1d03d8a48de2feb43c6897aba6afb2dd.ppt
- Количество слайдов: 66

Prof. Grobéty B. , Inst. de Minéralogie et Pétrographie, Univ. de Fribourg Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
Introduction Cementitous materials Definition: Material, which binds together with solid bodies (aggregates) by hardening from a plastic state. Examples: organic polymers inorganic cements Inorganic cements - mixed with water plastic state - hydration of the components development of rigidity (setting) - steady increase of strength (hardening) - Examples: Portland cement, gypsum plasters, phosphate cements - when hardening occurs also under water: hydraulic cement - Example: Portland cement Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008

Introduction Historical background I (www. auburn. edu/academic/architecture/bsc/classes/bsc 314/timeline. htm) 12 M BC: 3000 BC: 300 BC: Natural production of clinker through the spontaneous combustion of oil shales (Israel) Egyptians used sulfate and lime based plasters Use of cementitous materials in China (Great Wall) Concrete and mortars based on lime and pozzolanic material (volcanic ashes). Pliny reported a mortar mix of 1 part of lime and 4 part of sand. Examples: 193 BC: Porticu House, Amaelia, 200 AD: Pantheon, Rome (www. romanconcrete. com) http: //www. greatbuildings. com/buildings/Pantheon. html Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
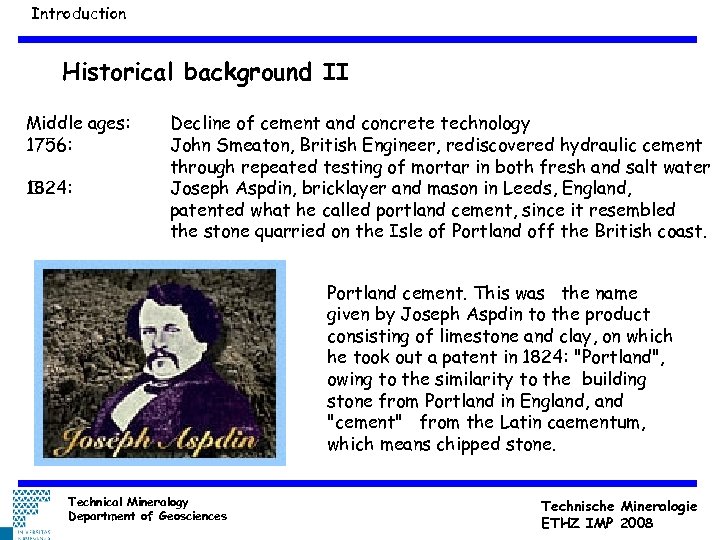
Introduction Historical background II Middle ages: 1756: 1824: Decline of cement and concrete technology John Smeaton, British Engineer, rediscovered hydraulic cement through repeated testing of mortar in both fresh and salt water Joseph Aspdin, bricklayer and mason in Leeds, England, patented what he called portland cement, since it resembled the stone quarried on the Isle of Portland off the British coast. Portland cement. This was the name given by Joseph Aspdin to the product consisting of limestone and clay, on which he took out a patent in 1824: "Portland", owing to the similarity to the building stone from Portland in England, and "cement" from the Latin caementum, which means chipped stone. Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
Introduction Cement: definitions Portland cement: Hydraulic cementitous material based on clinker, a material composed of calcium silicates and aluminates, and a small amount of added gypsum/anhydrite. The clinker is made by burning mixtures of limestone and argilaceous rocks (slates). Mortar: Mixture of Portland cement, fine sand water (used f. ex. for the construction of brick walls) Neat paste: Mixture of Portland cement and water alone (used for filling cracks and sealing small spaces) Concrete: Mixture of Portland cement, coarse and fine aggregates (rock pebbles, sand), water and chemical additives. The mechanical strength can be reinforced by the insertion of steel bars. Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
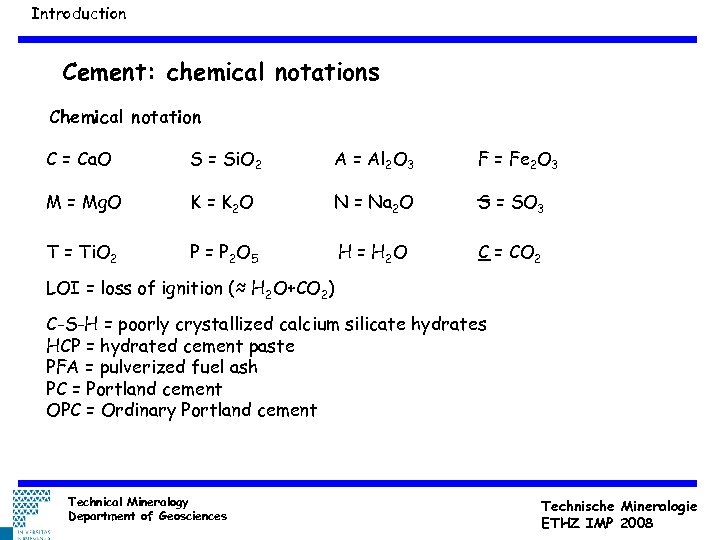
Introduction Cement: chemical notations Chemical notation C = Ca. O S = Si. O 2 A = Al 2 O 3 F = Fe 2 O 3 M = Mg. O K = K 2 O N = Na 2 O S = SO 3 T = Ti. O 2 P = P 2 O 5 H = H 2 O C = CO 2 LOI = loss of ignition (≈ H 2 O+CO 2) C-S-H = poorly crystallized calcium silicate hydrates HCP = hydrated cement paste PFA = pulverized fuel ash PC = Portland cement OPC = Ordinary Portland cement Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
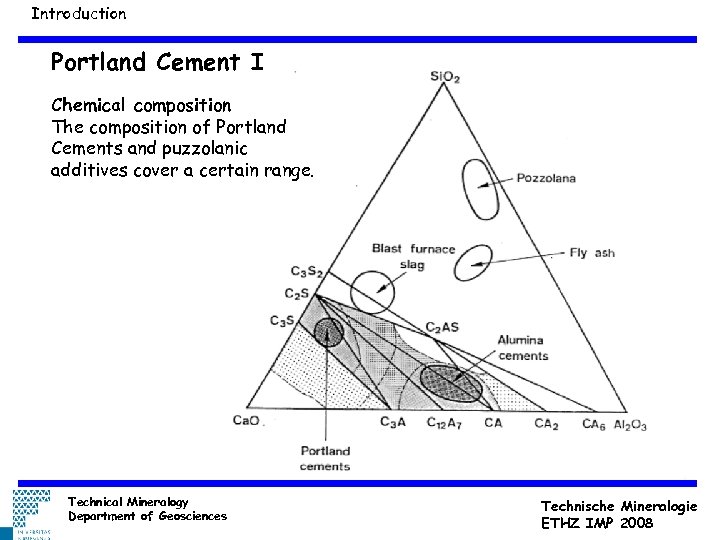
Introduction Portland Cement I Chemical composition The composition of Portland Cements and puzzolanic additives cover a certain range. Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
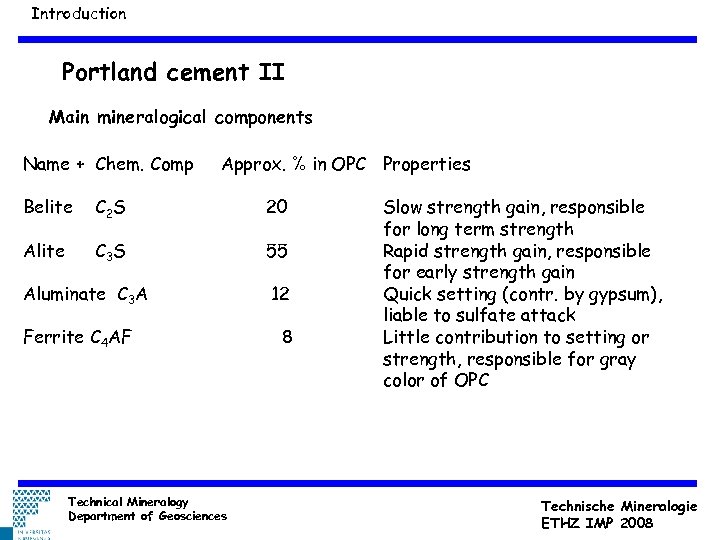
Introduction Portland cement II Main mineralogical components Name + Chem. Comp Approx. % in OPC Properties Belite C 2 S 20 Alite C 3 S 55 Aluminate C 3 A Ferrite C 4 AF Technical Mineralogy Department of Geosciences 12 8 Slow strength gain, responsible for long term strength Rapid strength gain, responsible for early strength gain Quick setting (contr. by gypsum), liable to sulfate attack Little contribution to setting or strength, responsible for gray color of OPC Technische Mineralogie ETHZ IMP 2008
Introduction Portland Cement III Main production steps (http: //www. ppc. co. za/Cement/c_cement_manprocess. asp) Quarrying chalk in northern Jutland (Aalborg Cement) Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
Introduction Portland Cement IV Main production steps (cont. ) Chalk slurry tank (Aalborg cement) Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
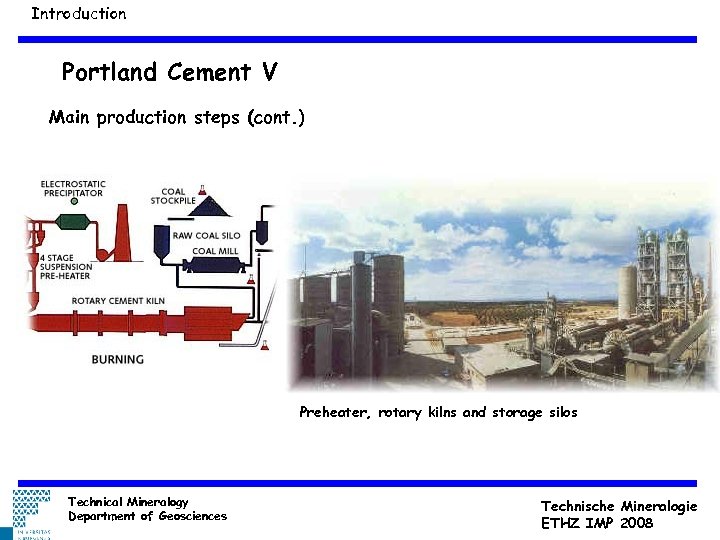
Introduction Portland Cement V Main production steps (cont. ) Preheater, rotary kilns and storage silos Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
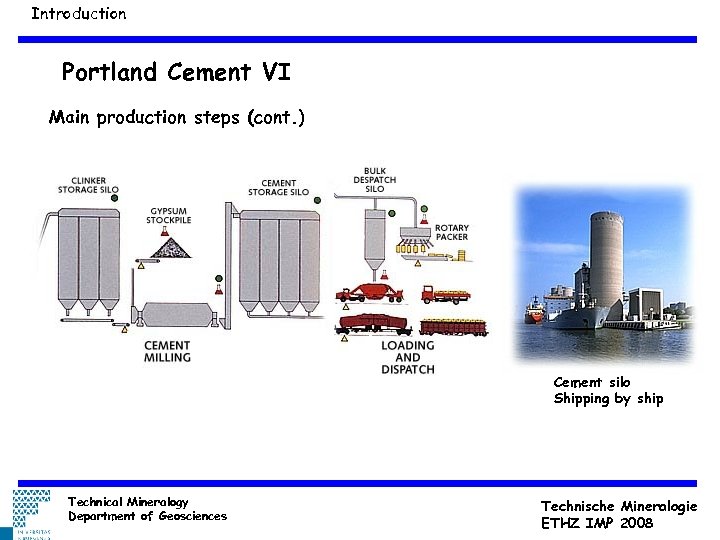
Introduction Portland Cement VI Main production steps (cont. ) Cement silo Shipping by ship Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
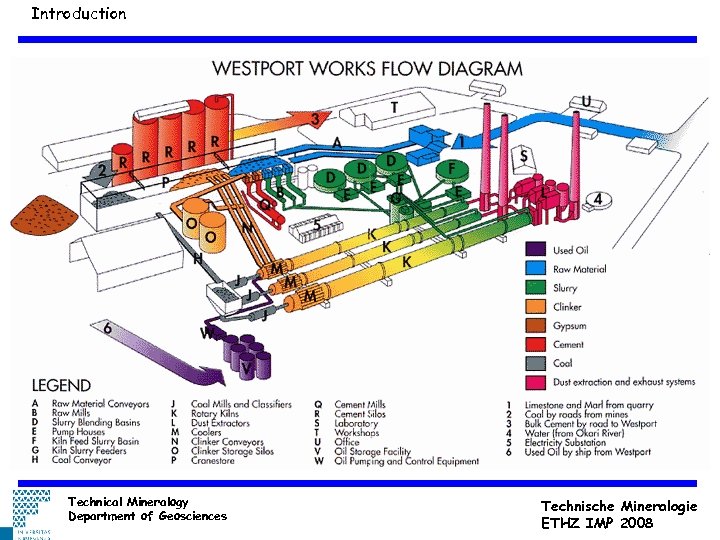
Introduction Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
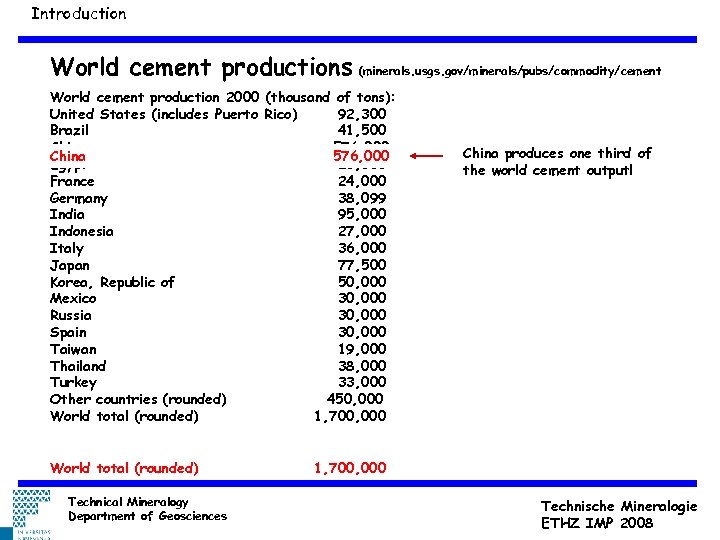
Introduction World cement productions (minerals. usgs. gov/minerals/pubs/commodity/cement World cement production 2000 (thousand of tons): United States (includes Puerto Rico) 92, 300 Brazil 41, 500 China 576, 000 Egypt 23, 000 France 24, 000 Germany 38, 099 India 95, 000 Indonesia 27, 000 Italy 36, 000 Japan 77, 500 Korea, Republic of 50, 000 Mexico 30, 000 Russia 30, 000 Spain 30, 000 Taiwan 19, 000 Thailand 38, 000 Turkey 33, 000 Other countries (rounded) 450, 000 World total (rounded) 1, 700, 000 World total (rounded) Technical Mineralogy Department of Geosciences China produces one third of the world cement output! 1, 700, 000 Technische Mineralogie ETHZ IMP 2008
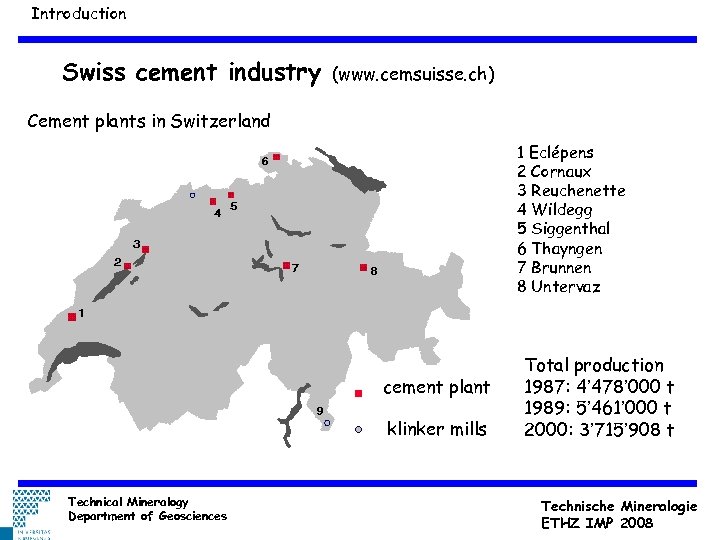
Introduction Swiss cement industry (www. cemsuisse. ch) Cement plants in Switzerland 1 Eclépens 2 Cornaux 3 Reuchenette 4 Wildegg 5 Siggenthal 6 Thayngen 7 Brunnen 8 Untervaz cement plant klinker mills Technical Mineralogy Department of Geosciences Total production 1987: 4’ 478’ 000 t 1989: 5’ 461’ 000 t 2000: 3’ 715’ 908 t Technische Mineralogie ETHZ IMP 2008
Raw materials Main raw materials Calcareous lime stones: - calcite-rich - low in dolomite Shales: - clay rich, usually dominated by illite, smectite and kaolinite. Ideal bulk composition ranges: 55 -60 wt% Si. O 2, 15 -25 wt% Al 2 O 3, 5 -10 wt% Fe 2 O 3 Corrective constituents Sand, flyash: Ironores, bauxite: - adjust Si. O 2 -content in quartz-poor shales - adjust Fe resp. Al content Additional reactive constituents, which have to be considered, may be introduced through impurities in the fuel. Up of 30% of ash is produced by the firing of brown coal. Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
Raw materials Composition of ordinary Portland cements Major components Si. O 2 Al 2 O 3 Fe 2 O 3 Ca. O Mg. O K 2 O Na 2 O SO 3 LOI (H 2 O+CO 2) 19. 0 - 23. 0 - 7. 0 1. 5 - 4. 5 63. 0 - 67. 0 0. 5 - 2. 5 0. 1 - 1. 2 0. 1 - 0. 4 2. 5 - 3. 5 1. 0 - 3. 0 Minor components and traces (deleterious) few %: Mg. O, Sr. O 2 few tenth of a %: P 2 O 5, Ca. F 2 , alkalis traces: heavy metals The composition of different cements, their minimum mechanical properties and their application is regulated by Norm SIA Norm 215. 001/002 (http: //www. vicem. ch/produits/normes/2_7 d. htm) which corresponds to the European Norm ENV 197 (http: //www. readymix-beton. de/service/betontechnische_daten/kap_1_1. pdf) Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
Raw materials Proportioning of raw materials Targets for an ordinary Portland cement (OPC) - Lime saturation factor (LSF) close to 100% - Free lime content under 1. 5 wt% - Silica ratio (SR module) between 2. 0 and 3. 0 - Alumina ratio (AR module) between 1. 0 and 2. 0 - Hydraulic index (IH) ≈ 2. 0 - Low concentration of deleterious components Lime saturation factor The calcium present in the raw materials should be completely bound in the silicate and aluminate phases of the cement clinker. The amount of different oxide components necessary to saturate the amount of lime is given by(in wt%): Ca. O = 2. 8 Si. O 2 + 1. 2 Al 2 O 3 + 0. 65 Fe 2 O 3 Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
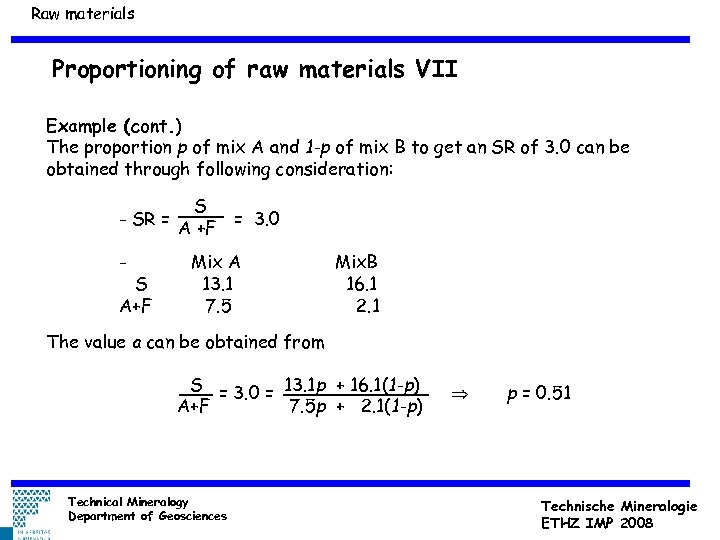
Raw materials Proportioning of raw materials VII Example (cont. ) The proportion p of mix A and 1 -p of mix B to get an SR of 3. 0 can be obtained through following consideration: S - SR = A +F = 3. 0 - S A+F Mix A 13. 1 7. 5 Mix. B 16. 1 2. 1 The value a can be obtained from S = 3. 0 = 13. 1 p + 16. 1(1 -p) A+F 7. 5 p + 2. 1(1 -p) Technical Mineralogy Department of Geosciences p = 0. 51 Technische Mineralogie ETHZ IMP 2008
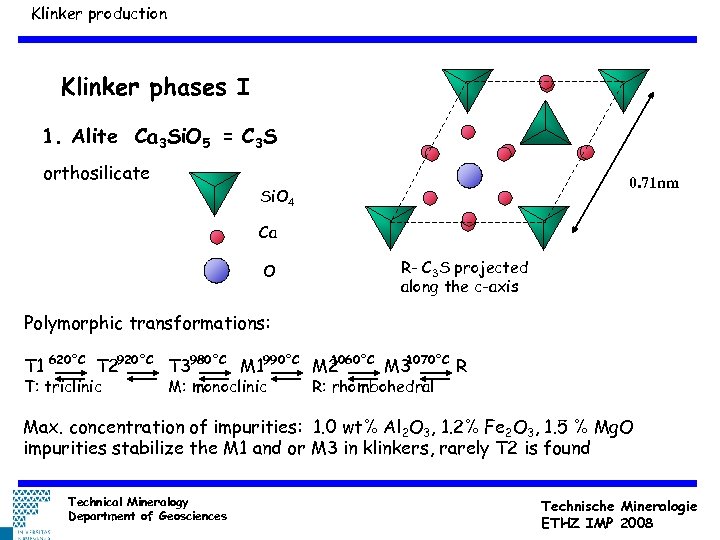
Klinker production Klinker phases I 1. Alite Ca 3 Si. O 5 = C 3 S orthosilicate 0. 71 nm Si. O 4 Ca O R- C 3 S projected along the c-axis Polymorphic transformations: 1060°C 1070°C T 1 620°C T 2920°C T 3980°C M 1990°C M 2 M 3 R T: triclinic M: monoclinic R: rhombohedral Max. concentration of impurities: 1. 0 wt% Al 2 O 3, 1. 2% Fe 2 O 3, 1. 5 % Mg. O impurities stabilize the M 1 and or M 3 in klinkers, rarely T 2 is found Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
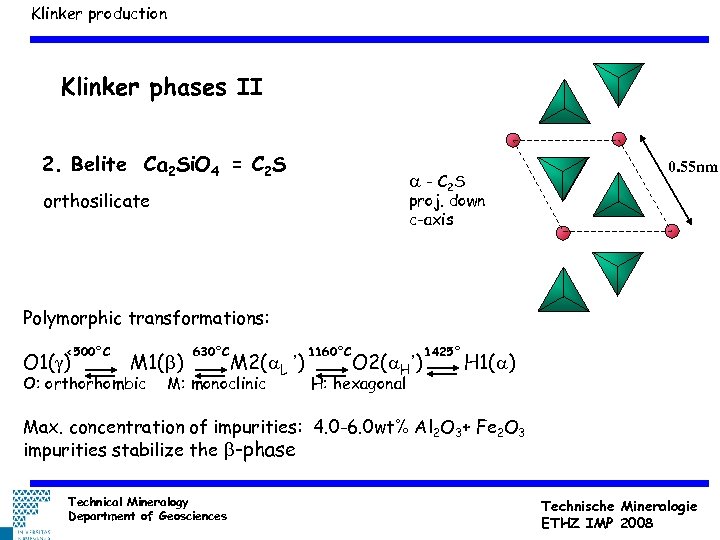
Klinker production Klinker phases II 2. Belite Ca 2 Si. O 4 = C 2 S a - C 2 S orthosilicate 0. 55 nm proj. down c-axis Polymorphic transformations: <500°C O 1(g) M 1(b) O: orthorhombic 630°C M 2(a. L ’) M: monoclinic 1160°C O 2(a. H’) H: hexagonal 1425° H 1(a) Max. concentration of impurities: 4. 0 -6. 0 wt% Al 2 O 3+ Fe 2 O 3 impurities stabilize the b-phase Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
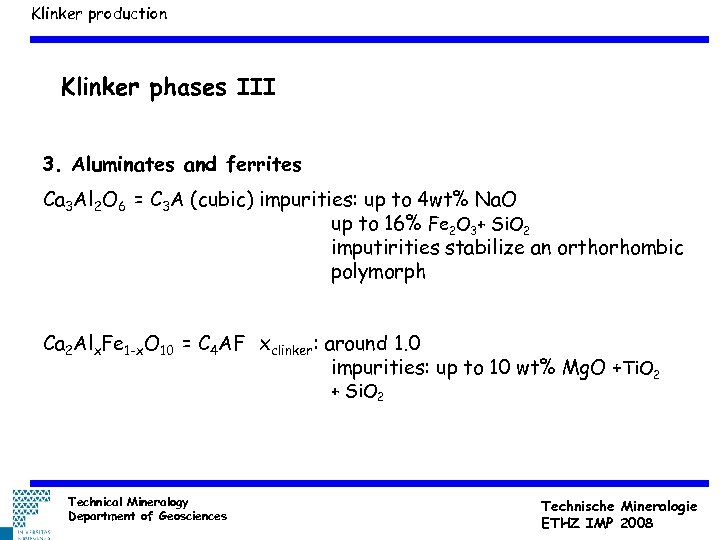
Klinker production Klinker phases III 3. Aluminates and ferrites Ca 3 Al 2 O 6 = C 3 A (cubic) impurities: up to 4 wt% Na. O up to 16% Fe 2 O 3+ Si. O 2 imputirities stabilize an orthorhombic polymorph Ca 2 Alx. Fe 1 -x. O 10 = C 4 AF xclinker: around 1. 0 impurities: up to 10 wt% Mg. O +Ti. O 2 + Si. O 2 Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
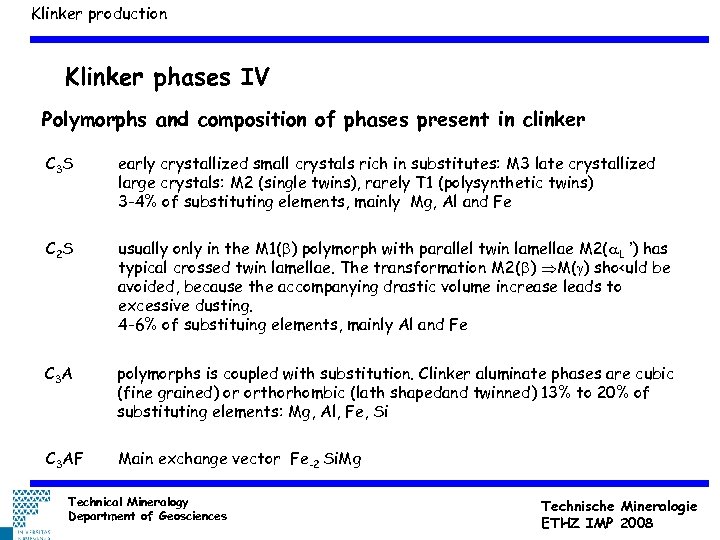
Klinker production Klinker phases IV Polymorphs and composition of phases present in clinker C 3 S early crystallized small crystals rich in substitutes: M 3 late crystallized large crystals: M 2 (single twins), rarely T 1 (polysynthetic twins) 3 -4% of substituting elements, mainly Mg, Al and Fe C 2 S usually only in the M 1(b) polymorph with parallel twin lamellae M 2(a. L ’) has typical crossed twin lamellae. The transformation M 2(b) M(g) sho<uld be avoided, because the accompanying drastic volume increase leads to excessive dusting. 4 -6% of substituing elements, mainly Al and Fe C 3 A polymorphs is coupled with substitution. Clinker aluminate phases are cubic (fine grained) or orthorhombic (lath shapedand twinned) 13% to 20% of substituting elements: Mg, Al, Fe, Si C 3 AF Main exchange vector Fe-2 Si. Mg Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
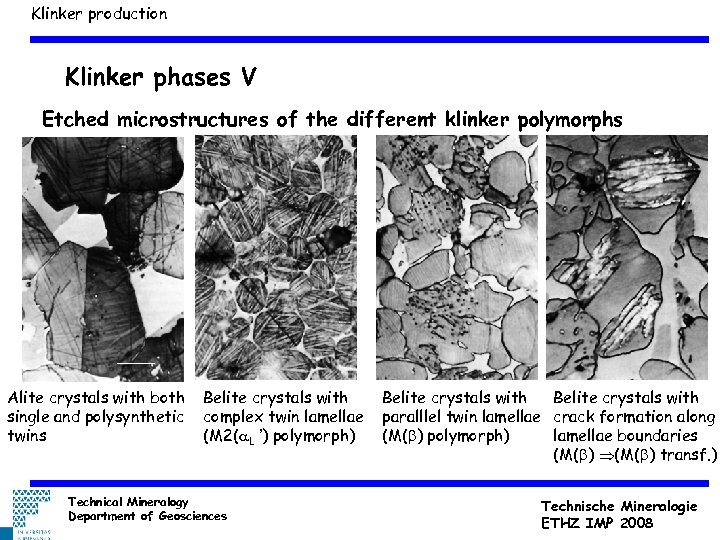
Klinker production Klinker phases V Etched microstructures of the different klinker polymorphs Alite crystals with both single and polysynthetic twins Belite crystals with complex twin lamellae (M 2(a. L ’) polymorph) Technical Mineralogy Department of Geosciences Belite crystals with paralllel twin lamellae crack formation along (M(b) polymorph) lamellae boundaries (M(b) transf. ) Technische Mineralogie ETHZ IMP 2008
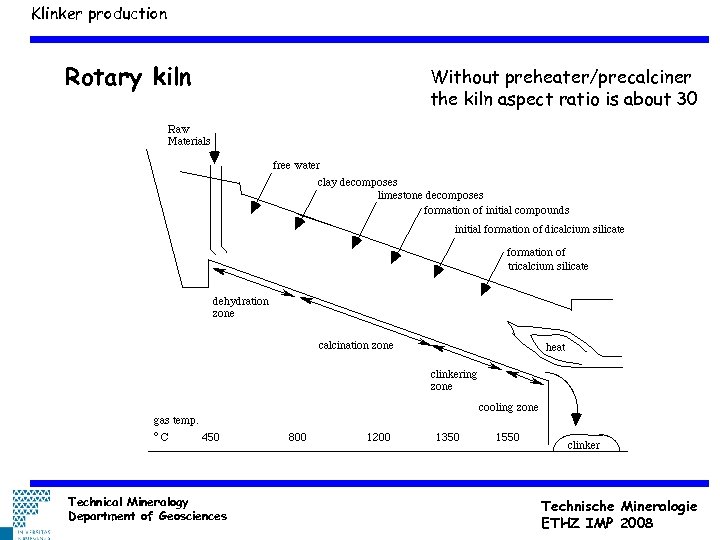
Klinker production Rotary kiln Technical Mineralogy Department of Geosciences Without preheater/precalciner the kiln aspect ratio is about 30 Technische Mineralogie ETHZ IMP 2008
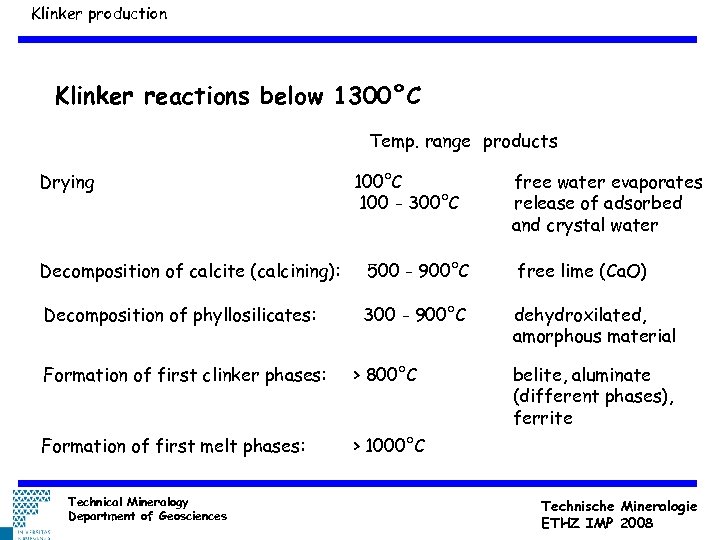
Klinker production Klinker reactions below 1300°C Temp. range products Drying 100°C 100 - 300°C free water evaporates release of adsorbed and crystal water Decomposition of calcite (calcining): 500 - 900°C free lime (Ca. O) Decomposition of phyllosilicates: 300 - 900°C dehydroxilated, amorphous material Formation of first clinker phases: > 800°C Formation of first melt phases: > 1000°C Technical Mineralogy Department of Geosciences belite, aluminate (different phases), ferrite Technische Mineralogie ETHZ IMP 2008
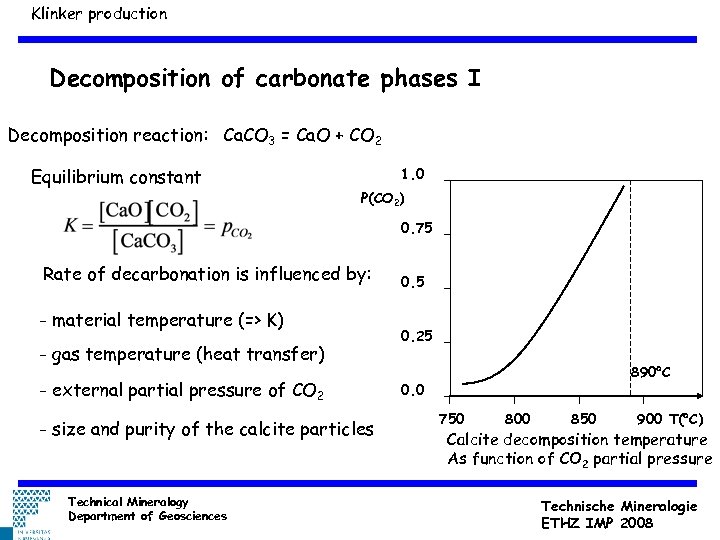
Klinker production Decomposition of carbonate phases I Decomposition reaction: Ca. CO 3 = Ca. O + CO 2 Equilibrium constant 1. 0 P(CO 2) 0. 75 Rate of decarbonation is influenced by: - material temperature (=> K) - gas temperature (heat transfer) - external partial pressure of CO 2 - size and purity of the calcite particles Technical Mineralogy Department of Geosciences 0. 5 0. 25 890 C 0. 0 750 800 850 900 T( C) Calcite decomposition temperature As function of CO 2 partial pressure Technische Mineralogie ETHZ IMP 2008
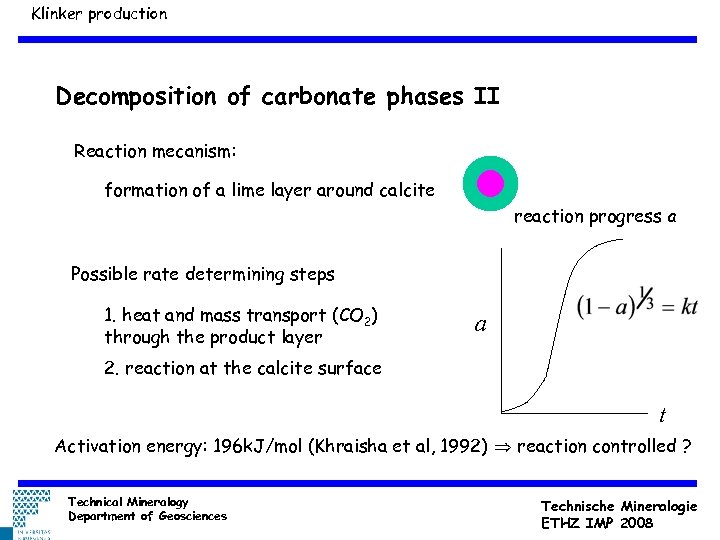
Klinker production Decomposition of carbonate phases II Reaction mecanism: formation of a lime layer around calcite reaction progress a Possible rate determining steps 1. heat and mass transport (CO 2) through the product layer a 2. reaction at the calcite surface t Activation energy: 196 k. J/mol (Khraisha et al, 1992) reaction controlled ? Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
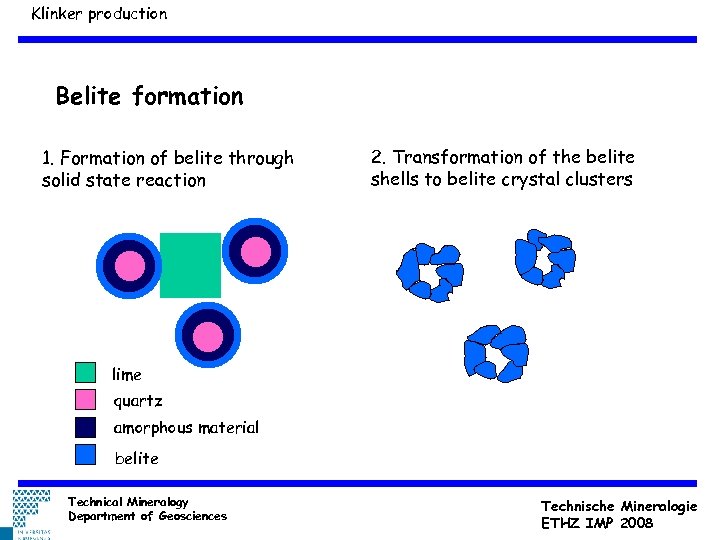
Klinker production Belite formation 1. Formation of belite through solid state reaction 2. Transformation of the belite shells to belite crystal clusters lime quartz amorphous material belite Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
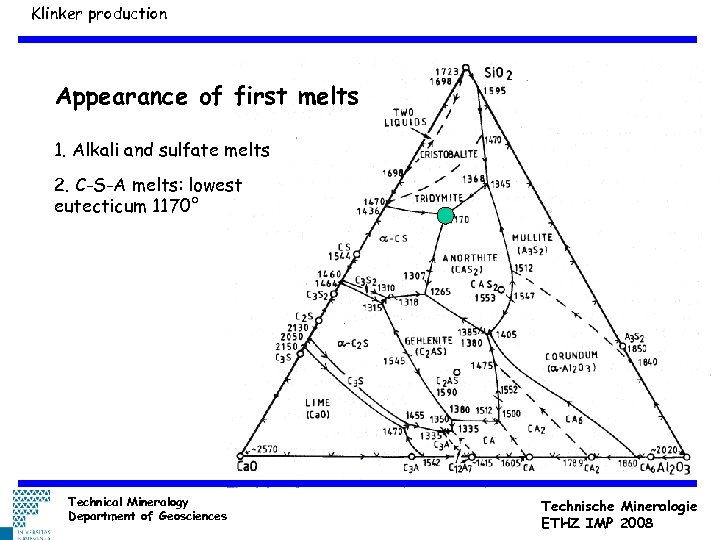
Klinker production Appearance of first melts 1. Alkali and sulfate melts 2. C-S-A melts: lowest eutecticum 1170° Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
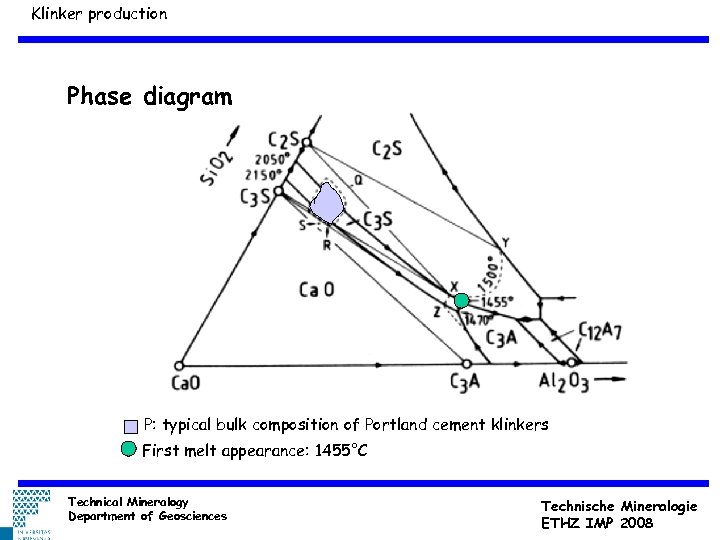
Klinker production Phase diagram P: typical bulk composition of Portland cement klinkers First melt appearance: 1455°C Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
Klinker production Klinker reactions between 1300°C and 1450°C 1. Melting reactions - Melting of ferrite and aluminate phases - Melting of part of the early formed belite 2. Formation of new phases Reaction of melt, free lime, unreacted silica and remaining belite to alite 3. Polymorphic transformation of belite 4. Recrystallization of alite and belite 5. Nodulization (clinkering) Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
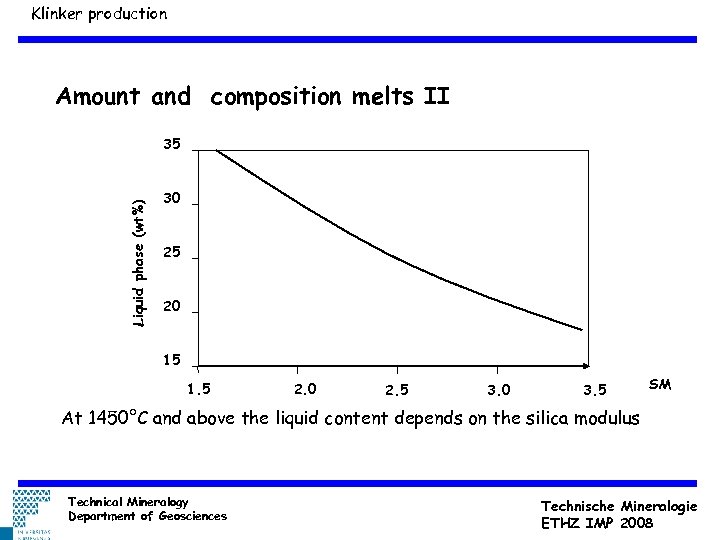
Klinker production Amount and composition melts II Liquid phase (wt%) 35 30 25 20 15 1. 5 2. 0 2. 5 3. 0 3. 5 SM At 1450°C and above the liquid content depends on the silica modulus Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
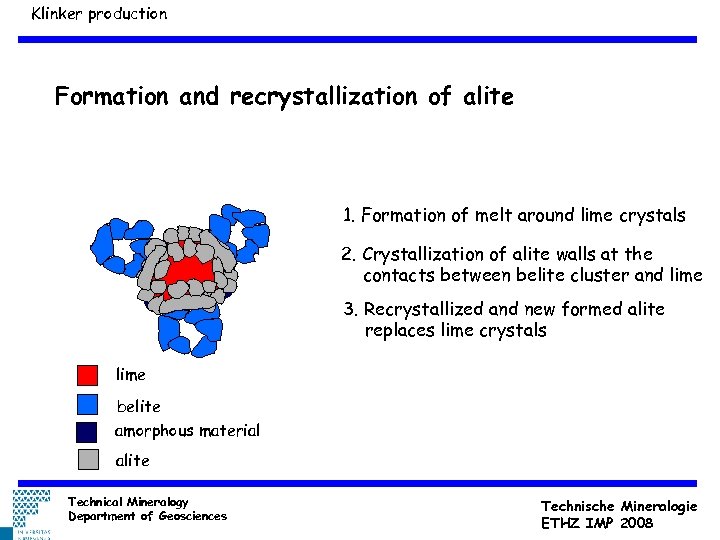
Klinker production Formation and recrystallization of alite 1. Formation of melt around lime crystals 2. Crystallization of alite walls at the contacts between belite cluster and lime 3. Recrystallized and new formed alite replaces lime crystals lime belite amorphous material alite Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
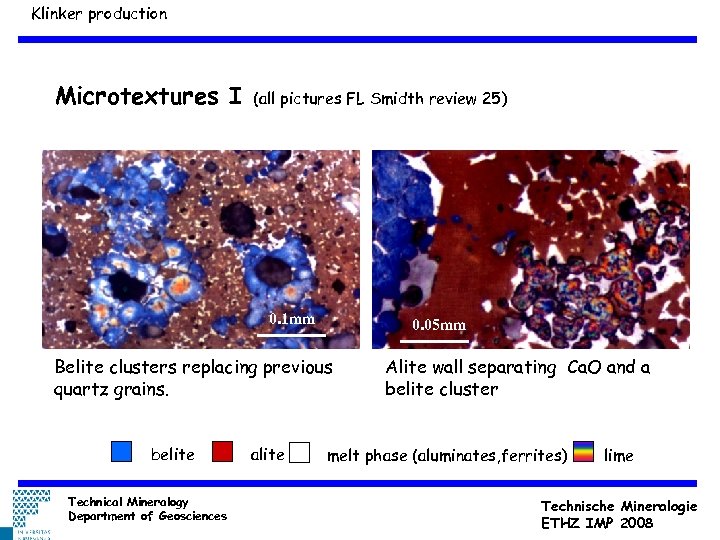
Klinker production Microtextures I (all pictures FL Smidth review 25) 0. 1 mm 0. 05 mm Belite clusters replacing previous quartz grains. belite Technical Mineralogy Department of Geosciences alite Alite wall separating Ca. O and a belite cluster melt phase (aluminates, ferrites) lime Technische Mineralogie ETHZ IMP 2008
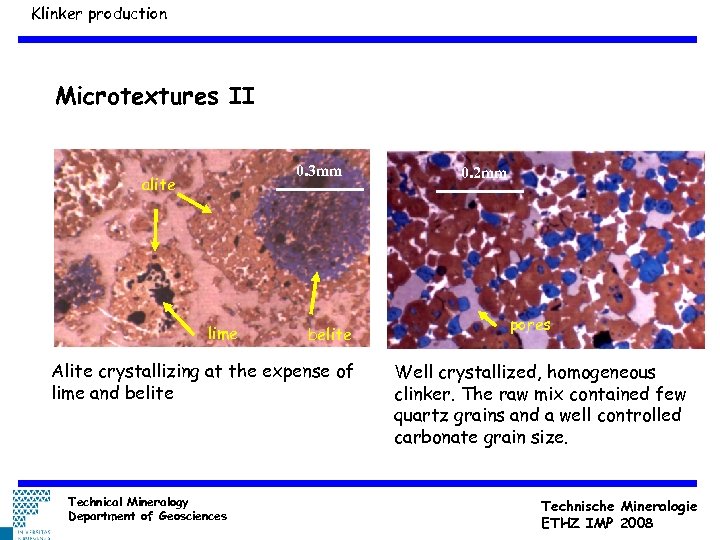
Klinker production Microtextures II 0. 3 mm alite lime belite Alite crystallizing at the expense of lime and belite Technical Mineralogy Department of Geosciences 0. 2 mm pores Well crystallized, homogeneous clinker. The raw mix contained few quartz grains and a well controlled carbonate grain size. Technische Mineralogie ETHZ IMP 2008
Klinker production Klinker reactions during cooling 1. Crystallization of the restitic melt. Products: aluminates (C 3 A) and ferrites (C 4 AF) 2. Polymorphic transformations of alite and belite If cooling is too slow 3. Backreaction of alite to belite + lime 4. Recrystallization aluminates and ferrites Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
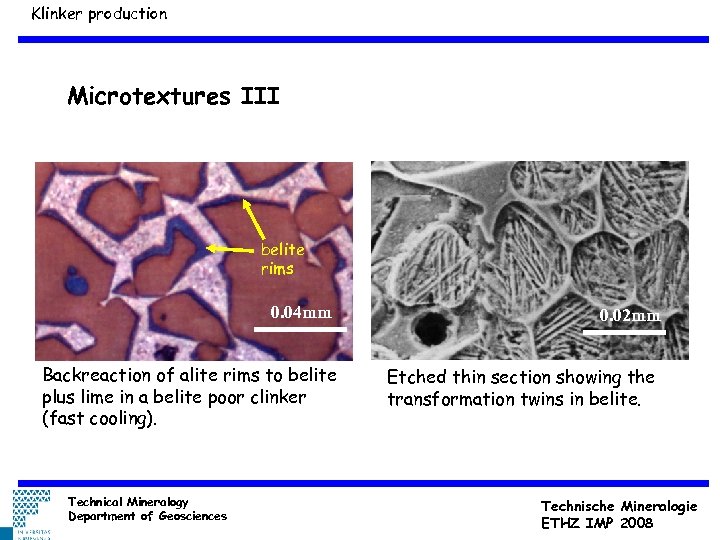
Klinker production Microtextures III belite rims 0. 04 mm Backreaction of alite rims to belite plus lime in a belite poor clinker (fast cooling). Technical Mineralogy Department of Geosciences 0. 02 mm Etched thin section showing the transformation twins in belite. Technische Mineralogie ETHZ IMP 2008
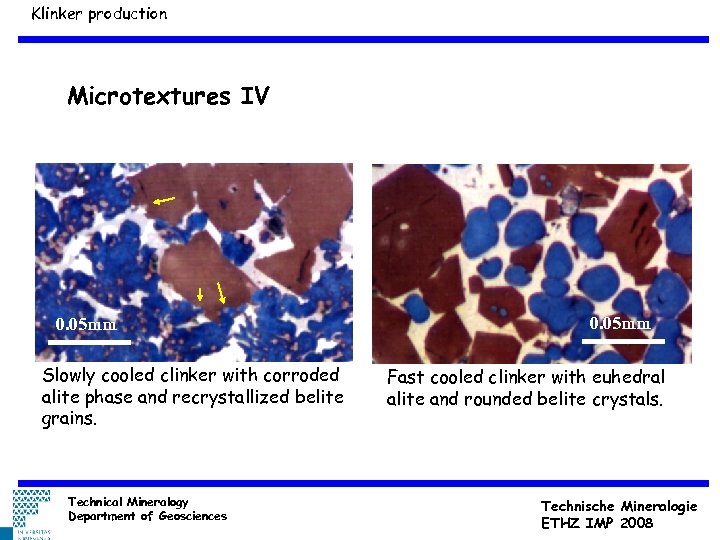
Klinker production Microtextures IV 0. 05 mm Slowly cooled clinker with corroded alite phase and recrystallized belite grains. Technical Mineralogy Department of Geosciences 0. 05 mm Fast cooled clinker with euhedral alite and rounded belite crystals. Technische Mineralogie ETHZ IMP 2008
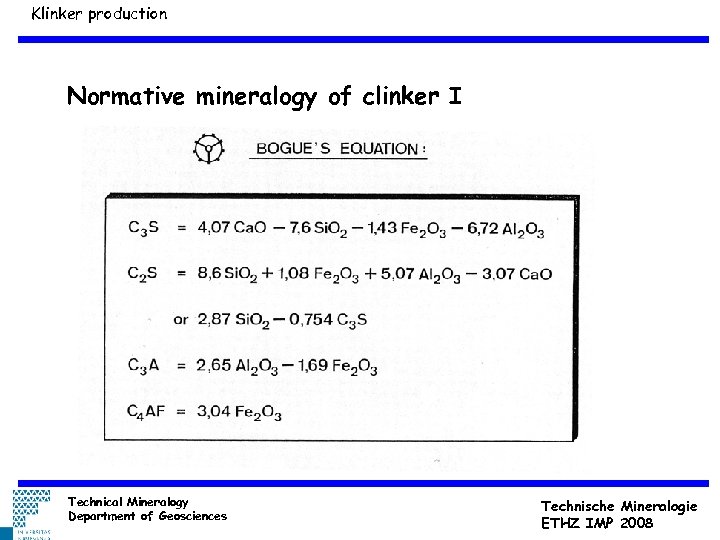
Klinker production Normative mineralogy of clinker I Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
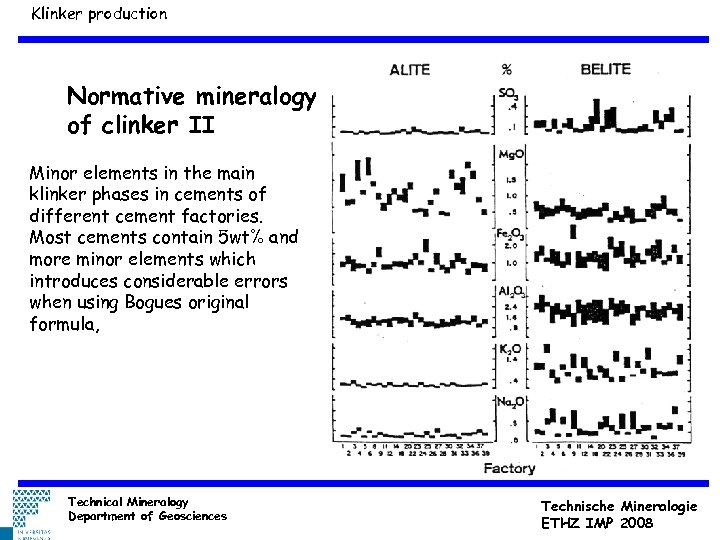
Klinker production Normative mineralogy of clinker II Minor elements in the main klinker phases in cements of different cement factories. Most cements contain 5 wt% and more minor elements which introduces considerable errors when using Bogues original formula, Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
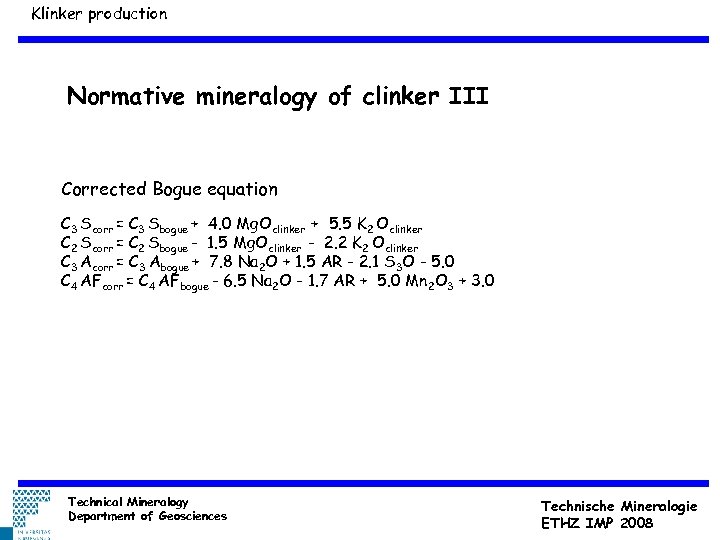
Klinker production Normative mineralogy of clinker III Corrected Bogue equation C 3 Scorr = C 3 Sbogue + 4. 0 Mg. Oclinker + 5. 5 K 2 Oclinker C 2 Scorr = C 2 Sbogue - 1. 5 Mg. Oclinker - 2. 2 K 2 Oclinker C 3 Acorr = C 3 Abogue + 7. 8 Na 2 O + 1. 5 AR - 2. 1 S 3 O - 5. 0 C 4 AFcorr = C 4 AFbogue - 6. 5 Na 2 O - 1. 7 AR + 5. 0 Mn 2 O 3 + 3. 0 0. 05 mm Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
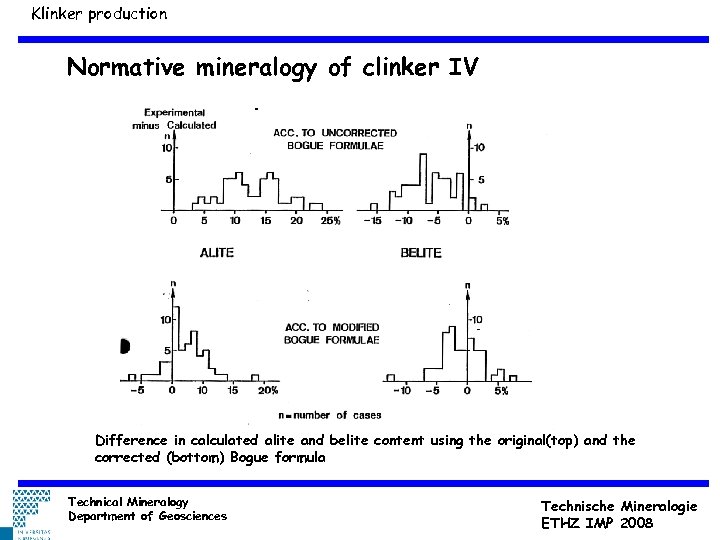
Klinker production Normative mineralogy of clinker IV 0. 05 mm Difference in calculated alite and belite content using the original(top) and the corrected (bottom) Bogue formula Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
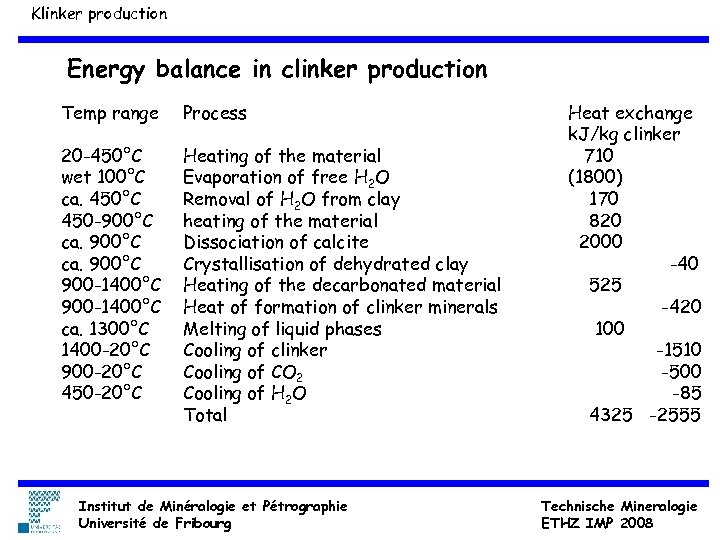
Klinker production Energy balance in clinker production Temp range Process 20 -450°C wet 100°C ca. 450°C 450 -900°C ca. 900°C 900 -1400°C ca. 1300°C 1400 -20°C 900 -20°C 450 -20°C Heating of the material Evaporation of free H 2 O Removal of H 2 O from clay heating of the material Dissociation of calcite Crystallisation of dehydrated clay Heating of the decarbonated material Heat of formation of clinker minerals Melting of liquid phases Cooling of clinker Cooling of CO 2 Cooling of H 2 O Total Institut de Minéralogie et Pétrographie Université de Fribourg Heat exchange k. J/kg clinker 710 (1800) 170 820 2000 -40 525 -420 100 -1510 -500 -85 4325 -2555 Technische Mineralogie ETHZ IMP 2008
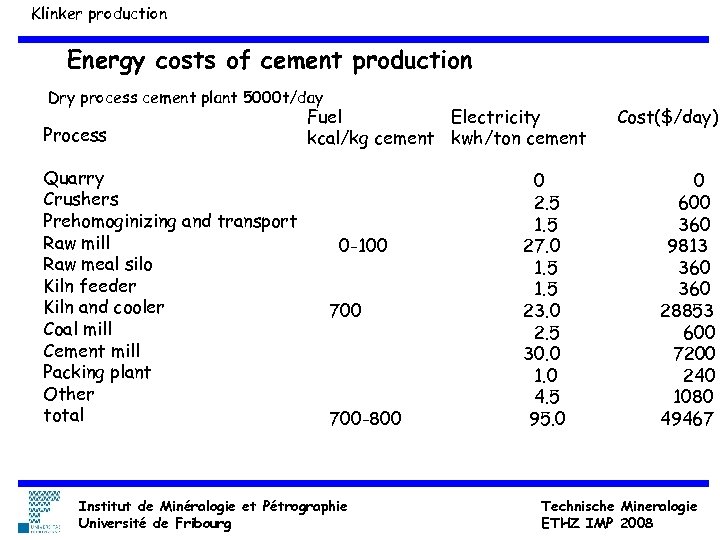
Klinker production Energy costs of cement production Dry process cement plant 5000 t/day Process Quarry Crushers Prehomoginizing and transport Raw mill Raw meal silo Kiln feeder Kiln and cooler Coal mill Cement mill Packing plant Other total Fuel Electricity kcal/kg cement kwh/ton cement 0 -100 700 -800 Institut de Minéralogie et Pétrographie Université de Fribourg 0 2. 5 1. 5 27. 0 1. 5 23. 0 2. 5 30. 0 1. 0 4. 5 95. 0 Cost($/day) 0 600 360 9813 360 28853 600 7200 240 1080 49467 Technische Mineralogie ETHZ IMP 2008
Mineralized cement Improvements in klinker manufacturing 1. Energy savings through: - lowering the melting point of the system. - increasing the burning rate - better insulation, improved heat exchanger etc. - use of alternative raw materials 2. Reduction of CO 2 , SO 3 NOx etc output through: - use of alternative raw materials Institut de Minéralogie et Pétrographie Université de Fribourg Technische Mineralogie ETHZ IMP 2008
Mineralized cement Improvements in klinker manufacturing 1. Energy savings through: - lowering the melting point of the system. - increasing the burning rate - better insulation, improved heat exchanger etc. - use of alternative raw materials 2. Reduction of CO 2 , SO 3 NOx etc output through: - use of alternative raw materials Institut de Minéralogie et Pétrographie Université de Fribourg Cours bloc 2006
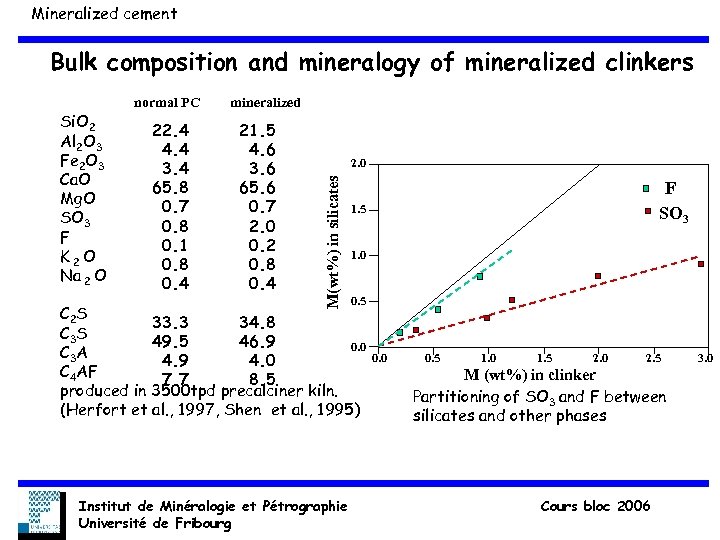
Mineralized cement Bulk composition and mineralogy of mineralized clinkers 22. 4 4. 4 3. 4 65. 8 0. 7 0. 8 0. 1 0. 8 0. 4 mineralized 21. 5 4. 6 3. 6 65. 6 0. 7 2. 0 0. 2 0. 8 0. 4 2. 0 M(wt%) in silicates Si. O 2 Al 2 O 3 Fe 2 O 3 Ca. O Mg. O SO 3 F K 2 O Na 2 O normal PC 1. 5 1. 0 0. 5 C 2 S 33. 3 34. 8 C 3 S 49. 5 46. 9 0. 0 C 3 A 4. 9 4. 0 C 4 AF 7. 7 8. 5 produced in 3500 tpd precalciner kiln. (Herfort et al. , 1997, Shen et al. , 1995) Institut de Minéralogie et Pétrographie Université de Fribourg F SO 3 0. 0 0. 5 1. 0 1. 5 2. 0 2. 5 M (wt%) in clinker Partitioning of SO 3 and F between silicates and other phases Cours bloc 2006 3. 0
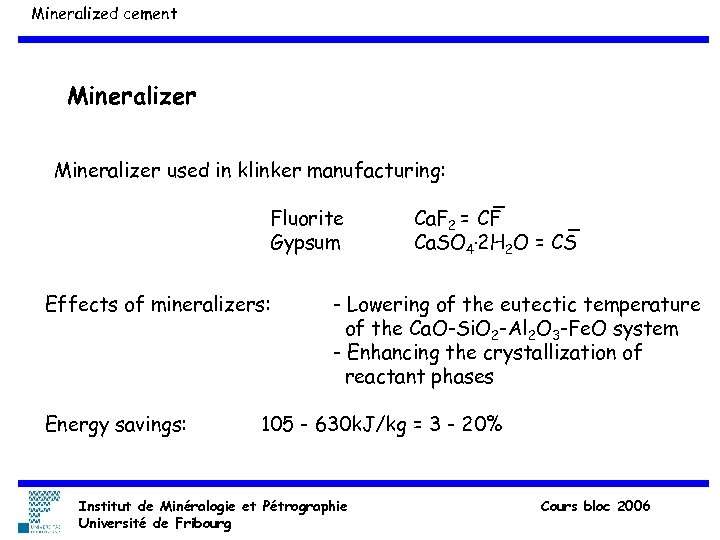
Mineralized cement Mineralizer used in klinker manufacturing: Fluorite Gypsum Effects of mineralizers: Energy savings: Ca. F 2 = CF Ca. SO 4. 2 H 2 O = CS - Lowering of the eutectic temperature of the Ca. O-Si. O 2 -Al 2 O 3 -Fe. O system - Enhancing the crystallization of reactant phases 105 - 630 k. J/kg = 3 - 20% Institut de Minéralogie et Pétrographie Université de Fribourg Cours bloc 2006
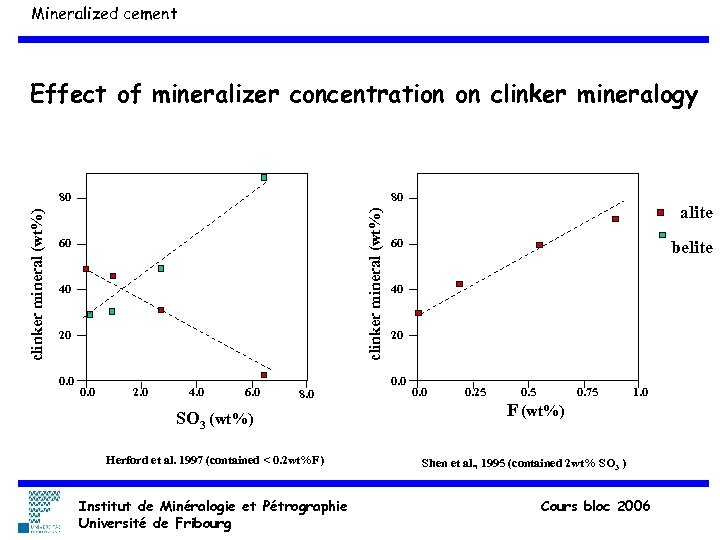
Mineralized cement Effect of mineralizer concentration on clinker mineralogy 80 clinker mineral (wt%) 80 60 40 20 0. 0 2. 0 4. 0 60 SO 3 (wt%) Herford et al. 1997 (contained < 0. 2 wt%F) Institut de Minéralogie et Pétrographie Université de Fribourg belite 40 20 0. 0 8. 0 alite 0. 0 0. 25 0. 75 1. 0 F (wt%) Shen et al. , 1995 (contained 2 wt% SO 3 ) Cours bloc 2006
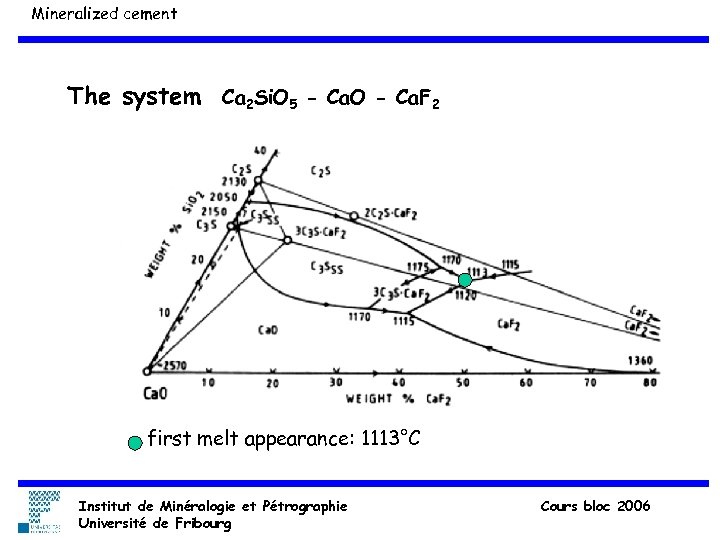
Mineralized cement The system Ca 2 Si. O 5 - Ca. O - Ca. F 2 first melt appearance: 1113°C Institut de Minéralogie et Pétrographie Université de Fribourg Cours bloc 2006
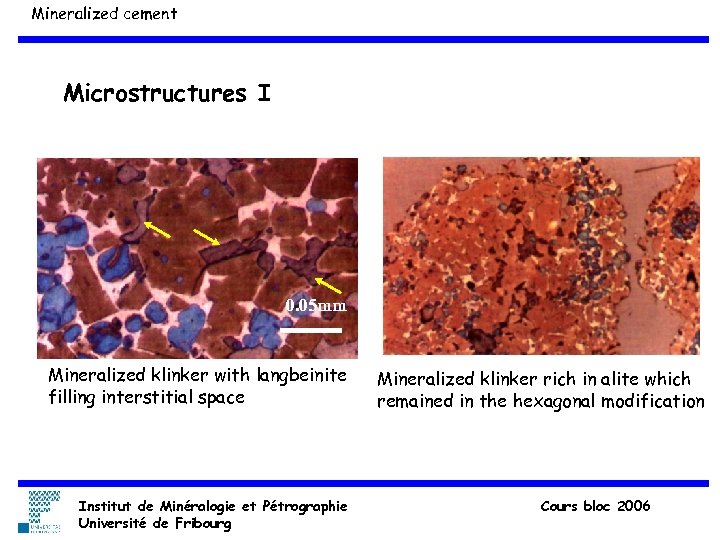
Mineralized cement Microstructures I 0. 05 mm Mineralized klinker with langbeinite filling interstitial space Institut de Minéralogie et Pétrographie Université de Fribourg Mineralized klinker rich in alite which remained in the hexagonal modification Cours bloc 2006
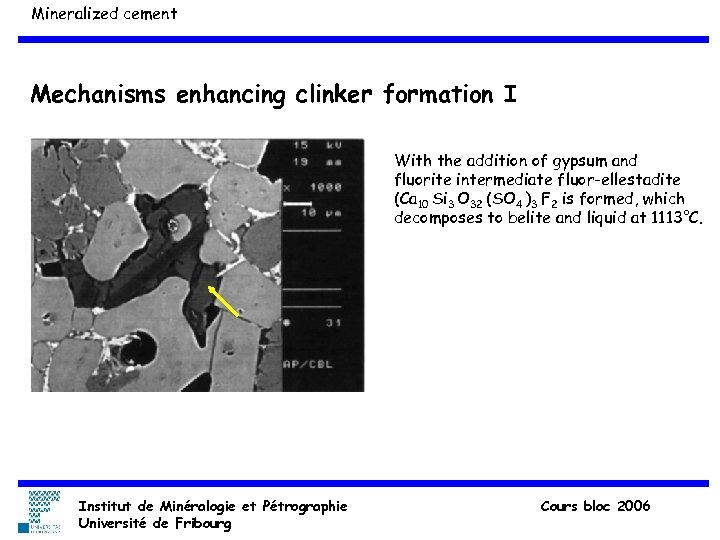
Mineralized cement Mechanisms enhancing clinker formation I With the addition of gypsum and fluorite intermediate fluor-ellestadite (Ca 10 Si 3 O 32 (SO 4 )3 F 2 is formed, which decomposes to belite and liquid at 1113°C. Institut de Minéralogie et Pétrographie Université de Fribourg Cours bloc 2006
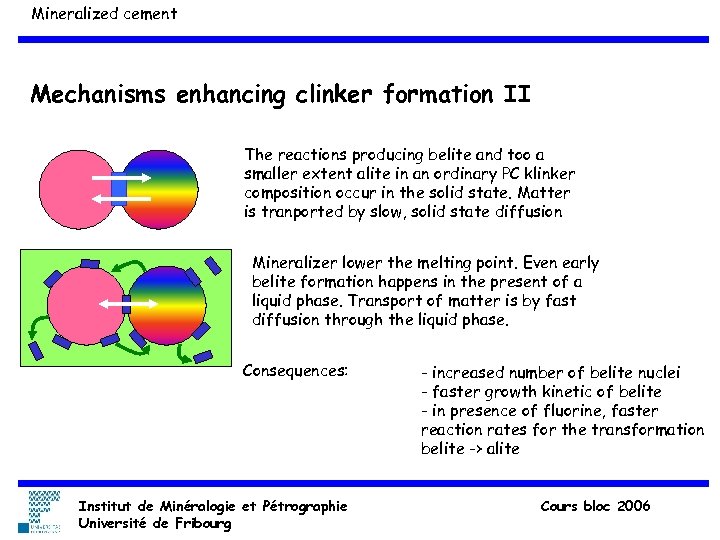
Mineralized cement Mechanisms enhancing clinker formation II The reactions producing belite and too a smaller extent alite in an ordinary PC klinker composition occur in the solid state. Matter is tranported by slow, solid state diffusion Mineralizer lower the melting point. Even early belite formation happens in the present of a liquid phase. Transport of matter is by fast diffusion through the liquid phase. Consequences: Institut de Minéralogie et Pétrographie Université de Fribourg - increased number of belite nuclei - faster growth kinetic of belite - in presence of fluorine, faster reaction rates for the transformation belite -> alite Cours bloc 2006
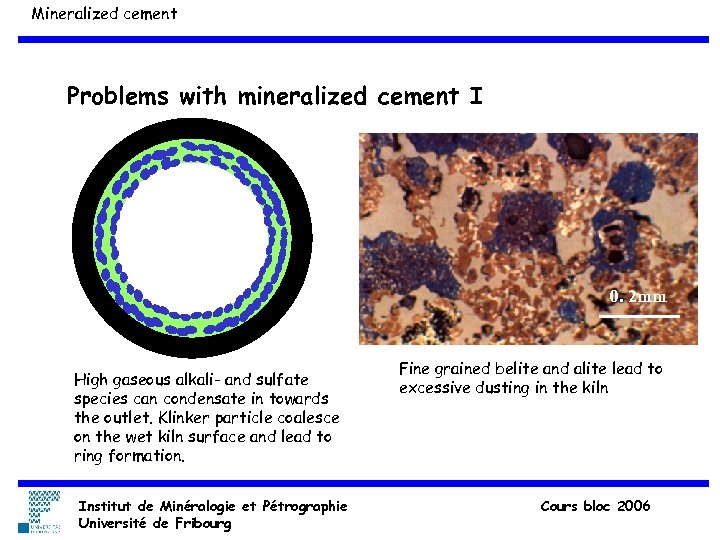
Mineralized cement Problems with mineralized cement I 0. 2 mm High gaseous alkali- and sulfate species can condensate in towards the outlet. Klinker particle coalesce on the wet kiln surface and lead to ring formation. Institut de Minéralogie et Pétrographie Université de Fribourg Fine grained belite and alite lead to excessive dusting in the kiln Cours bloc 2006
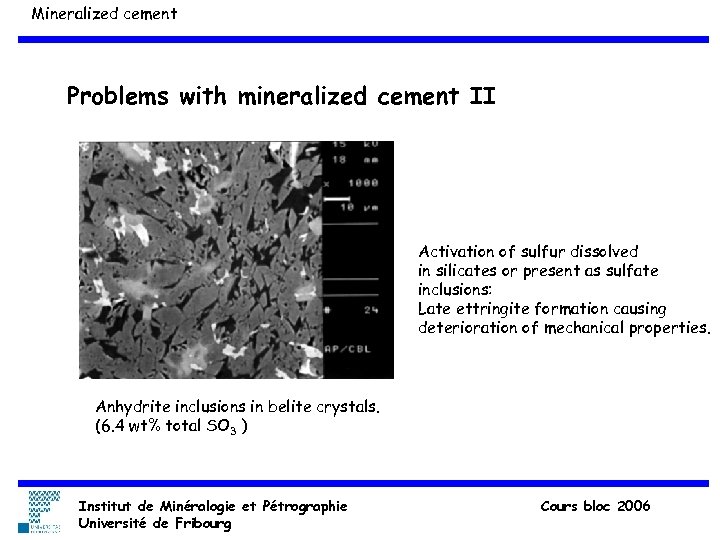
Mineralized cement Problems with mineralized cement II Activation of sulfur dissolved in silicates or present as sulfate inclusions: Late ettringite formation causing deterioration of mechanical properties. Anhydrite inclusions in belite crystals. (6. 4 wt% total SO 3 ) Institut de Minéralogie et Pétrographie Université de Fribourg Cours bloc 2006
Mineralized cement Pro and cons of mineralized klinker Pro: - lowering of burning temperature - increase of alite content - formation of the rhombohedral, hydraulic more active polymorph of alite - stabilization of the hydraulic more active a phase of belite Cons: - Ring formation and excessive dusting in the kiln - with too low fluorine content: increase in belite content - Presence of phases deletrious to mechanical properties Institut de Minéralogie et Pétrographie Université de Fribourg Cours bloc 2006
Rapid burning Consequences of steep temperature ramps: - Decomposition and new phase formation occur simultaneously - New phases are formed through metastable reactions having larger reaction free energies - Decomposition products are much smaller and have a higher surface activity Institut de Minéralogie et Pétrographie Université de Fribourg Cours bloc 2006
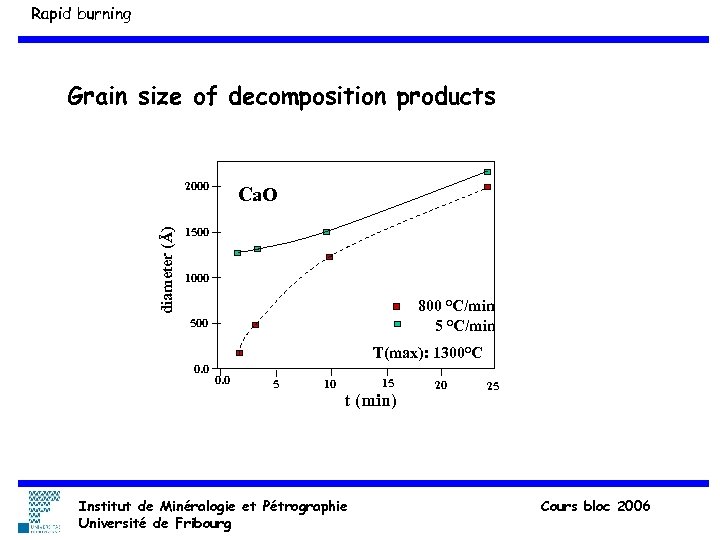
Rapid burning Grain size of decomposition products diameter (Å) 2000 Ca. O 1500 1000 800 °C/min 500 T(max): 1300°C 0. 0 5 10 15 t (min) Institut de Minéralogie et Pétrographie Université de Fribourg 20 25 Cours bloc 2006
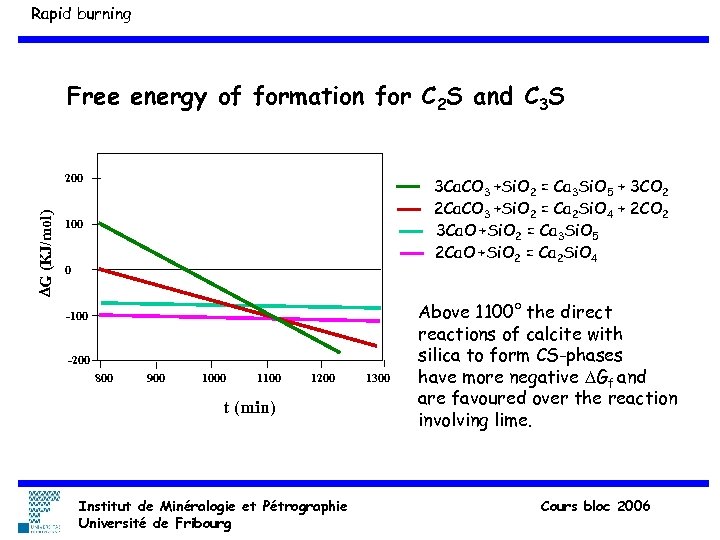
Rapid burning Free energy of formation for C 2 S and C 3 S DG (KJ/mol) 200 3 Ca. CO 3 +Si. O 2 = Ca 3 Si. O 5 + 3 CO 2 2 Ca. CO 3 +Si. O 2 = Ca 2 Si. O 4 + 2 CO 2 3 Ca. O +Si. O 2 = Ca 3 Si. O 5 2 Ca. O +Si. O 2 = Ca 2 Si. O 4 100 0 -100 -200 800 900 1000 1100 1200 t (min) Institut de Minéralogie et Pétrographie Université de Fribourg 1300 Above 1100° the direct reactions of calcite with silica to form CS-phases have more negative DGf and are favoured over the reaction involving lime. Cours bloc 2006
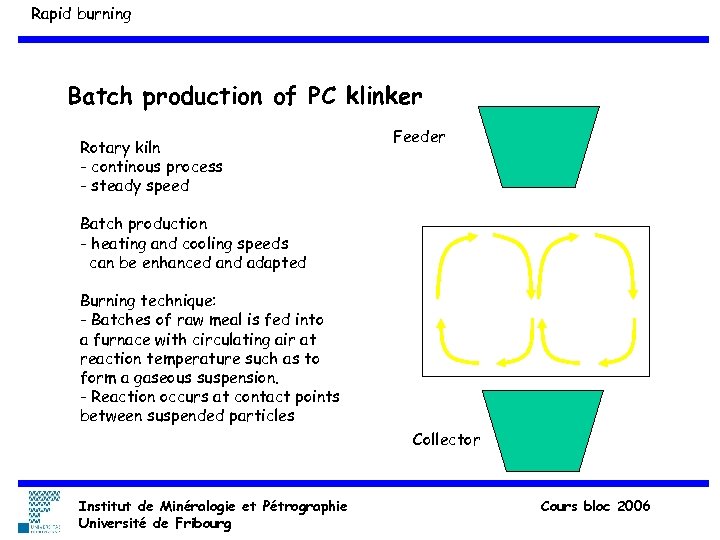
Rapid burning Batch production of PC klinker Rotary kiln - continous process - steady speed Feeder Batch production - heating and cooling speeds can be enhanced and adapted Burning technique: - Batches of raw meal is fed into a furnace with circulating air at reaction temperature such as to form a gaseous suspension. - Reaction occurs at contact points between suspended particles Collector Institut de Minéralogie et Pétrographie Université de Fribourg Cours bloc 2006
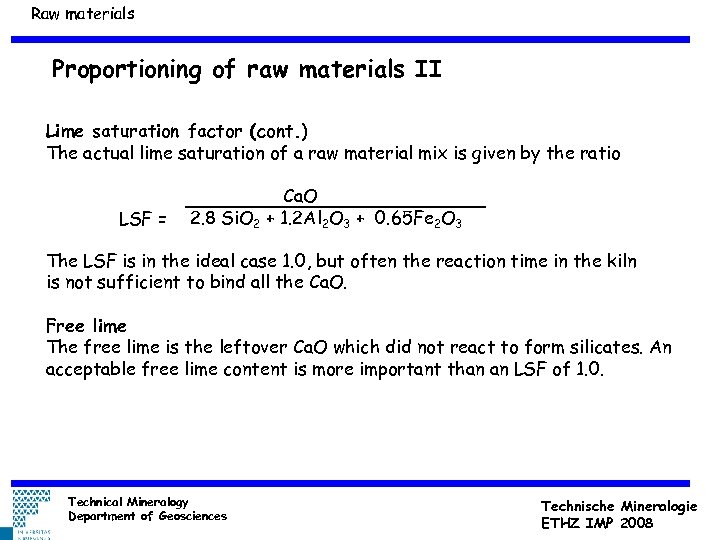
Raw materials Proportioning of raw materials II Lime saturation factor (cont. ) The actual lime saturation of a raw material mix is given by the ratio LSF = Ca. O 2. 8 Si. O 2 + 1. 2 Al 2 O 3 + 0. 65 Fe 2 O 3 The LSF is in the ideal case 1. 0, but often the reaction time in the kiln is not sufficient to bind all the Ca. O. Free lime The free lime is the leftover Ca. O which did not react to form silicates. An acceptable free lime content is more important than an LSF of 1. 0. Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
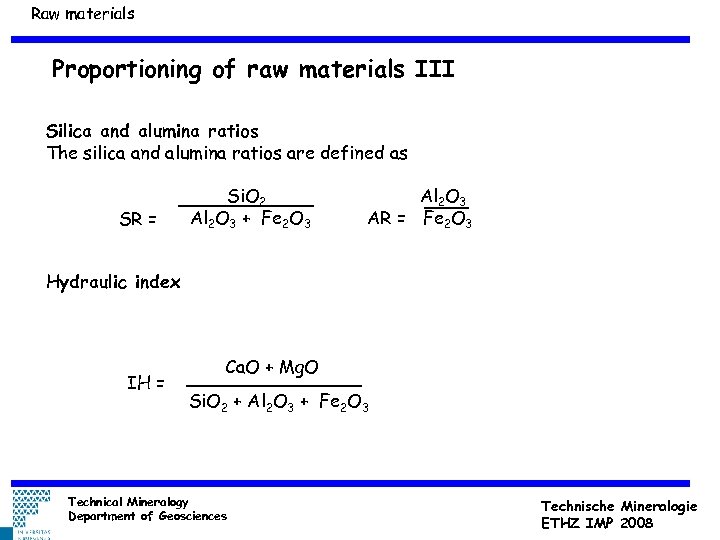
Raw materials Proportioning of raw materials III Silica and alumina ratios The silica and alumina ratios are defined as SR = Si. O 2 Al 2 O 3 + Fe 2 O 3 Al 2 O 3 AR = Fe 2 O 3 Hydraulic index IH = Ca. O + Mg. O Si. O 2 + Al 2 O 3 + Fe 2 O 3 Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
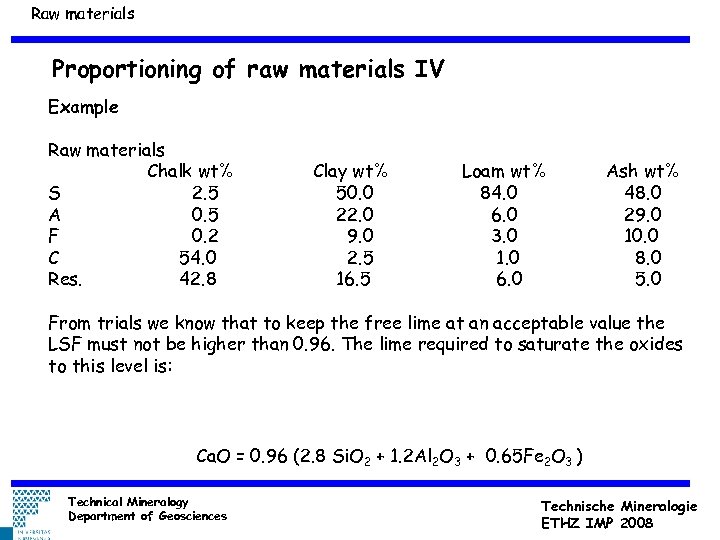
Raw materials Proportioning of raw materials IV Example Raw materials Chalk wt% S 2. 5 A 0. 5 F 0. 2 C 54. 0 Res. 42. 8 Clay wt% 50. 0 22. 0 9. 0 2. 5 16. 5 Loam wt% 84. 0 6. 0 3. 0 1. 0 6. 0 Ash wt% 48. 0 29. 0 10. 0 8. 0 5. 0 From trials we know that to keep the free lime at an acceptable value the LSF must not be higher than 0. 96. The lime required to saturate the oxides to this level is: Ca. O = 0. 96 (2. 8 Si. O 2 + 1. 2 Al 2 O 3 + 0. 65 Fe 2 O 3 ) Technical Mineralogy Department of Geosciences Technische Mineralogie ETHZ IMP 2008
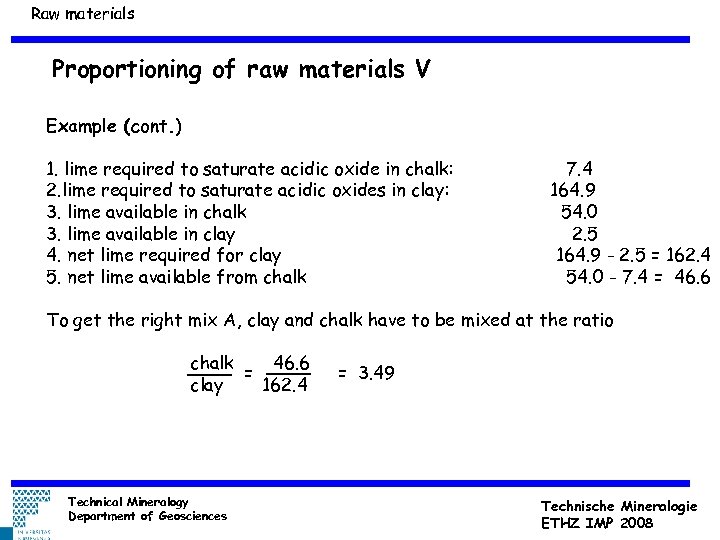
Raw materials Proportioning of raw materials V Example (cont. ) 1. lime required to saturate acidic oxide in chalk: 2. lime required to saturate acidic oxides in clay: 3. lime available in chalk 3. lime available in clay 4. net lime required for clay 5. net lime available from chalk 7. 4 164. 9 54. 0 2. 5 164. 9 - 2. 5 = 162. 4 54. 0 - 7. 4 = 46. 6 To get the right mix A, clay and chalk have to be mixed at the ratio chalk 46. 6 = clay 162. 4 Technical Mineralogy Department of Geosciences = 3. 49 Technische Mineralogie ETHZ IMP 2008
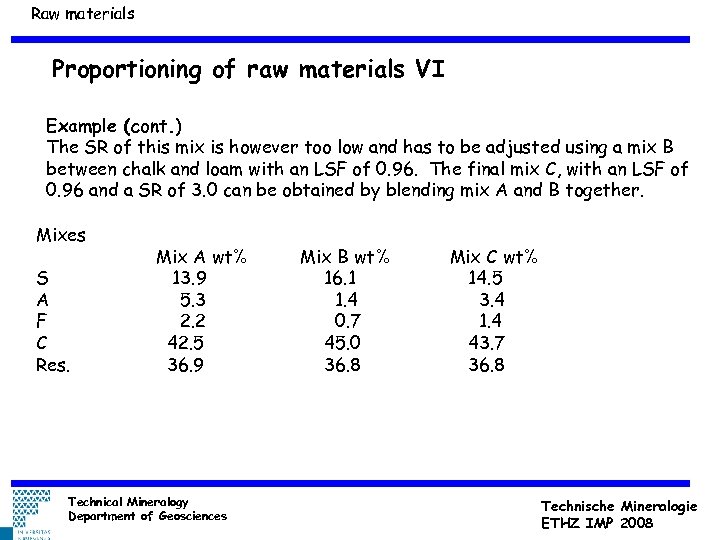
Raw materials Proportioning of raw materials VI Example (cont. ) The SR of this mix is however too low and has to be adjusted using a mix B between chalk and loam with an LSF of 0. 96. The final mix C, with an LSF of 0. 96 and a SR of 3. 0 can be obtained by blending mix A and B together. Mixes S A F C Res. Mix A wt% 13. 9 5. 3 2. 2 42. 5 36. 9 Technical Mineralogy Department of Geosciences Mix B wt% 16. 1 1. 4 0. 7 45. 0 36. 8 Mix C wt% 14. 5 3. 4 1. 4 43. 7 36. 8 Technische Mineralogie ETHZ IMP 2008
1d03d8a48de2feb43c6897aba6afb2dd.ppt