrastructure & Resources for the IACUC • Staffing • Training • Oversight Programs – Conflicts of Interest • Institution vs. IACUC vs. Faculty – Commitment to AAALAC accreditation RCR 12 -29 -07
 Promoting Integrity The IACUC Active and rigorous promotion of the “ 3 R” • Reduction. – Science Review for experimental design • Controls • Sample size determination. • Pilot studies • Replacement – Literature review for models with less ethical cost. • Refinement – New approaches to mitigate against pain and distress. • • • Analgesia strategies Enrichment Better documentation for waivers of analgesia. – Technical refinement to reduce model failure. RCR 12 -29 -07
Promoting Integrity The IACUC Active and rigorous promotion of the “ 3 R” • Reduction. – Science Review for experimental design • Controls • Sample size determination. • Pilot studies • Replacement – Literature review for models with less ethical cost. • Refinement – New approaches to mitigate against pain and distress. • • • Analgesia strategies Enrichment Better documentation for waivers of analgesia. – Technical refinement to reduce model failure. RCR 12 -29 -07
 Promoting Integrity The IACUC Membership • IACUC membership – Member education and training. • • • New member training. Continuing education, refresher courses. IACUC Members need to understand the expectations of the regulators. • Include educational sessions at meetings. • Participation in national meeting and OLAW, USDA sponsored events. – Enhance the role of the Community Member • Insure Community Member understands the process and his/her role. • Encourage questions. • Multiple Community Members. – Non Science members • Statistician • Librarian RCR 12 -29 -07
Promoting Integrity The IACUC Membership • IACUC membership – Member education and training. • • • New member training. Continuing education, refresher courses. IACUC Members need to understand the expectations of the regulators. • Include educational sessions at meetings. • Participation in national meeting and OLAW, USDA sponsored events. – Enhance the role of the Community Member • Insure Community Member understands the process and his/her role. • Encourage questions. • Multiple Community Members. – Non Science members • Statistician • Librarian RCR 12 -29 -07
 Promoting Integrity The IACUC Membership • IACUC membership – Science members • Anesthesiologist or pain researcher – Evaluate your IACUC member performance. • • • Attendance Protocol Review Participation in Subcommittee Activities – Excuse non-participatory members. – Retain responsible members. RCR 12 -29 -07
Promoting Integrity The IACUC Membership • IACUC membership – Science members • Anesthesiologist or pain researcher – Evaluate your IACUC member performance. • • • Attendance Protocol Review Participation in Subcommittee Activities – Excuse non-participatory members. – Retain responsible members. RCR 12 -29 -07
 Promoting Integrity IACUC Procedures and Process • Protocol Review – Designated Review vs. Full Committee Review • Clear written policies. • Choice of Reviewers – – Science Review Literature Search Review Statistical Review Analgesia review • Efficient communication between the “ 3 I’s” – IACUC, IBC, and IRB. • Confidentiality • Manage conflicts of interest. RCR 12 -29 -07
Promoting Integrity IACUC Procedures and Process • Protocol Review – Designated Review vs. Full Committee Review • Clear written policies. • Choice of Reviewers – – Science Review Literature Search Review Statistical Review Analgesia review • Efficient communication between the “ 3 I’s” – IACUC, IBC, and IRB. • Confidentiality • Manage conflicts of interest. RCR 12 -29 -07
 Promoting Integrity The IACUC Issues of non-compliance • Dealing with non-compliance. – Provide a easy way for the reporting of concerns. – Clear policies and SOPs for reviewing allegations of noncompliance. • Communicate those polices to investigators. • Follow the policies. – – RCR 12 -29 -07 Get the IO involved early. Confidentiality. Protection of whistleblowers. Reporting promptly to the regulators, AAALAC, sponsors.
Promoting Integrity The IACUC Issues of non-compliance • Dealing with non-compliance. – Provide a easy way for the reporting of concerns. – Clear policies and SOPs for reviewing allegations of noncompliance. • Communicate those polices to investigators. • Follow the policies. – – RCR 12 -29 -07 Get the IO involved early. Confidentiality. Protection of whistleblowers. Reporting promptly to the regulators, AAALAC, sponsors.
 Promoting Integrity Investigators • Education and Communication. – Simple and effective means of communication between the IACUC and investigators. • When policies change alert the investigators. • Have a mechanism in place. – Training and education • • • Basic Animal Welfare Instruction Refresher or continuing education. Wet labs. – Documentation of training. – Enforcement of the training mandates. • Monitor Adherence to the protocols RCR 12 -29 -07
Promoting Integrity Investigators • Education and Communication. – Simple and effective means of communication between the IACUC and investigators. • When policies change alert the investigators. • Have a mechanism in place. – Training and education • • • Basic Animal Welfare Instruction Refresher or continuing education. Wet labs. – Documentation of training. – Enforcement of the training mandates. • Monitor Adherence to the protocols RCR 12 -29 -07
 Promoting Integrity in Your Animal Research Program. The Post Approval Monitor (PAM) Integrity in the research program requires oversight. RCR 12 -29 -07
Promoting Integrity in Your Animal Research Program. The Post Approval Monitor (PAM) Integrity in the research program requires oversight. RCR 12 -29 -07
 Post Approval Monitor (PAM) “We are the IACUC and we are here to help you. ” • PAM - lab science training and customer service demeanor. – Arranges meeting with PI & the research team. (annually) – Reviews all active protocols. • • Unreported changes to the protocol. New personnel, training issues. Record keeping procedures. Provides Information. – Training opportunities, – Reporting concerns – Amendments • We are the IACUC and we are here to help you. – Report at the monthly IACUC meeting. RCR 12 -29 -07
Post Approval Monitor (PAM) “We are the IACUC and we are here to help you. ” • PAM - lab science training and customer service demeanor. – Arranges meeting with PI & the research team. (annually) – Reviews all active protocols. • • Unreported changes to the protocol. New personnel, training issues. Record keeping procedures. Provides Information. – Training opportunities, – Reporting concerns – Amendments • We are the IACUC and we are here to help you. – Report at the monthly IACUC meeting. RCR 12 -29 -07
 Promoting Integrity in Your Animal Research Program. Investigator Training RCR 12 -29 -07
Promoting Integrity in Your Animal Research Program. Investigator Training RCR 12 -29 -07
 Promoting Integrity Investigator Training • PHS Policy and USDA Regulation – Basic Training Lab Animal Welfare – Continuing Education • Investigators, staff, students • IACUC members – Procedure or model specific training • Training vehicles • • • 1 on 1 instruction Classroom sessions Web based instruction – – – “Home Built” AALAS Learning Library, IACUC. org CITI Program. • Combination of approaches. • Evaluation of the training program RCR 12 -29 -07
Promoting Integrity Investigator Training • PHS Policy and USDA Regulation – Basic Training Lab Animal Welfare – Continuing Education • Investigators, staff, students • IACUC members – Procedure or model specific training • Training vehicles • • • 1 on 1 instruction Classroom sessions Web based instruction – – – “Home Built” AALAS Learning Library, IACUC. org CITI Program. • Combination of approaches. • Evaluation of the training program RCR 12 -29 -07
 CITI Program Lab Animal Welfare Educational Program opened 5 -15 -07 Anonymous Voluntary User Satisfaction Survey Data 8 -27 -07 to 4 -2 -08
CITI Program Lab Animal Welfare Educational Program opened 5 -15 -07 Anonymous Voluntary User Satisfaction Survey Data 8 -27 -07 to 4 -2 -08
 Training Program Evaluation • • About how many hrs did it take you to complete your Lab Animal Welfare Course requirements? • The quiz questions addressed the relevant issues in the modules? • RCR 12 -29 -07 Age, gender, course completed, years conducting L. A. Research I think that the Internet is an appropriate tool to deliver basic instruction in the ethical conduct of research with animal subjects.
Training Program Evaluation • • About how many hrs did it take you to complete your Lab Animal Welfare Course requirements? • The quiz questions addressed the relevant issues in the modules? • RCR 12 -29 -07 Age, gender, course completed, years conducting L. A. Research I think that the Internet is an appropriate tool to deliver basic instruction in the ethical conduct of research with animal subjects.
 Training Program Evaluation • • Now that I have completed the course, I am more confident in my ability to advise a student or a colleague on how to apply the "3 Rs" to insure that their lab animal studies are conducted to the highest ethical standards? • Now that I have completed this course on the ethical conduct of studies with laboratory animals, I intend to take a more active role in assuring that lab animal research at my institution is conducted to the highest ethical standards by pursuing professional certification, an ethics committee or by joining an Institutional Animal Care and Use Committee (IACUC) • RCR 12 -29 -07 After completing this instruction, I now have a better understanding of how to apply the "3 Rs" to insure that my lab animal studies are conducted to the highest ethical standards? How do you rate the Course?
Training Program Evaluation • • Now that I have completed the course, I am more confident in my ability to advise a student or a colleague on how to apply the "3 Rs" to insure that their lab animal studies are conducted to the highest ethical standards? • Now that I have completed this course on the ethical conduct of studies with laboratory animals, I intend to take a more active role in assuring that lab animal research at my institution is conducted to the highest ethical standards by pursuing professional certification, an ethics committee or by joining an Institutional Animal Care and Use Committee (IACUC) • RCR 12 -29 -07 After completing this instruction, I now have a better understanding of how to apply the "3 Rs" to insure that my lab animal studies are conducted to the highest ethical standards? How do you rate the Course?
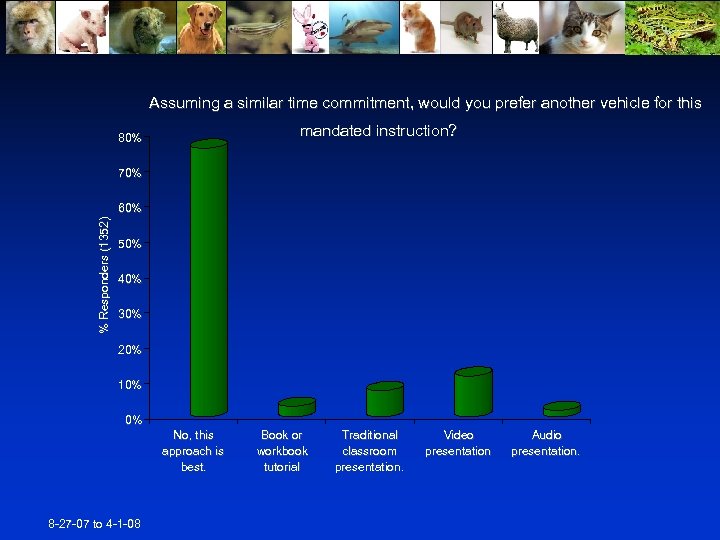 Assuming a similar time commitment, would you prefer another vehicle for this mandated instruction? 80% 70% % Responders (1352) 60% 50% 40% 30% 20% 10% 0% No, this approach is best. 8 -27 -07 to 4 -1 -08 Book or workbook tutorial Traditional classroom presentation. Video presentation Audio presentation.
Assuming a similar time commitment, would you prefer another vehicle for this mandated instruction? 80% 70% % Responders (1352) 60% 50% 40% 30% 20% 10% 0% No, this approach is best. 8 -27 -07 to 4 -1 -08 Book or workbook tutorial Traditional classroom presentation. Video presentation Audio presentation.
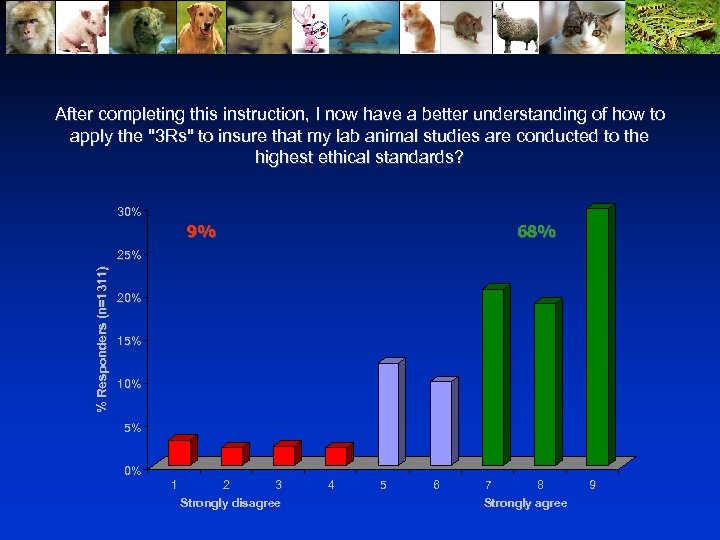 After completing this instruction, I now have a better understanding of how to apply the "3 Rs" to insure that my lab animal studies are conducted to the highest ethical standards? 30% 9% 68% % Responders (n=1311) 25% 20% 15% 10% 5% 0% 1 2 3 Strongly disagree 4 5 6 7 8 Strongly agree 9
After completing this instruction, I now have a better understanding of how to apply the "3 Rs" to insure that my lab animal studies are conducted to the highest ethical standards? 30% 9% 68% % Responders (n=1311) 25% 20% 15% 10% 5% 0% 1 2 3 Strongly disagree 4 5 6 7 8 Strongly agree 9
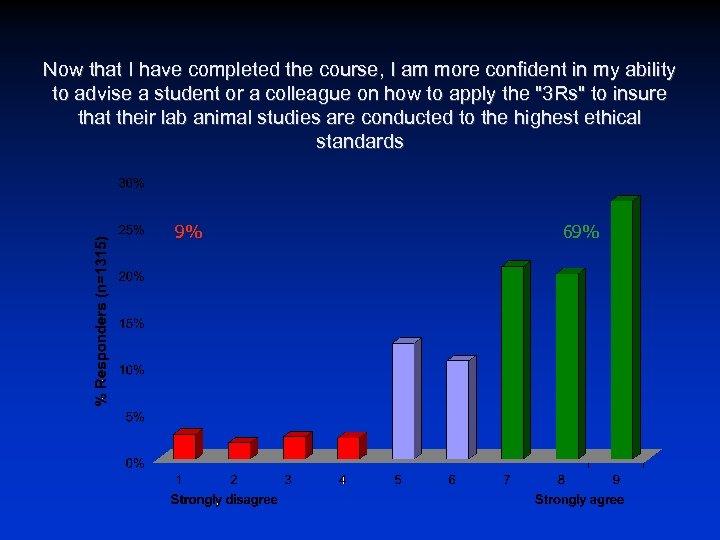 Now that I have completed the course, I am more confident in my ability to advise a student or a colleague on how to apply the "3 Rs" to insure that their lab animal studies are conducted to the highest ethical standards 9% 69%
Now that I have completed the course, I am more confident in my ability to advise a student or a colleague on how to apply the "3 Rs" to insure that their lab animal studies are conducted to the highest ethical standards 9% 69%
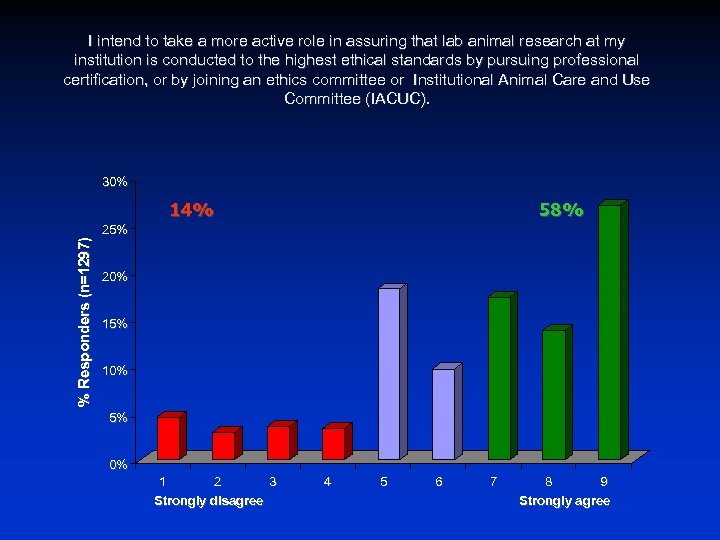 I intend to take a more active role in assuring that lab animal research at my institution is conducted to the highest ethical standards by pursuing professional certification, or by joining an ethics committee or Institutional Animal Care and Use Committee (IACUC). 30% % Responders (n=1297) 14% 58% 25% 20% 15% 10% 5% 0% 1 2 3 Strongly disagree 4 5 6 7 8 9 Strongly agree
I intend to take a more active role in assuring that lab animal research at my institution is conducted to the highest ethical standards by pursuing professional certification, or by joining an ethics committee or Institutional Animal Care and Use Committee (IACUC). 30% % Responders (n=1297) 14% 58% 25% 20% 15% 10% 5% 0% 1 2 3 Strongly disagree 4 5 6 7 8 9 Strongly agree
 Summary • The active promotion of integrity in the research enterprise is essential to maintain the Public Trust. – Without the Public Trust there can be now research • Institutional Support provides the backbone for the Animal Research Program. – Educate the IO – AAALAC accreditation • Management of conflicts of interest between – Investigators, the institution and the animal subjects is crucial to well run animal care program. “Integrity” by Joris Plu 2005
Summary • The active promotion of integrity in the research enterprise is essential to maintain the Public Trust. – Without the Public Trust there can be now research • Institutional Support provides the backbone for the Animal Research Program. – Educate the IO – AAALAC accreditation • Management of conflicts of interest between – Investigators, the institution and the animal subjects is crucial to well run animal care program. “Integrity” by Joris Plu 2005
 Summary • Institution and the IACUC should make it easy for investigators to be compliant. – Take a look at your process. Reduce the time between submission and approval. – Institute Post Approval Protocol Monitor system • “We are the IACUC, yes we are the cops, but we are here to help you. ” • Marked reduction in non-compliance. – The foundation of research integrity is education • Early, often, multiple formats • Evaluate your Animal use training program. Is it working for you? • Listen to the feedback from your learners. • • Promoting Integrity is everyone’s responsibility. – Leads to good animal care – Leads to good science. – Compliance The Responsible Conduct of Research is beyond simply being compliant with Federal regulations. It is just The “right thing to do”. “New Integrity” by Artibella Avanti
Summary • Institution and the IACUC should make it easy for investigators to be compliant. – Take a look at your process. Reduce the time between submission and approval. – Institute Post Approval Protocol Monitor system • “We are the IACUC, yes we are the cops, but we are here to help you. ” • Marked reduction in non-compliance. – The foundation of research integrity is education • Early, often, multiple formats • Evaluate your Animal use training program. Is it working for you? • Listen to the feedback from your learners. • • Promoting Integrity is everyone’s responsibility. – Leads to good animal care – Leads to good science. – Compliance The Responsible Conduct of Research is beyond simply being compliant with Federal regulations. It is just The “right thing to do”. “New Integrity” by Artibella Avanti
 Joseph Wright (September 3, 1734 - August 29, 1797),
Joseph Wright (September 3, 1734 - August 29, 1797),
 “An Experiment on a Bird in The Air Pump “ by Joseph Wright (1734 -1797) British National Gallery- London
“An Experiment on a Bird in The Air Pump “ by Joseph Wright (1734 -1797) British National Gallery- London
 Promoting Integrity in Your Animal Research Program Paul Braunschweiger Ph. D Professor Radiation Oncology Chair, University of Miami IACUC Director, Office of the IACUC Co-Founder, CITI Program.
Promoting Integrity in Your Animal Research Program Paul Braunschweiger Ph. D Professor Radiation Oncology Chair, University of Miami IACUC Director, Office of the IACUC Co-Founder, CITI Program.


