bf546fe198f6b6faef203ac6a995e40c.ppt
- Количество слайдов: 29

Production of bioactive myostaitn propeptide of variouis animal species in E. coli Yong soo Kim, Professor Department of Human Nutrition, Food and Animal Sciences University of Hawaii, Manoa

Myostatin (MSTN) n n 1997, Se-jin Lee’s Lab at the Johns Hopkins University A member of the TGF-β superfamily growth and differentiation proteins, also called GDF-8 Natural, non-functional mutation
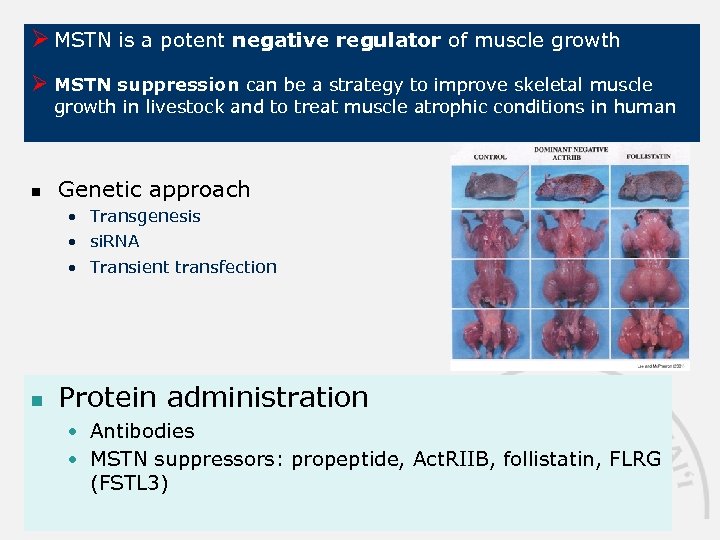
Ø MSTN is a potent negative regulator of muscle growth Ø MSTN suppression can be a strategy to improve skeletal muscle growth in livestock and to treat muscle atrophic conditions in human n Genetic approach • Transgenesis • si. RNA • Transient transfection n Protein administration • Antibodies • MSTN suppressors: propeptide, Act. RIIB, follistatin, FLRG (FSTL 3)
• Production of Bioactive MSTN-suppressing proteins in E. coli • Myostatin Propeptide (MSTNPro) • Follistatin MSTNPro: $329/25 ug Follistatin: $329/25 ug Mouse study: 2, 400 ug ($31, 584) 6 animals x 4 injections x 100 ug/10 g mouse (10 mg/kg body wt) Pig study: 204 mg ($2. 68 Mil) 6 animals x 2 injections x 17 mg/1. 7 kg new-born pig (10 mg/kg body wt)
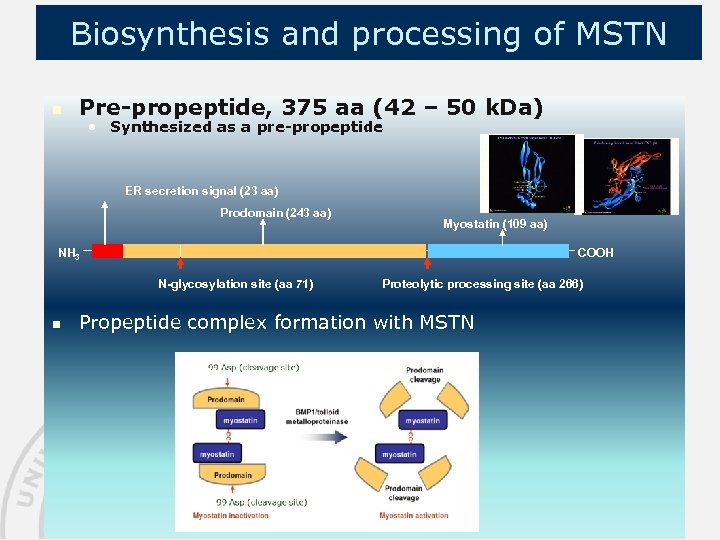
Biosynthesis and processing of MSTN n Pre-propeptide, 375 aa (42 – 50 k. Da) • Synthesized as a pre-propeptide ER secretion signal (23 aa) Prodomain (243 aa) Myostatin (109 aa) COOH NH 3 N-glycosylation site (aa 71) n Proteolytic processing site (aa 266) Propeptide complex formation with MSTN
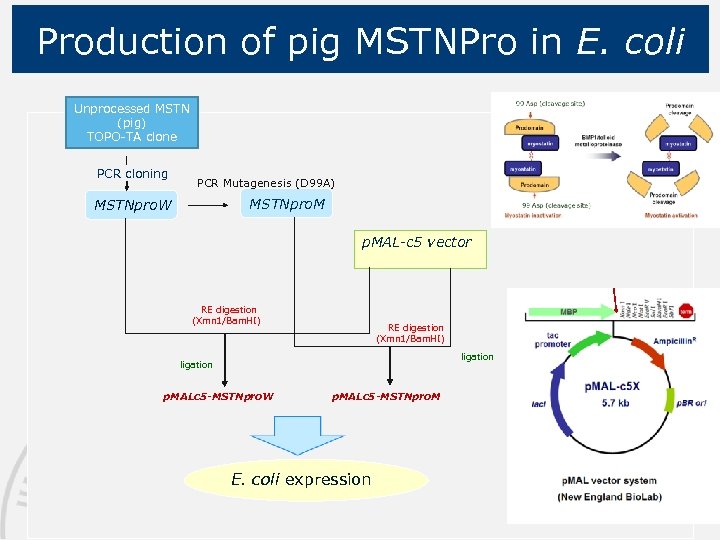
Production of pig MSTNPro in E. coli Unprocessed MSTN (pig) TOPO-TA clone PCR cloning PCR Mutagenesis (D 99 A) MSTNpro. M MSTNpro. W p. MAL-c 5 vector RE digestion (Xmn 1/Bam. HI) ligation p. MALc 5 -MSTNpro. W p. MALc 5 -MSTNpro. M E. coli expression
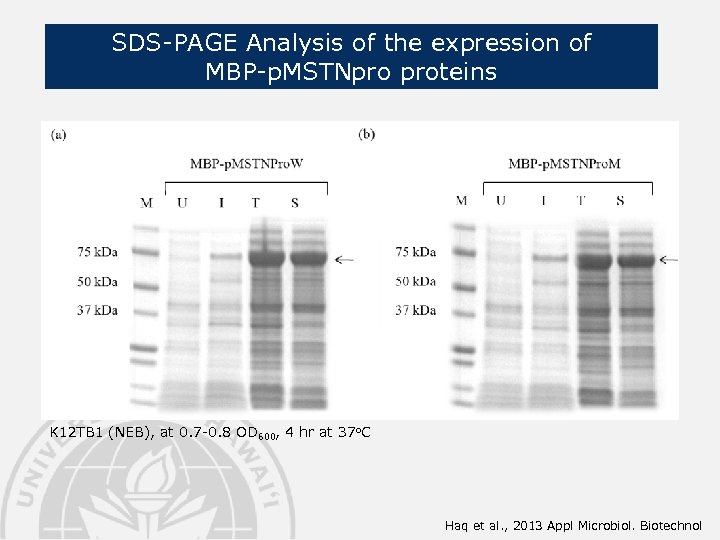
SDS-PAGE Analysis of the expression of MBP-p. MSTNpro proteins K 12 TB 1 (NEB), at 0. 7 -0. 8 OD 600, 4 hr at 37 o. C Haq et al. , 2013 Appl Microbiol. Biotechnol
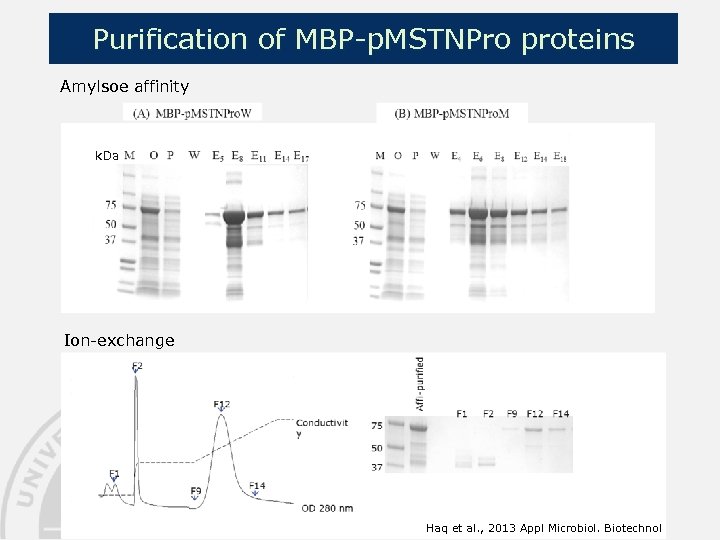
Purification of MBP-p. MSTNPro proteins Amylsoe affinity k. Da Ion-exchange Haq et al. , 2013 Appl Microbiol. Biotechnol
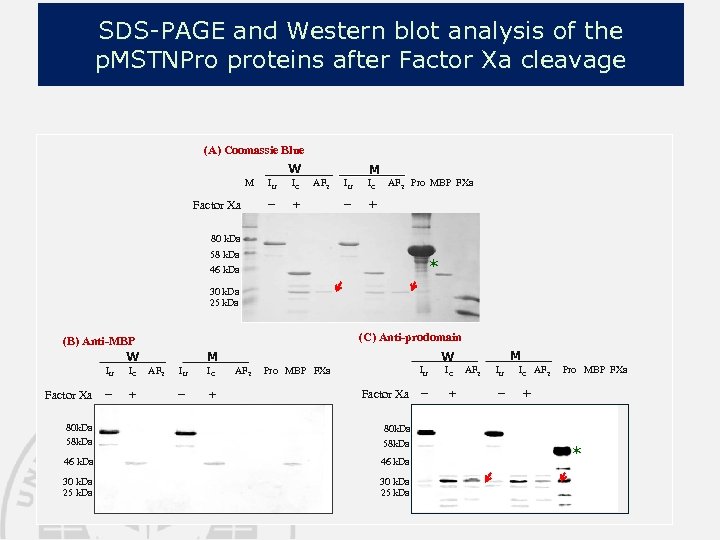
SDS-PAGE and Western blot analysis of the p. MSTNPro proteins after Factor Xa cleavage (A) Coomassie Blue W M Factor Xa IU IC − M AF 2 + IU IC − AF 2 Pro MBP FXa + 80 k. Da 58 k. Da * 46 k. Da 30 k. Da 25 k. Da (C) Anti-prodomain (B) Anti-MBP W IU Factor Xa IC − + M AF 2 IU IC − + M W AF 2 IU Pro MBP FXa Factor Xa 80 k. Da 58 k. Da 46 k. Da 30 k. Da 25 k. Da IC − + AF 2 IU − IC AF 2 Pro MBP FXa + *
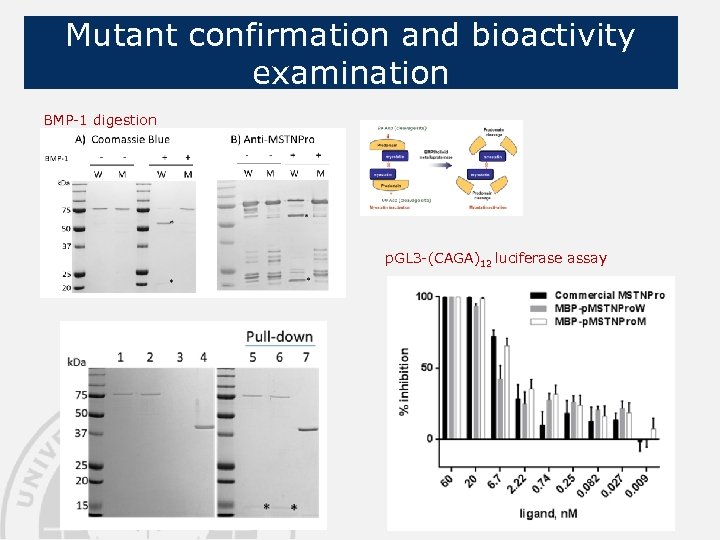
Mutant confirmation and bioactivity examination BMP-1 digestion p. GL 3 -(CAGA)12 luciferase assay
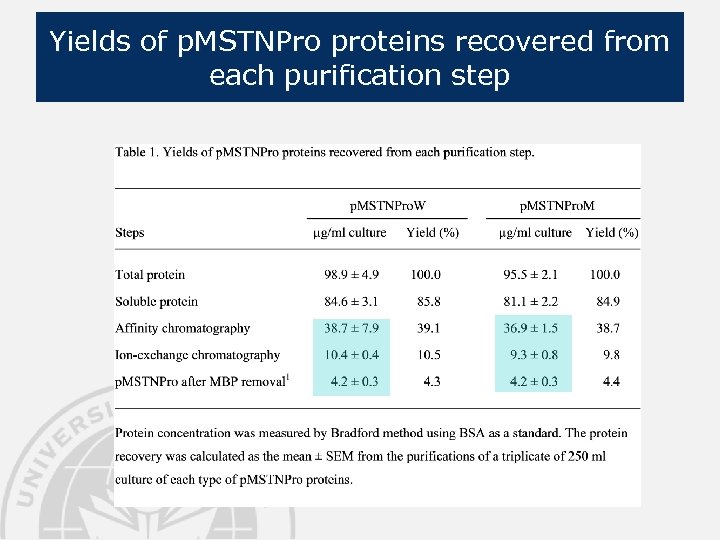
Yields of p. MSTNPro proteins recovered from each purification step
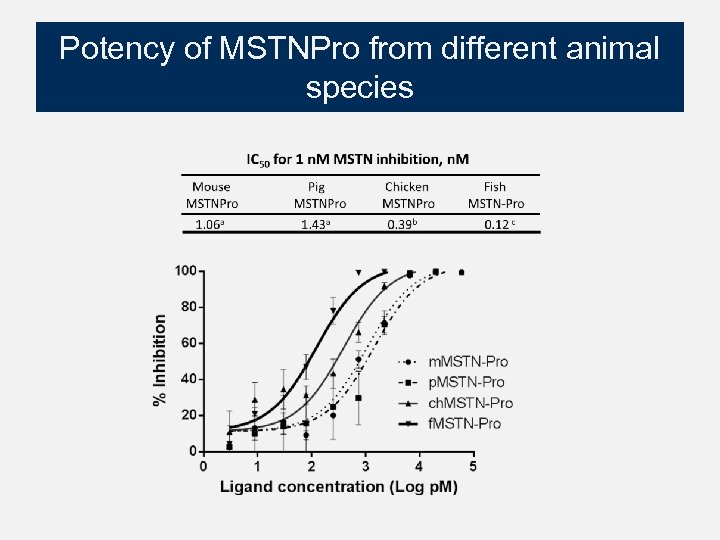
Potency of MSTNPro from different animal species
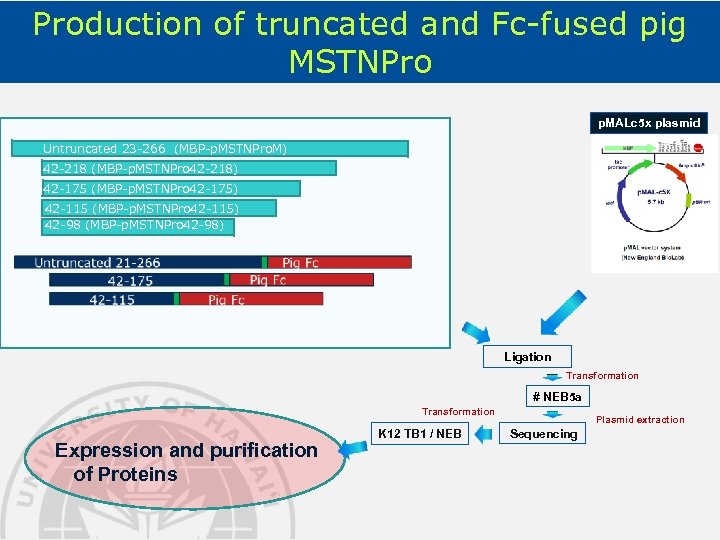
Production of truncated and Fc-fused pig MSTNPro p. MALc 5 x plasmid Untruncated 23 -266 (MBP-p. MSTNPro. M) 42 -218 (MBP-p. MSTNPro 42 -218) 42 -175 (MBP-p. MSTNPro 42 -175) 42 -115 (MBP-p. MSTNPro 42 -115) 42 -98 (MBP-p. MSTNPro 42 -98) Ligation Transformation # NEB 5 a Transformation Expression and purification of Proteins K 12 TB 1 / NEB Plasmid extraction Sequencing
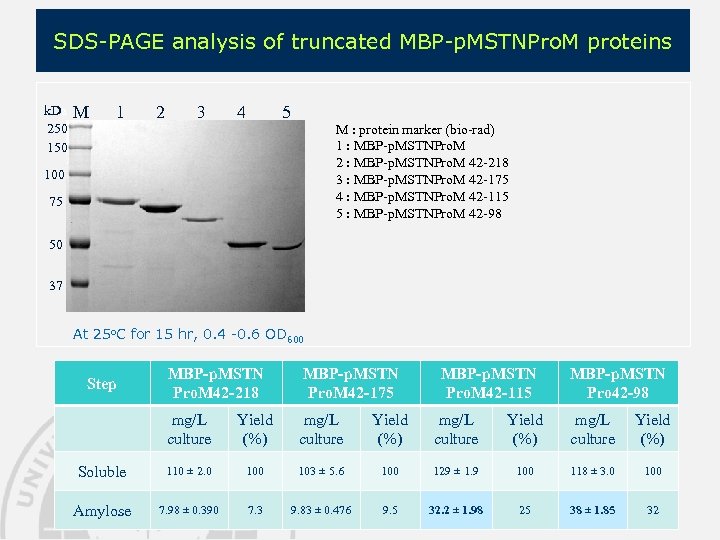
SDS-PAGE analysis of truncated MBP-p. MSTNPro. M proteins k. Da 250 150 M 1 2 3 4 5 M : protein marker (bio-rad) 1 : MBP-p. MSTNPro. M 2 : MBP-p. MSTNPro. M 42 -218 3 : MBP-p. MSTNPro. M 42 -175 4 : MBP-p. MSTNPro. M 42 -115 5 : MBP-p. MSTNPro. M 42 -98 100 75 50 37 At 25 o. C for 15 hr, 0. 4 -0. 6 OD 600 MBP-p. MSTN Pro. M 42 -218 MBP-p. MSTN Pro. M 42 -175 mg/L culture Yield (%) Soluble 110 ± 2. 0 103 ± 5. 6 100 129 ± 1. 9 100 118 ± 3. 0 100 Amylose 7. 98 ± 0. 390 7. 3 9. 83 ± 0. 476 9. 5 32. 2 ± 1. 98 25 38 ± 1. 85 32 Step MBP-p. MSTN Pro. M 42 -115 MBP-p. MSTN Pro 42 -98
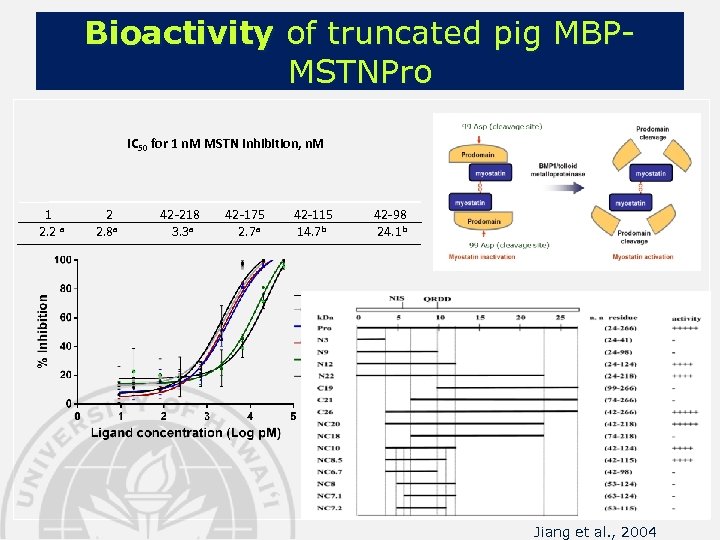
Bioactivity of truncated pig MBPMSTNPro IC 50 for 1 n. M MSTN inhibition, n. M 1 2. 2 a 2 2. 8 a 42 -218 3. 3 a 42 -175 2. 7 a 42 -115 14. 7 b 42 -98 24. 1 b Jiang et al. , 2004
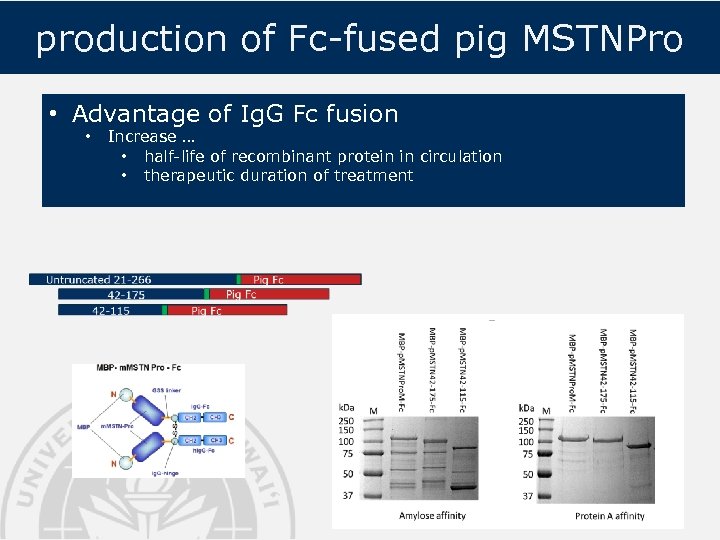
production of Fc-fused pig MSTNPro • Advantage of Ig. G Fc fusion • Increase … • half-life of recombinant protein in circulation • therapeutic duration of treatment
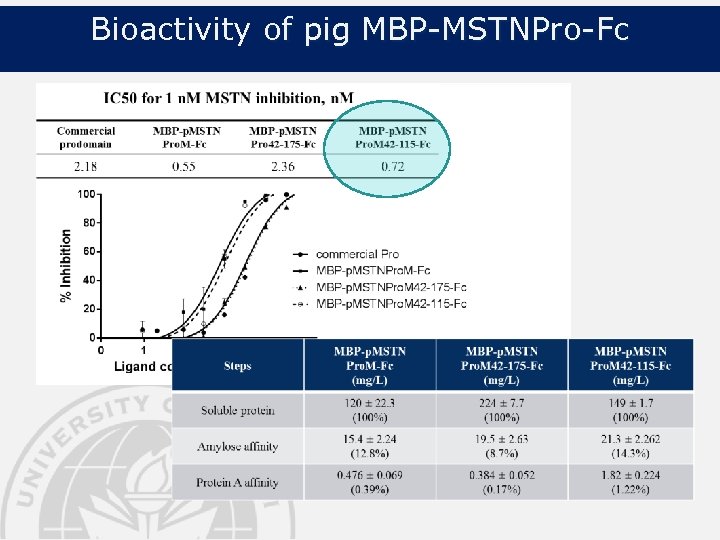
Bioactivity of pig MBP-MSTNPro-Fc
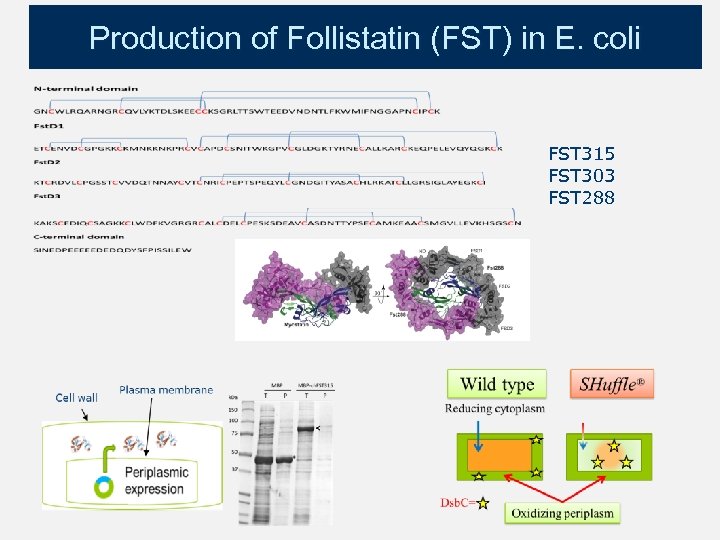
Production of Follistatin (FST) in E. coli FST 315 FST 303 FST 288
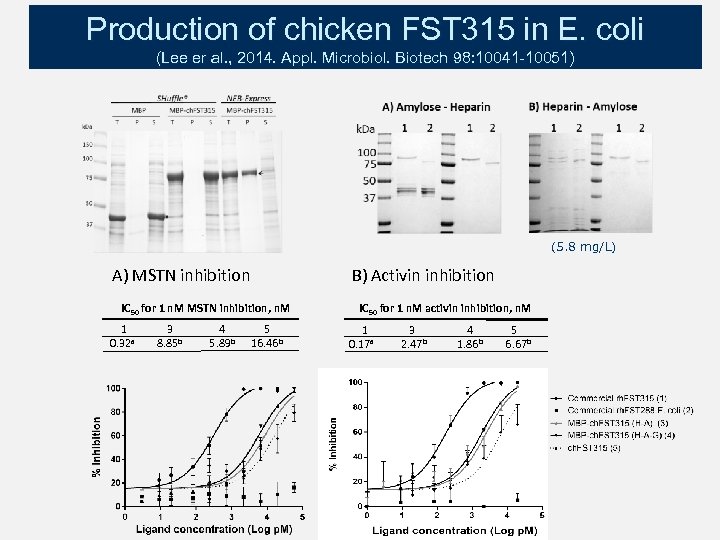
Production of chicken FST 315 in E. coli (Lee er al. , 2014. Appl. Microbiol. Biotech 98: 10041 -10051) (5. 8 mg/L) A) MSTN inhibition B) Activin inhibition IC 50 for 1 n. M MSTN inhibition, n. M 1 0. 32 a 3 8. 85 b 4 5. 89 b 5 16. 46 b IC 50 for 1 n. M activin inhibition, n. M 1 0. 17 a 3 2. 47 b 4 1. 86 b 5 6. 67 b

Conclusion • Myostatin propetide (MSTNPro) can be produced in a cost-effective way in an E. coli system • The easy availability of MSTPro will allow us to investigate the potentials of MSTNPro as an agent to improve skeletal muscle growth of meat-producing animals.

Acknowledgement Dr. Dr. Shihuan Kuang, Purdue University Bruria Funkenstein, Israel Oceanographic and Limnological Research Hyung. Joo Jin’s lab, Gangneung-Wonju National University, Korea Yun. Jaie Choi’s lab, Seoul National University, Korea Dr. Sang. Beum Lee Dr. Yunkyung Lee Naveen Bobbili Mandy Haq Rocky Choi Thanks 谢谢

Effects of In-ovo Injection of Monoclonal Anti-myostatin Antibody (m. Ab-c 134) on Post-hatch Chicken Growth and Muscle Mass Y S Kim, N K Bobbili, K S Paek and H J Jin Poultry Sci. 85: 1062 -1071 (2006)
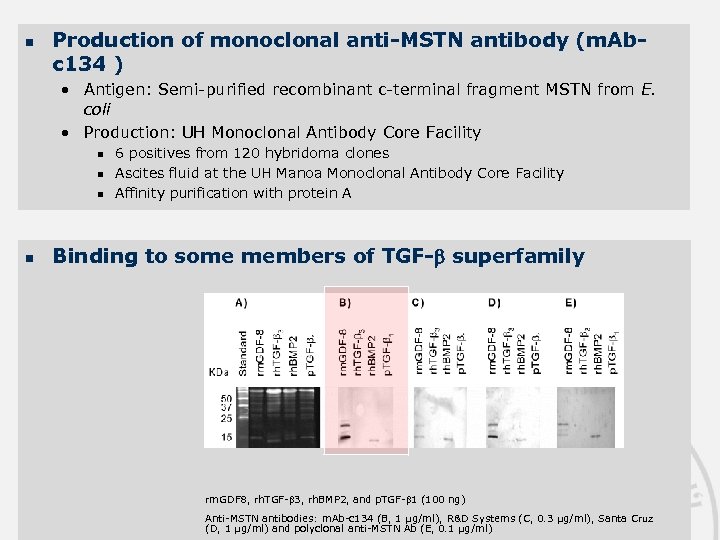
n Production of monoclonal anti-MSTN antibody (m. Abc 134 ) • Antigen: Semi-purified recombinant c-terminal fragment MSTN from E. coli • Production: UH Monoclonal Antibody Core Facility n n 6 positives from 120 hybridoma clones Ascites fluid at the UH Manoa Monoclonal Antibody Core Facility Affinity purification with protein A Binding to some members of TGF- superfamily rm. GDF 8, rh. TGF- 3, rh. BMP 2, and p. TGF- 1 (100 ng) Anti-MSTN antibodies: m. Ab-c 134 (B, 1 µg/ml), R&D Systems (C, 0. 3 µg/ml), Santa Cruz (D, 1 µg/ml) and polyclonal anti-MSTN Ab (E, 0. 1 µg/ml)
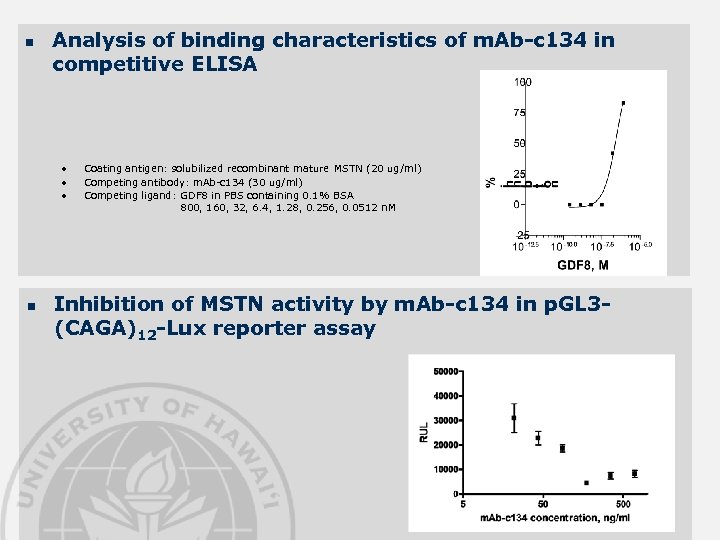
n Analysis of binding characteristics of m. Ab-c 134 in competitive ELISA • • • n Coating antigen: solubilized recombinant mature MSTN (20 ug/ml) Competing antibody: m. Ab-c 134 (30 ug/ml) Competing ligand: GDF 8 in PBS containing 0. 1% BSA 800, 160, 32, 6. 4, 1. 28, 0. 256, 0. 0512 n. M Inhibition of MSTN activity by m. Ab-c 134 in p. GL 3(CAGA)12 -Lux reporter assay
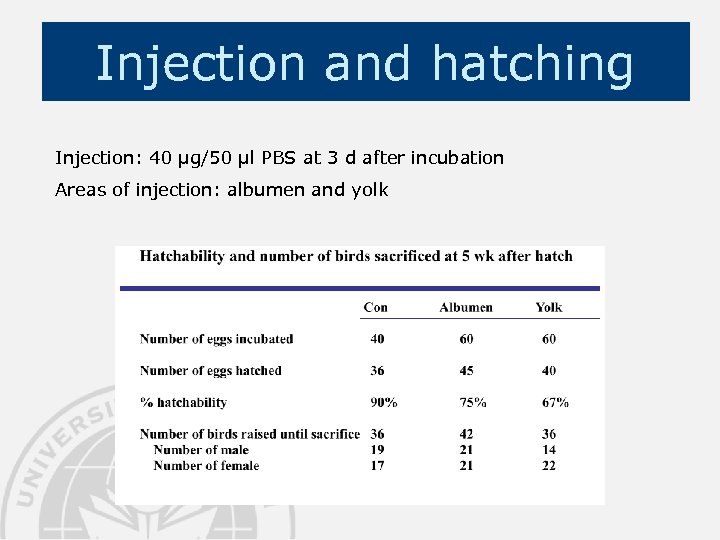
Injection and hatching Injection: 40 µg/50 µl PBS at 3 d after incubation Areas of injection: albumen and yolk
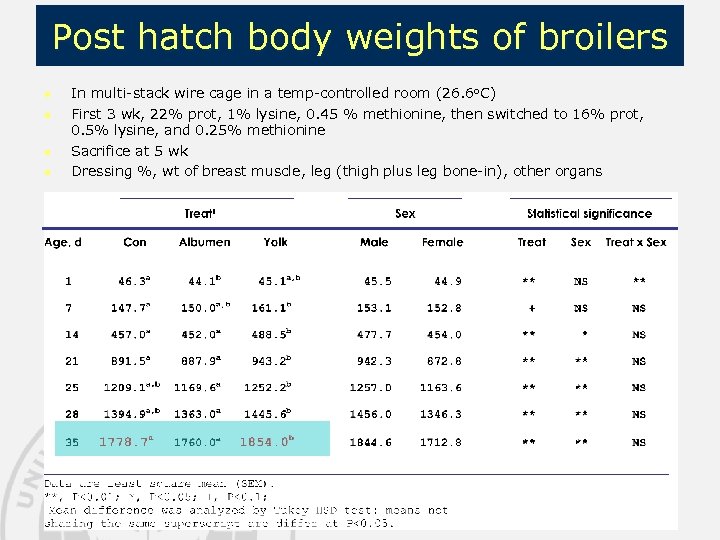
Post hatch body weights of broilers n n In multi-stack wire cage in a temp-controlled room (26. 6 o. C) First 3 wk, 22% prot, 1% lysine, 0. 45 % methionine, then switched to 16% prot, 0. 5% lysine, and 0. 25% methionine Sacrifice at 5 wk Dressing %, wt of breast muscle, leg (thigh plus leg bone-in), other organs
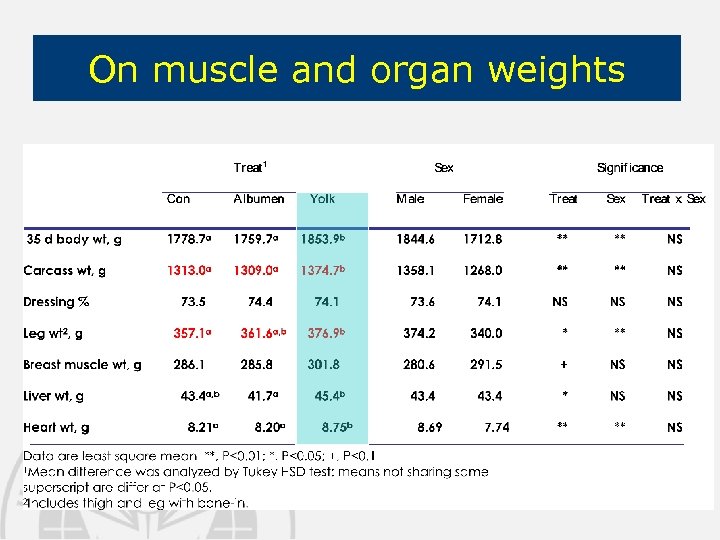
On muscle and organ weights
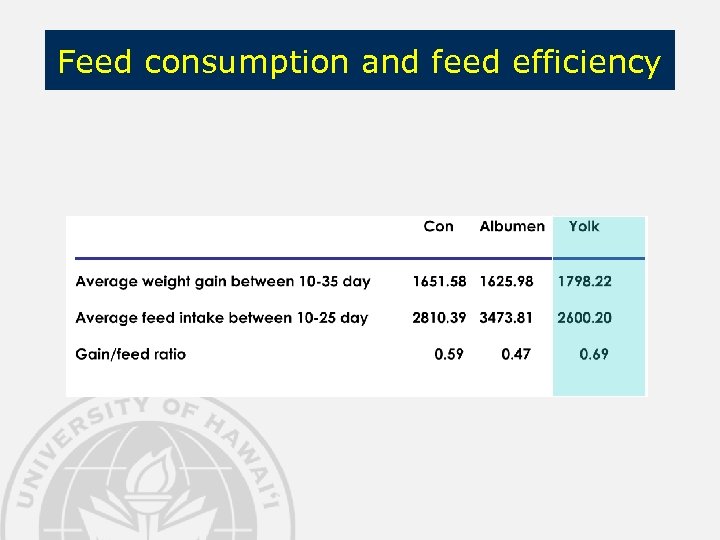
Feed consumption and feed efficiency

bf546fe198f6b6faef203ac6a995e40c.ppt