97cd383d59f0dea3b0435bf8976a23c7.ppt
- Количество слайдов: 13
 Prometheus & Myriad The Future of Diagnostic & Gene Claims Mercedes Meyer, Ph. D. Kevin Noonan, Ph. D.
Prometheus & Myriad The Future of Diagnostic & Gene Claims Mercedes Meyer, Ph. D. Kevin Noonan, Ph. D.
 Discussion Points § § § A rear view of Classen and Labcorp. AIA 35 USC § 101 change. USPTO Memo Guidance March 21, 2012 The tension – Mc. Kesson & Akamai 35 U. S. C. § 287(c) Smartgene – March 30, 2012 2 | The Future of Diagnostic Claims
Discussion Points § § § A rear view of Classen and Labcorp. AIA 35 USC § 101 change. USPTO Memo Guidance March 21, 2012 The tension – Mc. Kesson & Akamai 35 U. S. C. § 287(c) Smartgene – March 30, 2012 2 | The Future of Diagnostic Claims
 More Discussion Points § The politics – The public – The stakeholders – The politician – Congresswoman Wasserman-Schultz • AIA S. 27 • The Hearings Feb. 16 & Mar. 9 – The White House, DOJ, USPTO, NIH – LDTs, CLIA, and the FDA • Stakeholders: Pharma, Consumers/Patients, Companion diagnostic manufacturers, Payers, Physicians, Regulatory, and Testing Labs 3 | The Future of Diagnostic Claims
More Discussion Points § The politics – The public – The stakeholders – The politician – Congresswoman Wasserman-Schultz • AIA S. 27 • The Hearings Feb. 16 & Mar. 9 – The White House, DOJ, USPTO, NIH – LDTs, CLIA, and the FDA • Stakeholders: Pharma, Consumers/Patients, Companion diagnostic manufacturers, Payers, Physicians, Regulatory, and Testing Labs 3 | The Future of Diagnostic Claims
 Some myths on gene patents § Patents and Costs – No evidence was found demonstrating that patents accelerated or inhibited test development for certain conditions (e. g. hearing loss). – Price for genetic tests did not appear to correlate with patent status. Some for-profit providers had the same cost as not-forprofit testing providers. – Patents have been found not impede consumer utilization of the tests. – The marketplace is the driver of gene testing. – Incorrect data – the myth that 20% of the human genes are patented is false. • Whole Genome Sequencing – does not infringe a gene patent. 4 | The Future of Diagnostic Claims
Some myths on gene patents § Patents and Costs – No evidence was found demonstrating that patents accelerated or inhibited test development for certain conditions (e. g. hearing loss). – Price for genetic tests did not appear to correlate with patent status. Some for-profit providers had the same cost as not-forprofit testing providers. – Patents have been found not impede consumer utilization of the tests. – The marketplace is the driver of gene testing. – Incorrect data – the myth that 20% of the human genes are patented is false. • Whole Genome Sequencing – does not infringe a gene patent. 4 | The Future of Diagnostic Claims
 Some more myths on gene patents § Patents and Innovation – No evidence for a “tragedy of the anticommons” regarding basic research • >9, 000 scientific journal articles on BRCA genes post-patenting – “Isolated DNA” claims not infringed by genetic diagnostic testing • Claims require isolation of full-length genes capable of producing encoded protein • Isolation not required for genetic diagnostic tests – Oligomer claims of questionable validity – Gene patents do not inhibit information about genes • Genetic information is not patented and gene patent claims not infringed by in silico analyses 5 | The Future of Diagnostic Claims
Some more myths on gene patents § Patents and Innovation – No evidence for a “tragedy of the anticommons” regarding basic research • >9, 000 scientific journal articles on BRCA genes post-patenting – “Isolated DNA” claims not infringed by genetic diagnostic testing • Claims require isolation of full-length genes capable of producing encoded protein • Isolation not required for genetic diagnostic tests – Oligomer claims of questionable validity – Gene patents do not inhibit information about genes • Genetic information is not patented and gene patent claims not infringed by in silico analyses 5 | The Future of Diagnostic Claims
 A Slippery Slope & The Future § Some (Judge Moore) voice a concern that if DNA patents are attacked there will be a slippery slope effect to capture other compounds. – Antisense, si. RNA. – Proteins and peptides. • Antibodies and their fragments. § Method of treatment claims are recognized for Orange Book listings. § Companion Diagnostics. 6 | The Future of Diagnostic Claims
A Slippery Slope & The Future § Some (Judge Moore) voice a concern that if DNA patents are attacked there will be a slippery slope effect to capture other compounds. – Antisense, si. RNA. – Proteins and peptides. • Antibodies and their fragments. § Method of treatment claims are recognized for Orange Book listings. § Companion Diagnostics. 6 | The Future of Diagnostic Claims
 A Slippery Slope & The Future § Consider these examples: – Isolated chemical compound from crude oil useful as a lubricant – Isolated chemical compound from a plant useful as a drug – Isolated protein from an animal useful to cure/ameliorate human disease – From a human? 7 | The Future of Diagnostic Claims
A Slippery Slope & The Future § Consider these examples: – Isolated chemical compound from crude oil useful as a lubricant – Isolated chemical compound from a plant useful as a drug – Isolated protein from an animal useful to cure/ameliorate human disease – From a human? 7 | The Future of Diagnostic Claims
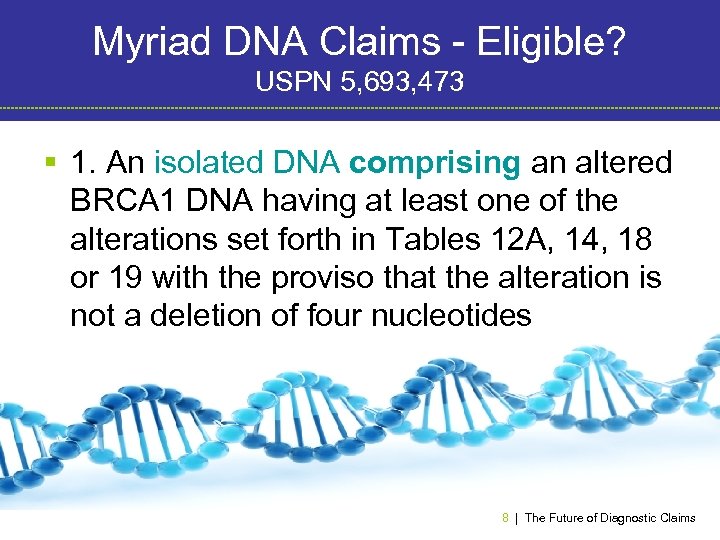 Myriad DNA Claims - Eligible? USPN 5, 693, 473 § 1. An isolated DNA comprising an altered BRCA 1 DNA having at least one of the alterations set forth in Tables 12 A, 14, 18 or 19 with the proviso that the alteration is not a deletion of four nucleotides corresponding to base numbers 41844187 in SEQ. ID. NO: 1. 8 | The Future of Diagnostic Claims
Myriad DNA Claims - Eligible? USPN 5, 693, 473 § 1. An isolated DNA comprising an altered BRCA 1 DNA having at least one of the alterations set forth in Tables 12 A, 14, 18 or 19 with the proviso that the alteration is not a deletion of four nucleotides corresponding to base numbers 41844187 in SEQ. ID. NO: 1. 8 | The Future of Diagnostic Claims
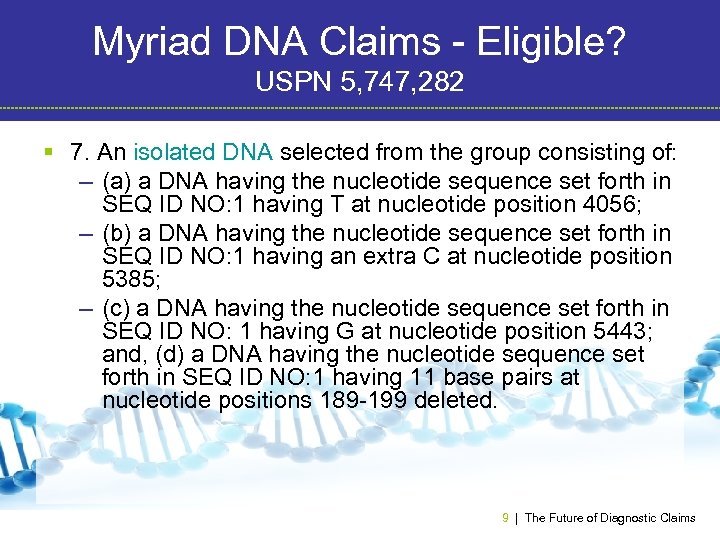 Myriad DNA Claims - Eligible? USPN 5, 747, 282 § 7. An isolated DNA selected from the group consisting of: – (a) a DNA having the nucleotide sequence set forth in SEQ ID NO: 1 having T at nucleotide position 4056; – (b) a DNA having the nucleotide sequence set forth in SEQ ID NO: 1 having an extra C at nucleotide position 5385; – (c) a DNA having the nucleotide sequence set forth in SEQ ID NO: 1 having G at nucleotide position 5443; and, (d) a DNA having the nucleotide sequence set forth in SEQ ID NO: 1 having 11 base pairs at nucleotide positions 189 -199 deleted. 9 | The Future of Diagnostic Claims
Myriad DNA Claims - Eligible? USPN 5, 747, 282 § 7. An isolated DNA selected from the group consisting of: – (a) a DNA having the nucleotide sequence set forth in SEQ ID NO: 1 having T at nucleotide position 4056; – (b) a DNA having the nucleotide sequence set forth in SEQ ID NO: 1 having an extra C at nucleotide position 5385; – (c) a DNA having the nucleotide sequence set forth in SEQ ID NO: 1 having G at nucleotide position 5443; and, (d) a DNA having the nucleotide sequence set forth in SEQ ID NO: 1 having 11 base pairs at nucleotide positions 189 -199 deleted. 9 | The Future of Diagnostic Claims
 Is Licensing “THE” Issue? § Single Providers – Myriad / BRCA 1 & 2 – Canavan Disease – Miami Children’s Hospital § Multiple Providers – Long QT Syndrome has two providers with separate blocking patents – Hearing loss – multiple providers for patents and unpatented genes – Hereditary hemochromatosis – multiple LDTs; no IP impact – Alzheimer’s Disease – Lynch Syndrome (HNPCC) – Familial adenomatous polyposis (FAP) – Spinocerebellar ataxia
Is Licensing “THE” Issue? § Single Providers – Myriad / BRCA 1 & 2 – Canavan Disease – Miami Children’s Hospital § Multiple Providers – Long QT Syndrome has two providers with separate blocking patents – Hearing loss – multiple providers for patents and unpatented genes – Hereditary hemochromatosis – multiple LDTs; no IP impact – Alzheimer’s Disease – Lynch Syndrome (HNPCC) – Familial adenomatous polyposis (FAP) – Spinocerebellar ataxia
 Myriad Internationally Gene Patent Attacks Globally
Myriad Internationally Gene Patent Attacks Globally
 International Stance § Canada – Myriad attacked the government. Today, Myriad’s patents are primarily ignored. § Europe – Opposed by Institut Curie. – UK – provides BRCA testing without payment to Myriad. § Australia – the government investigated and came close to banning gene patents. § Japan – exclusively licensed the rights to Falco. 12 | The Future of Diagnostic Claims
International Stance § Canada – Myriad attacked the government. Today, Myriad’s patents are primarily ignored. § Europe – Opposed by Institut Curie. – UK – provides BRCA testing without payment to Myriad. § Australia – the government investigated and came close to banning gene patents. § Japan – exclusively licensed the rights to Falco. 12 | The Future of Diagnostic Claims
 Questions? Mercedes K. Meyer, Ph. D. Drinker Biddle & Reath LLP Washington, DC 20005 Kevin E. Noonan, Ph. D. Mc. Donnell Boehnen Hulbert & Berghoff LLP Chicago, IL 60606
Questions? Mercedes K. Meyer, Ph. D. Drinker Biddle & Reath LLP Washington, DC 20005 Kevin E. Noonan, Ph. D. Mc. Donnell Boehnen Hulbert & Berghoff LLP Chicago, IL 60606


