detergents_presentation.pptx
- Количество слайдов: 7
 Production of synthetic detergents Sattarov A. , Cht-13 -6 ka 2
Production of synthetic detergents Sattarov A. , Cht-13 -6 ka 2
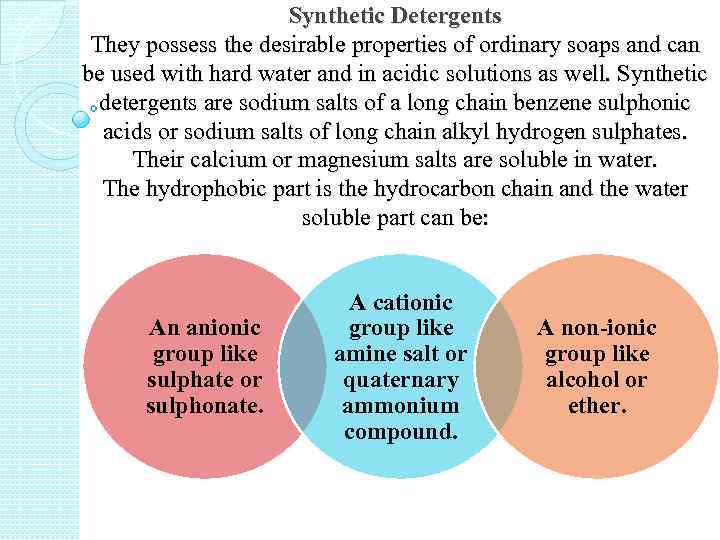 Synthetic Detergents They possess the desirable properties of ordinary soaps and can be used with hard water and in acidic solutions as well. Synthetic detergents are sodium salts of a long chain benzene sulphonic acids or sodium salts of long chain alkyl hydrogen sulphates. Their calcium or magnesium salts are soluble in water. The hydrophobic part is the hydrocarbon chain and the water soluble part can be: An anionic group like sulphate or sulphonate. A cationic group like amine salt or quaternary ammonium compound. A non-ionic group like alcohol or ether.
Synthetic Detergents They possess the desirable properties of ordinary soaps and can be used with hard water and in acidic solutions as well. Synthetic detergents are sodium salts of a long chain benzene sulphonic acids or sodium salts of long chain alkyl hydrogen sulphates. Their calcium or magnesium salts are soluble in water. The hydrophobic part is the hydrocarbon chain and the water soluble part can be: An anionic group like sulphate or sulphonate. A cationic group like amine salt or quaternary ammonium compound. A non-ionic group like alcohol or ether.
 Introduction In primitive societies, even today, clothes are cleaned by beating them on rocks near a stream. Certain plants, such as soapworts, have leaves that produce sapions, chemical compounds that give a soapy lather. These were probably the first detergents people used. If you look up detergent in a dictionary it is simply defined as cleaning agent. During the last two to three decades, however, the word detergent has tended to imply synthetic detergent, or syndet for short, rather than the older soap. In fact, commercial formulations consist of a number of components, and we shall use the term surface-active agent, or it's abbreviation surfactant, to describe the special active ingredients that give detergents their unusual properties. Soap, by this definition, is a surfactant. In fact, it is the oldest one and has been in use for over 4500 years. Some soap manufacture took place in Venice and Savona in the fifteenth century, and in Marseilles in the seventeenth century. By the eighteenth century, manufacture was widespread throughout Europe and North America, and by the nineteenth century the making of soap had become a major industry. As a matter of fact, soap became a detergent in 1907 when a German company put the product "Persil" on the market. In addition to the carboxylic acid soap, "Persil" contained sodium perborate, sodium silicate and sodium carbonate. Hence perborate + silicate = "PERSIL".
Introduction In primitive societies, even today, clothes are cleaned by beating them on rocks near a stream. Certain plants, such as soapworts, have leaves that produce sapions, chemical compounds that give a soapy lather. These were probably the first detergents people used. If you look up detergent in a dictionary it is simply defined as cleaning agent. During the last two to three decades, however, the word detergent has tended to imply synthetic detergent, or syndet for short, rather than the older soap. In fact, commercial formulations consist of a number of components, and we shall use the term surface-active agent, or it's abbreviation surfactant, to describe the special active ingredients that give detergents their unusual properties. Soap, by this definition, is a surfactant. In fact, it is the oldest one and has been in use for over 4500 years. Some soap manufacture took place in Venice and Savona in the fifteenth century, and in Marseilles in the seventeenth century. By the eighteenth century, manufacture was widespread throughout Europe and North America, and by the nineteenth century the making of soap had become a major industry. As a matter of fact, soap became a detergent in 1907 when a German company put the product "Persil" on the market. In addition to the carboxylic acid soap, "Persil" contained sodium perborate, sodium silicate and sodium carbonate. Hence perborate + silicate = "PERSIL".
 Properties Some of the synthetic detergents have very low biodegradability. They are resistant to bacterial attack and are not fully degraded in sewage treatment units. Therefore, they cause water pollution when they are discharged into a river or any other water body. Phosphate salts present in synthetic detergents cause rapid growth of algae that deplete the oxygen content in the water. (A condition known as eutrophication). Due to this aquatic animals die resulting in the imbalance of the ecosystem as well. These detergents lower the surface tension of water and act as cleansing agents (wetting agents). They can be used for delicate fabrics because they do not hydrolyse to give hydroxyl ions. They have equal action in both hard and soft water.
Properties Some of the synthetic detergents have very low biodegradability. They are resistant to bacterial attack and are not fully degraded in sewage treatment units. Therefore, they cause water pollution when they are discharged into a river or any other water body. Phosphate salts present in synthetic detergents cause rapid growth of algae that deplete the oxygen content in the water. (A condition known as eutrophication). Due to this aquatic animals die resulting in the imbalance of the ecosystem as well. These detergents lower the surface tension of water and act as cleansing agents (wetting agents). They can be used for delicate fabrics because they do not hydrolyse to give hydroxyl ions. They have equal action in both hard and soft water.
 Chemical Characteristics Synthetic detergents dissolve or tend to dissolve in water or other solvents. To enable them to do this, they require distinct chemical characteristics. Hydrophilic (water loving) groupings in their molecular structure, and hydrophobic (water hating) groupings, help the detergent in it’s “detergency” action. This detergency depends on the balance of the molecular weight of the hydrophobic to the hydrophilic portion. This is called the HLB value, and can range from 1 upwards. HLB is Hydrophilic-Lypophilic Balance. As the 0 HLB value increases, the product can tend towards being a paste or solid. The lower number HLB values tend to be less water soluble, and more oil soluble. The higher the HLB the more water soluble the product. Mixtures of low and high HLB detergents produce good detergents to handle oil, fat and grease, the higher HLB detergent helps solubilise the less water soluble, low HLB detergent into an aqueous system.
Chemical Characteristics Synthetic detergents dissolve or tend to dissolve in water or other solvents. To enable them to do this, they require distinct chemical characteristics. Hydrophilic (water loving) groupings in their molecular structure, and hydrophobic (water hating) groupings, help the detergent in it’s “detergency” action. This detergency depends on the balance of the molecular weight of the hydrophobic to the hydrophilic portion. This is called the HLB value, and can range from 1 upwards. HLB is Hydrophilic-Lypophilic Balance. As the 0 HLB value increases, the product can tend towards being a paste or solid. The lower number HLB values tend to be less water soluble, and more oil soluble. The higher the HLB the more water soluble the product. Mixtures of low and high HLB detergents produce good detergents to handle oil, fat and grease, the higher HLB detergent helps solubilise the less water soluble, low HLB detergent into an aqueous system.
 What's the Difference? What's the difference between a surfactant and soap? In general terms, the difference can be likened to the difference between cotton and nylon. On the one hand, soap and cotton are produced from natural products by a relatively small modification. On the other hand, synthetic surfactants and nylon are produced entirely in a chemical factory. Synthetic surfactants are not very new, either. Back in 1834 the first forerunner of today's synthetic surfactants was produced in the form of a sulfated castor oil, which was used in the textile industry. The development of the first detergents in an effort to overcome the reaction of soaps with hard water provides a good illustration of one of the standard chemical approaches. If a useful substance has some undesirable property, an attempt is made to prepare an analogue, a near chemical relation, which will prove more satisfactory. The petroleum industry had, as a waste product, the compound propylene, CH 3 -CH=CH 2, which used to be burnt off. By joining four of these propylene molecules together and if benzene is attached at the double bond, the resulting compound reacts with sulphuric acid. Then sodium hydroxide is added to neutralise the sulfonic acid and a sodium salt is obtained. The new substance is closely related to an ordinary soap, and is an excellent detergent.
What's the Difference? What's the difference between a surfactant and soap? In general terms, the difference can be likened to the difference between cotton and nylon. On the one hand, soap and cotton are produced from natural products by a relatively small modification. On the other hand, synthetic surfactants and nylon are produced entirely in a chemical factory. Synthetic surfactants are not very new, either. Back in 1834 the first forerunner of today's synthetic surfactants was produced in the form of a sulfated castor oil, which was used in the textile industry. The development of the first detergents in an effort to overcome the reaction of soaps with hard water provides a good illustration of one of the standard chemical approaches. If a useful substance has some undesirable property, an attempt is made to prepare an analogue, a near chemical relation, which will prove more satisfactory. The petroleum industry had, as a waste product, the compound propylene, CH 3 -CH=CH 2, which used to be burnt off. By joining four of these propylene molecules together and if benzene is attached at the double bond, the resulting compound reacts with sulphuric acid. Then sodium hydroxide is added to neutralise the sulfonic acid and a sodium salt is obtained. The new substance is closely related to an ordinary soap, and is an excellent detergent.
 References Threadingham, Desmond; Obrecht, Werner; Wieder, Wolfgang; Wachholz, Gerhard; Engehausen, Rüdiger (2011). Rubber, 3. Synthetic Rubbers, Introduction and Overview. Ullmann's Encyclopedia of Industrial Chemistry (Weinheim). doi: 10. 1002/14356007. a 23_239. pub 5. Market Study Synthetic Rubber, Ceresana, June 2013 The Moving Powers of Rubber, Leverkusen, Germany: LANXESS AG: 20 Michalovic, Mark (2000). "Destination Germany: A Poor Substitute". The Story of Rubber. Edwards, Douglas C. (2001). "Chap. 5 - Liquid Rubber". In Bhowmick, Anil K. ; Stephens, Howard. Handbook of Elastomers, Second Edition (First ed. ). Marcel Dekker Inc. p. 135. ISBN 0 -8247 -0383 -9. Retrieved 8 February 2015. Current Biography 1940, "SEMON, WALDO LONSBURY" pp 723 -24 Stormont, John W. (March 1946) [summer of 1945], AAFRH-19: The Combined Bomber Offensive; April through December 1943, Dwight D. Eisenhower Presidential Library: Collection of 20 th Century Military Records, 1918– 1950 Series I: Historical Studies Box 35: AAF Historical Office; Headquarters, Army Air Force, pp. 74– 5, 81, SECRET. . . Classification Cancelled. . . JUN 10 1959
References Threadingham, Desmond; Obrecht, Werner; Wieder, Wolfgang; Wachholz, Gerhard; Engehausen, Rüdiger (2011). Rubber, 3. Synthetic Rubbers, Introduction and Overview. Ullmann's Encyclopedia of Industrial Chemistry (Weinheim). doi: 10. 1002/14356007. a 23_239. pub 5. Market Study Synthetic Rubber, Ceresana, June 2013 The Moving Powers of Rubber, Leverkusen, Germany: LANXESS AG: 20 Michalovic, Mark (2000). "Destination Germany: A Poor Substitute". The Story of Rubber. Edwards, Douglas C. (2001). "Chap. 5 - Liquid Rubber". In Bhowmick, Anil K. ; Stephens, Howard. Handbook of Elastomers, Second Edition (First ed. ). Marcel Dekker Inc. p. 135. ISBN 0 -8247 -0383 -9. Retrieved 8 February 2015. Current Biography 1940, "SEMON, WALDO LONSBURY" pp 723 -24 Stormont, John W. (March 1946) [summer of 1945], AAFRH-19: The Combined Bomber Offensive; April through December 1943, Dwight D. Eisenhower Presidential Library: Collection of 20 th Century Military Records, 1918– 1950 Series I: Historical Studies Box 35: AAF Historical Office; Headquarters, Army Air Force, pp. 74– 5, 81, SECRET. . . Classification Cancelled. . . JUN 10 1959


