6ed63355825ba5f5c7b495ffe9242d00.ppt
- Количество слайдов: 7
Procleix® WNV Assay: A TMA-based Assay for Screening Blood Donations for West Nile Virus RNA So. GAT July 3, 2003 Jeff Linnen, Ph. D. Research and Development Gen-Probe Incorporated, San Diego, CA USA Procleix® is a registered trademark of Chiron Corporation 1
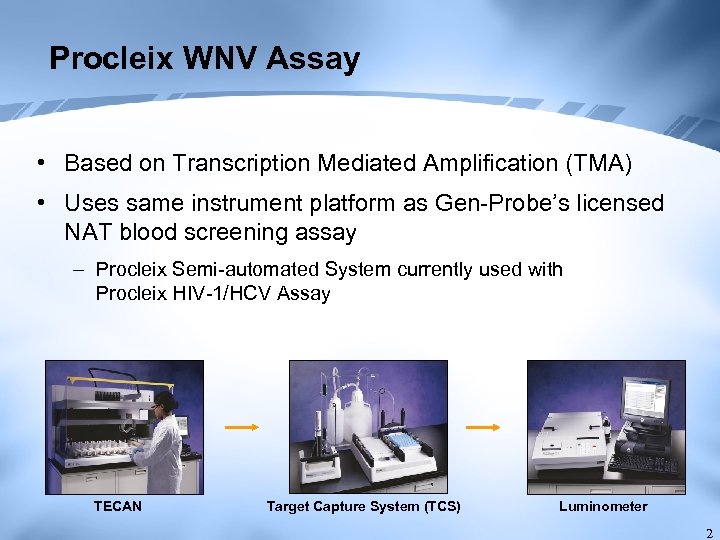
Procleix WNV Assay • Based on Transcription Mediated Amplification (TMA) • Uses same instrument platform as Gen-Probe’s licensed NAT blood screening assay – Procleix Semi-automated System currently used with Procleix HIV-1/HCV Assay TECAN Target Capture System (TCS) Luminometer 2
Specificity of the Procleix WNV Assay • 1, 680 normal blood donations were tested at Gen-Probe – 99. 8% initial specificity; 100% resolved specificity – over 40, 000 archived samples from 2002 high risk populations have been tested by American Red Cross (S. Stramer) • No cross reactivity to other blood borne viruses – Testing included HTLV, HIV-1/-2, HCV, HBV, HGV, Rubella, HAV, CMV, EBV, HCV, Parvo B 19 • Assay designed to specifically detect West Nile virus: – No cross reactivity to other flaviviruses: Dengue (1 -4), Yellow Fever Virus, and St. Louis Encephalitis virus; weak cross reactivity to Murray Valley Encephalitis virus – Detects Kunjin virus (Australian subtype of West Nile virus) 3
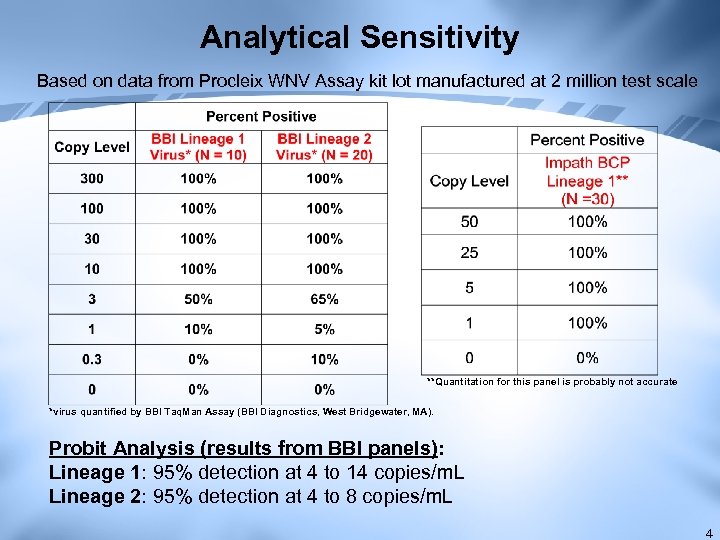
Analytical Sensitivity Based on data from Procleix WNV Assay kit lot manufactured at 2 million test scale **Quantitation for this panel is probably not accurate *virus quantified by BBI Taq. Man Assay (BBI Diagnostics, West Bridgewater, MA). Probit Analysis (results from BBI panels): Lineage 1: 95% detection at 4 to 14 copies/m. L Lineage 2: 95% detection at 4 to 8 copies/m. L 4
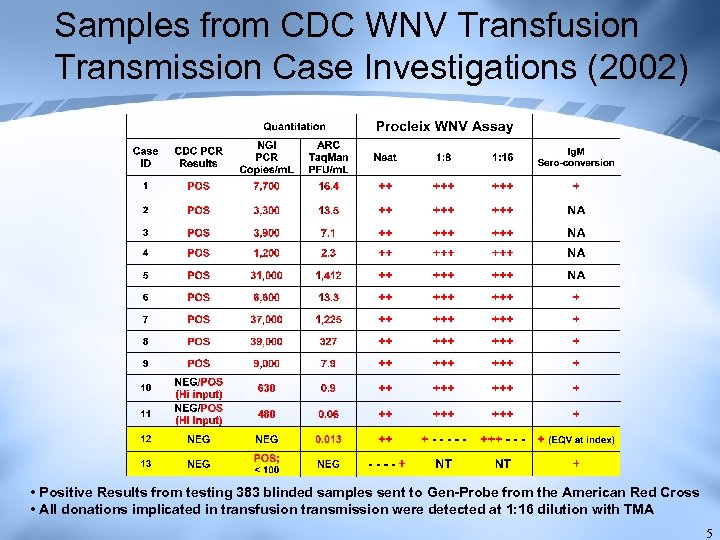
Samples from CDC WNV Transfusion Transmission Case Investigations (2002) • Positive Results from testing 383 blinded samples sent to Gen-Probe from the American Red Cross • All donations implicated in transfusion transmission were detected at 1: 16 dilution with TMA 5
Nationwide WNV Blood Screening in the United States, 2003 • Testing using the Procleix WNV Assay started on June 19 – Implemented nationwide on July 1 – Test development which normally takes 2 to 3 years was condensed into less than 9 months. – Procleix WNV Assay is being used to test over 80% of the US blood supply • Most donations are being tested in pools of 16 (some sites are testing individual donations) – reactive pools will be resolved to individual donation • Testing will reduce the risk of WNV transfusion transmission – will provide real time surveillance of human WNV activity in North America 6

Acknowledgements • Gen-Probe WNV Assay Development Team – (Front to back, left to right) Mike Shih, Josh Cary, Geoffrey Dennis, Janice Cline, Martha Alden, Wen Wu, Mackenzie Lewis, Michelle Cass, Amy Broulik, Jeff Linnen, Stephanie Miller • • • National Heart, Lung, and Blood Institute (NHLBI) for partial funding Susan Stramer, American Red Cross Chiron Corporation 7
6ed63355825ba5f5c7b495ffe9242d00.ppt