3d2b27b142253cb86006b48c2622e1cd.ppt
- Количество слайдов: 41

Prof. Dr. Enas Abdel Mageed Daef Professor Of Microbiology &Immunolog Director of Medical Research Center Faculty Of Medicine –Assiut University Email: Deafenas@yahoo. com Phone: 20 -1223971411 -01028820050 Webpage: www. aun. edu. eg/arabic/membercv. php? M_ID=1761 H index: http: //scholar. google. com. eg/citations? user=KM 5 c. M 7 k. AAAAJ&hl=ar ORCID: http: //orcid. org 0000 -0003 -1858 -7033

ﺑﺴﻢ ﺍﻟﻠﻪ ﺍﻟﺮﺣﻤﻦ ﺍﻟﺮﺣﻴﻢ ﺟﺎﻣﻌﻪ ﺃﺴﻴﻮﻁ 2
ACCESS MAP

Ongoing Research Support • 1 - Higher Education Council Fund (Center Of Medical Research Excellence Faculty of Medicine- Assiut University. • Applicant: Assiut University, Faculty of Medicine. • Code: CEPI_014_ASSU. • Duration: Two calendar Years starting from the date of Approval (1 -1 -2014). • Application Date: 16 -12 -2013. • Role: P. I • 2 -SCIENCE AND TECHNOLOGY DEVELOPMENT FUND (STDF) CAPACITY BUILDING GRANT • Molecular Virology Laboratory • Viral Hepatitis Research Unit (VHRU) • Liver Centre-Faculty of Medicine -Assiut University • Applicant: Assiut University, Faculty of Medicine. • Category A: Instrument Acquisition. • Duration: One calendar Year starting from the date of Approval. • Application Date: 31 -03 -2010.

The mission of the Medical Research Center • To provide access to the state-of-the art research equipments and core facilities to the basic and clinical medical researchers at the Assiut University.

The main goals of this center are : • To encourage Medical Research • To provide Academic and technical support to researchers. • To facilitate technical obstacles. • To Support the training of young researchers

• International experts, who are all working in the best research institutes in the world, have agreed to support the center, and facilitate collaborative research projects in the participating centers

• Several projects are already conducted in collaboration between scientists from several institutes in Assiut University and extended to other research institutes within Egypt and worldwide. • I-Memorandum of understanding of cooperation Agreement between the Medical Research Center and the Center of Excellence of Stem Cell research and Regenerative Medicine -Zewail City of science and Technology, Egypt

• II-Collaboration between the Medical Research Center-Faculty of Medicine-Assiut University &Dr. Mohamed Tarek Shata(Associate Prof. Viral Immunology Laboratory, Department of Internal Medicine. Division of Digestive Diseases- University of Cincinnati Medical Center).

Medical Research Center Units: • • Flow Cytometry Unit. Histopathology Unit Serology Unit Molecular Biology Unit Tissue and Cell Culture Unit Biorepository center&Cold Room Surveillance unit Animal House Unit
Flow cytometry core : Cellular analysis core:

Microscopy core and Histopathology core • Fluorescent microscopy services • Specialized immunohistochemistry services; • Histopathology core – fixation and processing of specimens for Frozen, paraffin and plastic embedded sectioning
Histopathology Unit • Microtome Leica (rm 2245)GERMANY

Fluorescent Microscope Olympus, U-LH 100 HG, Tokyo-Company: Optoscient • • Slide Moat

Serology unit ELISA reader and ELISA washer (Bioteck) New generation double beam UV-Visible spectrophotometer

• We have a well equipped Molecular Virology Lab. in the Medical Research Center –Faculty Of Medicine -Assiut University
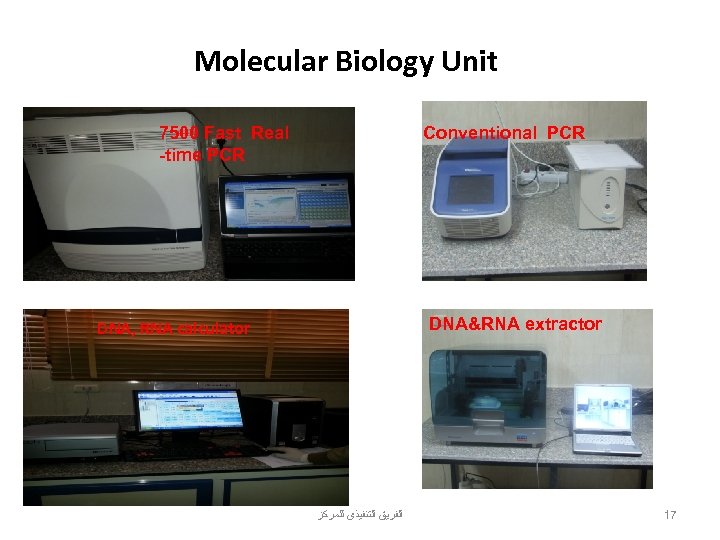
Molecular Biology Unit 7500 Fast Real -time PCR Conventional PCR DNA&RNA extractor DNA, RNA calculator DNA calculating(nanodrop) ﺍﻟﻔﺮﻳﻖ ﺍﻟﺘﻨﻔﻴﺬﻯ ﻟﻠﻤﺮﻛﺰ 17

Vertical electrophoresis Horizontal electrophoresis Documentation system Deep. Freez -80 °C ﺍﻟﻔﺮﻳﻖ ﺍﻟﺘﻨﻔﻴﺬﻯ ﻟﻠﻤﺮﻛﺰ 18
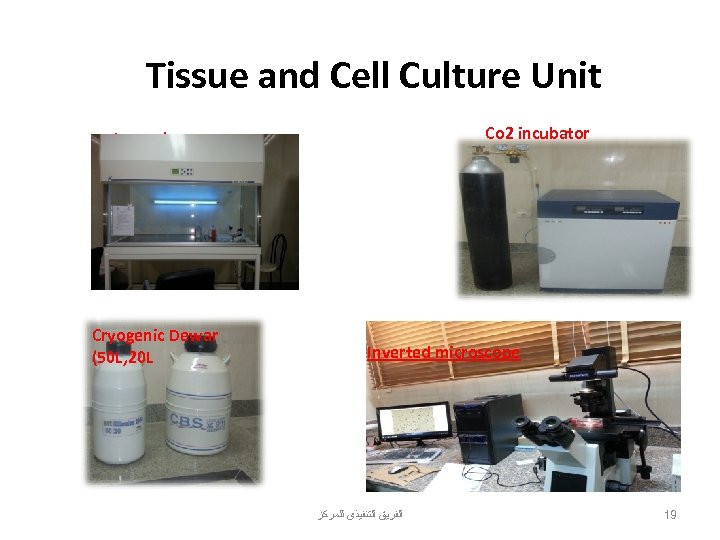
Tissue and Cell Culture Unit Co 2 incubator Ice maker Cryogenic Dewar (50 L, 20 L Inverted microscope ﺍﻟﻔﺮﻳﻖ ﺍﻟﺘﻨﻔﻴﺬﻯ ﻟﻠﻤﺮﻛﺰ 19

Stem cell culture Lab. Including laminar flow, Co 2 incubator
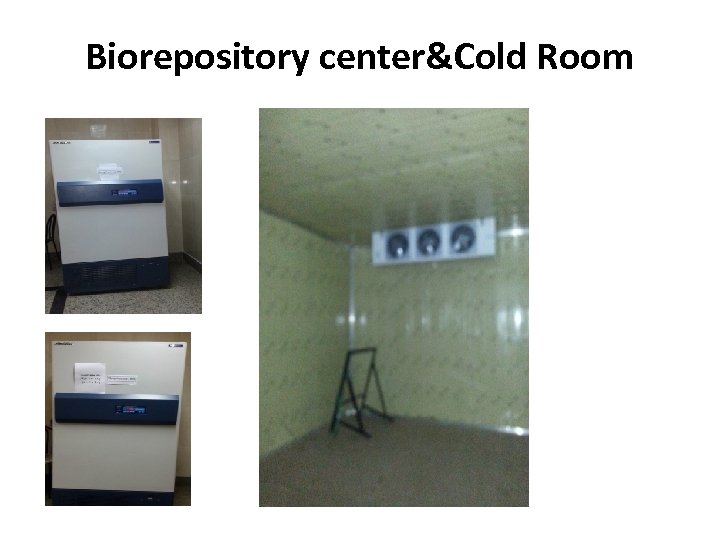
Biorepository center&Cold Room

Biorepository Center • We are going to establish a bank of samples from the enrolled chronic HCV infected patients before and after treatment. • Provides a centralized access point for assisting junior investigators to identify possible sample resources, select which samples to use in their projects and gather available data about the same individuals.

Database Repository

Database Repository

Database Repository

Database Repository

Database Repository

• The well-equipped molecular virology lab. and the biorepository center will be a key resources to apply for funds nationally and internationally and to train young scientists in Egypt on the modern techniques in epidemiology, molecular virology and viral immunology.

Animal House • Provide Technical support to investigators utilizing animal models of infectious disease , pathogenesis, resources and expertise to assist investigators in utilizing and/or developing new animal models to enhance or expand their research programs

Our Recent updated Publications (Viral Hepatitis) • • Helal F Hetta, Mohamed A Mekky, Nasr K Khalil, Wegdan A Mohamed, Mohamed A El-Feky, Shabaan H Ahmed, Enas A Daef, Mahmoud I Nassar, Ahmed Medhat, Kenneth E Sherman, Mohamed Tarek M Shata(2015). Colonic Treg cells are associated with HCV pathogenesis and Liver Pathology. J. of Gastroenterology and Hepatology(30): 2 -8. Helal F Hetta, Mohamed A Mekky, Nasr K Khalil, Wegdan A Mohamed, Mohamed A El-Feky, Shabaan H Ahmed, Enas A Daef, Mahmoud I Nassar, Ahmed M Nasr, Kenneth E Sherman, Mohamed Tarek M Shata (2013). Significant correlations between the frequency of colonic mucosal Treg cells with the viral load and liver inflammation in HCV-infected patients. HEPATOLOGY (58)1182 A-1192 A. Shata MT, Daef EA, Zaki ME, Abdelwahab SF, Marzuuk NM, Sobhy M, Rafaat M, Abdelbaki L, Nafeh MA, Hashem M, El-Kamary SS, Shardell MD, Mikhail NN, Strickland GT, Sherman KE. Protective role of humoral immune responses during an outbreak of hepatitis E in Egypt. Trans R Soc Trop Med Hyg. 2012 Oct; 106(10): 613 -8. Pub. Med Central PMCID (PMID: 22938992). Eldin SS, Seddik I, Daef EA, Shata MT, Raafat M, Abdel Baky L, Nafeh MA. Risk factors and immune response to hepatitis E viral infection among acute hepatitis patients in Assiut, Egypt J Immunol. 2010; 17(1): 73 -86. ( Pub. Med PMID: 22053611); Pub. Med Central PMCID: ( PMC 3399527).

• Blackard JT, Rouster SD, Nady S, Galal G, Marzuuk N, Rafaat MM, Daef E, El Din SS, Purcell RH, Emerson SU, Sherman KE, Shata MT. Genotypic characterization of symptomatic hepatitis E virus (HEV) infections in Egypt. J Clin Virol. 2009 Oct; 46(2): 140 -4. doi: 10. 1016/j. jcv. 2009. 07. 007. Epub 2009 Aug 3. Pub. Med (PMID: 19651539); Pub. Med Central PMCID: ( PMC 2753377). • Shata M. T. 1, Barrett A. 1, Shire N. J. 1, Abdel Wahab S 2. , Sobhy M. 3, Daef E 4, El-Kamary S 5, Hashem M. 3, 5, Engle R. 6, Purcell R. 6, Emerson S. 6, Strickland G. T. 5, Sherman K. E. ( 2007). Characterization of hepatitis E-specific cell-mediated immune response using IFN-gamma ELISPOT assay. J Immunol Methods. ; 328: 152 -161. (PMID: 17905301) • This work was presented in part at the 12 th International Symposium on Viral Hepatitis and Liver Disease in Paris, July 2006, Abstract No P 312.

Papers Presented in International Conferances : • Helal F. Hetta 1, 2, Mohamed A. Mekky 3, Nasr K. Khalil 5, Wegdan A. Mohamed 2, Mohamed A. EL-Feky 2, Shabaan H. Ahmed 2, Enas A. Daef 2, Mahmoud I. Nassar 4, Ahmed M. Nasr 3, Kenneth E. Sherman 1, Mohamed Tarek M. Shata 1. Detection of extrahepatic replication of HCV in the colon tissue and its relationship with HCV pathogenesis • Presented in the 2014 Digestive Health Center annual Scientific Symposium February 26, 2014 • Helal F. Hetta, Mohamed A. Mekky, Nasr K. Khalil , Wegdan A. Mohamed, Mohamed A. El-Feky , Shabaan H. Ahmed, Enas A. Daef, Mahmoud I. Nassar , Ahmed Medhat, Kenneth E. Sherman and Mohamed Tarek M. Shata (2014). “Extra-hepatic replication of HCV in the colon tissue and its relationship with HCV pathogenesis” Department of Internal Medicine “Trainees’ Research Grand Rounds” May 14, 2014. University of Cincinnati, OH. USA. • Poster presented at Department of Internal Medicine “Trainees’ Research Grand Rounds” May 14, 2014. University of Cincinnati, OH. USA

• Hetta HF, Mekky MA, Khalil NK. , Mohamed W, Elfekky MA, Ahmed SH, Daef E, Nassar MI, Nasr AM. , Sherman KE, and Shata MT (2013). Fox. P 3 expression patterns in human colonic tissue” and was presented at the Digestive Health Center Scientific Symposium, on Feb 26, 2013 Cincinnati • Helal F. Hetta, Mohamed A. Mekky, Nasr K. Khalil, Wegdan A. Mohamed, Mohamed A. EL-Feky, Shabaan H. Ahmed, Enas A. Daef, Mahmoud I. Nassar, Ahmed M. Nasr, Kenneth E. Sherman, Mohamed Tarek M. Shata. Significant correlations between the frequency of colonic mucosal Treg cells with the viral load and liver inflammation in HCV-infected patients. Presented in the 64 th Liver meeting of the American Association for the study of liver diseases (AASLD) Washington DC, November 1 to November 5, 2013, Washington DC, November 1 to November 5, 2013; 11/2013. • Shata M. T. 1, Barrett A. 1, Shire N. J. 1, Abdel Wahab S 2. , Sobhy M. , Daef E, El -Kamary S 5, Hashem M. 3, 5, Engle R. 6, Purcell R. 6, Emerson S. 6, Strickland G. T. 5, Sherman K. E. ( 2007). Characterization of hepatitis Especific cell-mediated immune response using IFN-gamma ELISPOT assay. This work was presented in part at the 12 th International Symposium on Viral Hepatitis and Liver Disease in Paris, July 2006, Abstract No P 312.
International publications Of faculty of medicine 2005/2013 34

Research Agenda For Viral Hepatitis Potential Areas of Research by Component Surveillance: Determine the incidence and prevalence of HBV and HCV infection in the general population and specific groups (e. g. , HCWs, dialysis patients, multi-transfused patients, street children, and school teachers, ) to assess HBV and HCV burden in Egypt and identify high risk groups. • Determine the incidence, prevalence, risk factors, and high risk groups associated with HAV infection to assess disease burden in Egypt. • Determine the incidence and prevalence of , liver cirrhosis, hepatocellular carcinoma , and their relation to HBV and HCV.

Infection Control • Evaluate infection-control practices, behaviors, and attitudes about HCV infection in different settings and groups. • Conduct qualitative research with HCWs to identify barriers (e. g. , nurse/patient ratio, work overflow, and lack of knowledge) to adhere to recommended practices and preventive measures (e. g. , HBV vaccination). • Conduct epidemiologic studies on : needle sticks, endoscopy, laparoscopy, cardiac catheterizations, obstetrical procedures and other known risk factors of healthcare-associated viral hepatitis. • Evaluate reuse of single-use items.

Blood Safety: • Determine the prevalence and incidence of HBV and HCV infection in the donor population and in transfused patients. • Identify risk factors for viral hepatitis infection among blood donors. • Identify the most efficient interventions to recruit and retain uninfected blood donors. • Analyze patient outcomes following transfusion of blood products (e. g. , adverse reactions and transfusion-transmitted infections). • Examine adverse reactions in blood donors

Vaccination: • Identify the most effective HBV vaccine strategies among HCWs. • Research non-responders to HBV vaccination. • Estimate HBV vaccination coverage in infants, children, and persons in high-risk populations. • Conduct cost-effectiveness studies on HAV vaccination. • Research need for a booster dose of HBV vaccine in Egypt.

Care and Treatment: • Estimate treatment response rates in “real-life” conditions, and identify factors associated with treatment response. • Estimate rates of relapse and re-infection following HCV treatment. • Identify safe and effective drugs or treatment regimens for persons with chronic viral hepatitis, whether naïve or former relapsers or non-responders. • Conduct a cost-effectiveness analysis of treatment strategies, including use of newly available antiviral therapies.


ﺭﺑﻨﺎ ﺃﺘﻨﺎﻓﻰ ﺍﻟﺪﻧﻴﺎﺣﺴﻨﺔﻭﻓﻰ ﺨﺮﺓ ﺍﻷ ﺣﺴﻨﺔ ﻭﻗﻨﺎﻋﺬﺍﺏ ﺍﻟﻨﺎﺭ 14 ﺍﻟﻔﺮﻳﻖ ﺍﻟﺘﻨﻔﻴﺬﻯ ﻟﻠﻤﺮﻛﺰ
3d2b27b142253cb86006b48c2622e1cd.ppt