07539eebff296b2ff970f25665c41f24.ppt
- Количество слайдов: 19
 Process Understanding and PAT D. Christopher Watts, Ph. D. Office of Pharmaceutical Science, CDER, FDA ACPS, Manufacturing Subcommittee July 21, 2004
Process Understanding and PAT D. Christopher Watts, Ph. D. Office of Pharmaceutical Science, CDER, FDA ACPS, Manufacturing Subcommittee July 21, 2004
 The Questions • What is PAT? • Who is involved with PAT? – Engine for Success • How will PAT benefit? – Industry – Agency – Public Health • Where are we going with PAT?
The Questions • What is PAT? • Who is involved with PAT? – Engine for Success • How will PAT benefit? – Industry – Agency – Public Health • Where are we going with PAT?
 What is PAT? A system for: – designing, analyzing, and controlling manufacturing – timely measurements (i. e. , during processing) – critical quality and performance attributes – raw and in-process materials – processes “Analytical“ includes: – chemical, physical, microbiological, mathematical, and risk analysis – conducted in an integrated manner
What is PAT? A system for: – designing, analyzing, and controlling manufacturing – timely measurements (i. e. , during processing) – critical quality and performance attributes – raw and in-process materials – processes “Analytical“ includes: – chemical, physical, microbiological, mathematical, and risk analysis – conducted in an integrated manner
 PAT = Process Understanding • A process is well understood when: – all critical sources of variability are identified and explained – variability is managed by the process – product quality attributes can be accurately and reliably predicted • Accurate and Reliable predictions reflect process understanding • Process Understanding inversely proportional to risk
PAT = Process Understanding • A process is well understood when: – all critical sources of variability are identified and explained – variability is managed by the process – product quality attributes can be accurately and reliably predicted • Accurate and Reliable predictions reflect process understanding • Process Understanding inversely proportional to risk
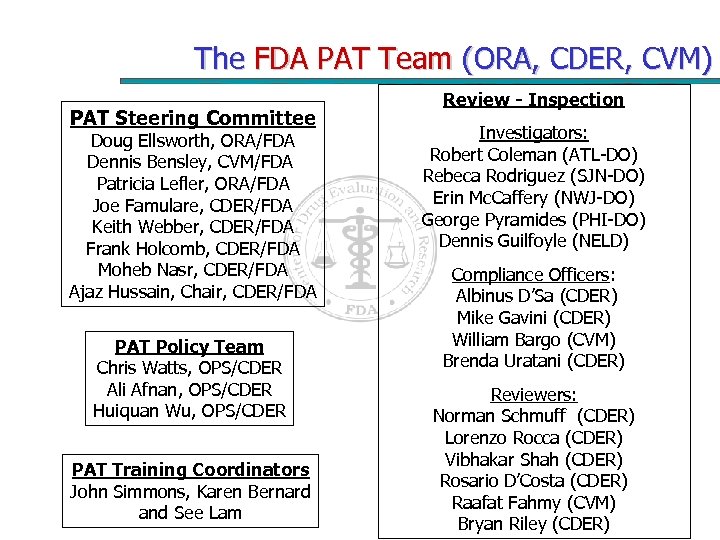 The FDA PAT Team (ORA, CDER, CVM) PAT Steering Committee Doug Ellsworth, ORA/FDA Dennis Bensley, CVM/FDA Patricia Lefler, ORA/FDA Joe Famulare, CDER/FDA Keith Webber, CDER/FDA Frank Holcomb, CDER/FDA Moheb Nasr, CDER/FDA Ajaz Hussain, Chair, CDER/FDA PAT Policy Team Chris Watts, OPS/CDER Ali Afnan, OPS/CDER Huiquan Wu, OPS/CDER PAT Training Coordinators John Simmons, Karen Bernard and See Lam Review - Inspection Investigators: Robert Coleman (ATL-DO) Rebeca Rodriguez (SJN-DO) Erin Mc. Caffery (NWJ-DO) George Pyramides (PHI-DO) Dennis Guilfoyle (NELD) Compliance Officers: Albinus D’Sa (CDER) Mike Gavini (CDER) William Bargo (CVM) Brenda Uratani (CDER) Reviewers: Norman Schmuff (CDER) Lorenzo Rocca (CDER) Vibhakar Shah (CDER) Rosario D’Costa (CDER) Raafat Fahmy (CVM) Bryan Riley (CDER)
The FDA PAT Team (ORA, CDER, CVM) PAT Steering Committee Doug Ellsworth, ORA/FDA Dennis Bensley, CVM/FDA Patricia Lefler, ORA/FDA Joe Famulare, CDER/FDA Keith Webber, CDER/FDA Frank Holcomb, CDER/FDA Moheb Nasr, CDER/FDA Ajaz Hussain, Chair, CDER/FDA PAT Policy Team Chris Watts, OPS/CDER Ali Afnan, OPS/CDER Huiquan Wu, OPS/CDER PAT Training Coordinators John Simmons, Karen Bernard and See Lam Review - Inspection Investigators: Robert Coleman (ATL-DO) Rebeca Rodriguez (SJN-DO) Erin Mc. Caffery (NWJ-DO) George Pyramides (PHI-DO) Dennis Guilfoyle (NELD) Compliance Officers: Albinus D’Sa (CDER) Mike Gavini (CDER) William Bargo (CVM) Brenda Uratani (CDER) Reviewers: Norman Schmuff (CDER) Lorenzo Rocca (CDER) Vibhakar Shah (CDER) Rosario D’Costa (CDER) Raafat Fahmy (CVM) Bryan Riley (CDER)
 The FDA PAT Team: Training & Certification • Team Building – FDA PAT Team (CDER, ORA, CVM) • Two Didactic Sessions – FDA • Three Practica – University of Washington (CPAC) – Purdue University (CPPR) – The University of Tennessee (MCEC)
The FDA PAT Team: Training & Certification • Team Building – FDA PAT Team (CDER, ORA, CVM) • Two Didactic Sessions – FDA • Three Practica – University of Washington (CPAC) – Purdue University (CPPR) – The University of Tennessee (MCEC)
 The FDA PAT Team: Training & Certification • Completed Initial Training Program – “Lessons Learned” – Continuing Education – Involve in Next Training – Guidance Finalization • Team Approach /Inspection – Review – Inspection – Peer Review
The FDA PAT Team: Training & Certification • Completed Initial Training Program – “Lessons Learned” – Continuing Education – Involve in Next Training – Guidance Finalization • Team Approach /Inspection – Review – Inspection – Peer Review
 Team Approach to Review/Inspection: Implementation Options • Supplement (CBE, CBE-30, or PAS) can be submitted – if necessary, an inspection can be performed • Implemented under the facility's own quality system – CGMP inspections by the PAT Team or PAT certified Investigator may follow • Implemented following an inspection – by the FDA PAT Team or a PAT certified Investigator – recommendations in the inspection report serve as a summary basis of final approval • Comparability Protocol can be submitted – one or a combination of the above regulatory pathways can be adopted for implementation
Team Approach to Review/Inspection: Implementation Options • Supplement (CBE, CBE-30, or PAS) can be submitted – if necessary, an inspection can be performed • Implemented under the facility's own quality system – CGMP inspections by the PAT Team or PAT certified Investigator may follow • Implemented following an inspection – by the FDA PAT Team or a PAT certified Investigator – recommendations in the inspection report serve as a summary basis of final approval • Comparability Protocol can be submitted – one or a combination of the above regulatory pathways can be adopted for implementation
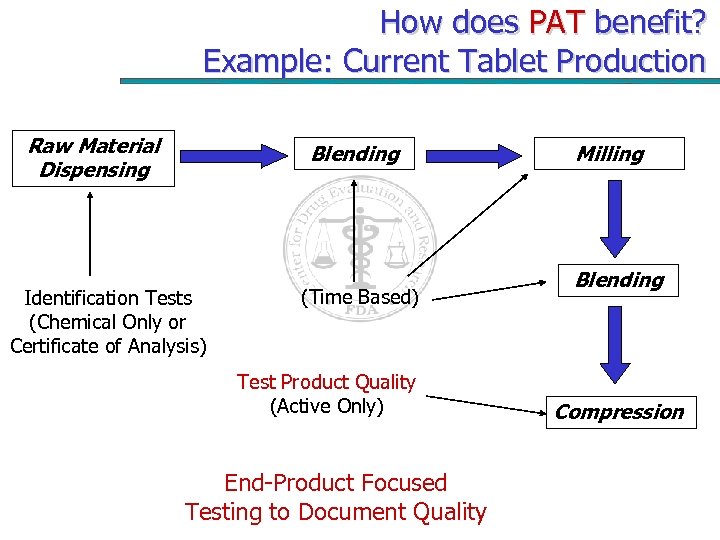 How does PAT benefit? Example: Current Tablet Production Raw Material Dispensing Blending Identification Tests (Chemical Only or Certificate of Analysis) (Time Based) Test Product Quality (Active Only) End-Product Focused Testing to Document Quality Milling Blending Compression
How does PAT benefit? Example: Current Tablet Production Raw Material Dispensing Blending Identification Tests (Chemical Only or Certificate of Analysis) (Time Based) Test Product Quality (Active Only) End-Product Focused Testing to Document Quality Milling Blending Compression
 PAT Approach: Quality by Design Focus on Process Understanding • What parameters are critical to Product Quality? (How? Why? ) – Experimental Design • How do we analyze these parameters? – Appropriate Instrumentation • How do we control these parameters throughout the process? – Control Strategy
PAT Approach: Quality by Design Focus on Process Understanding • What parameters are critical to Product Quality? (How? Why? ) – Experimental Design • How do we analyze these parameters? – Appropriate Instrumentation • How do we control these parameters throughout the process? – Control Strategy
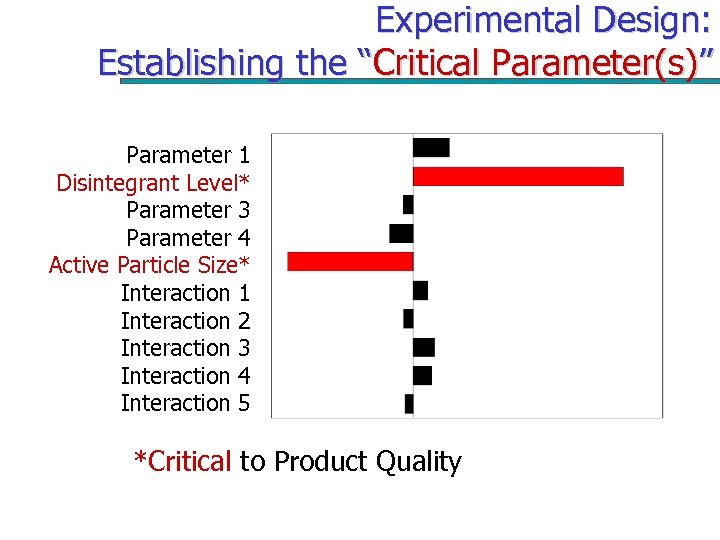 Experimental Design: Establishing the “Critical Parameter(s)” Parameter 1 Disintegrant Level* Parameter 3 Parameter 4 Active Particle Size* Interaction 1 Interaction 2 Interaction 3 Interaction 4 Interaction 5 *Critical to Product Quality
Experimental Design: Establishing the “Critical Parameter(s)” Parameter 1 Disintegrant Level* Parameter 3 Parameter 4 Active Particle Size* Interaction 1 Interaction 2 Interaction 3 Interaction 4 Interaction 5 *Critical to Product Quality
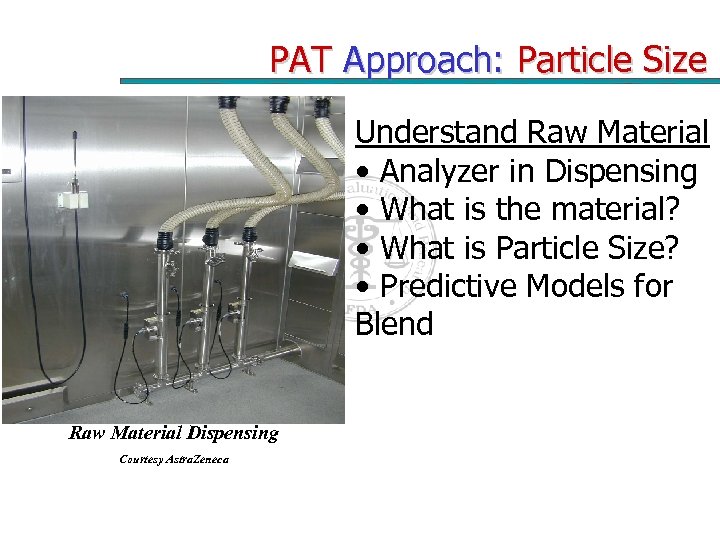 PAT Approach: Particle Size Understand Raw Material • Analyzer in Dispensing • What is the material? • What is Particle Size? • Predictive Models for Blend Raw Material Dispensing Courtesy Astra. Zeneca
PAT Approach: Particle Size Understand Raw Material • Analyzer in Dispensing • What is the material? • What is Particle Size? • Predictive Models for Blend Raw Material Dispensing Courtesy Astra. Zeneca
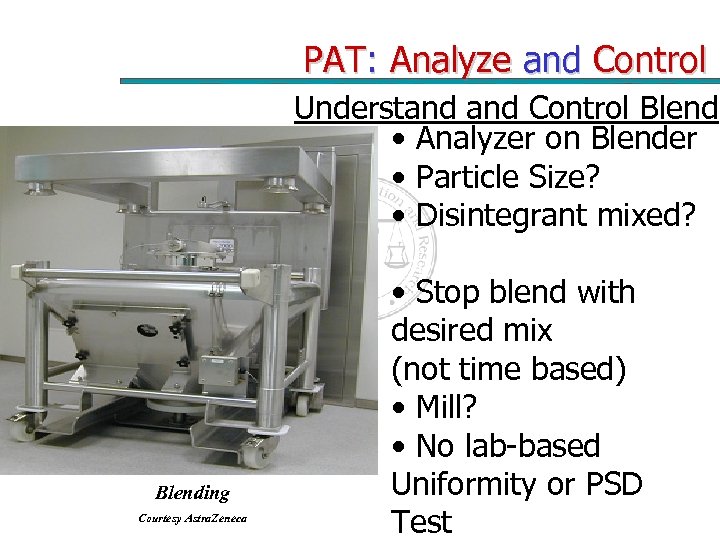 PAT: Analyze and Control Understand Control Blend • Analyzer on Blender • Particle Size? • Disintegrant mixed? Blending Courtesy Astra. Zeneca • Stop blend with desired mix (not time based) • Mill? • No lab-based Uniformity or PSD Test
PAT: Analyze and Control Understand Control Blend • Analyzer on Blender • Particle Size? • Disintegrant mixed? Blending Courtesy Astra. Zeneca • Stop blend with desired mix (not time based) • Mill? • No lab-based Uniformity or PSD Test
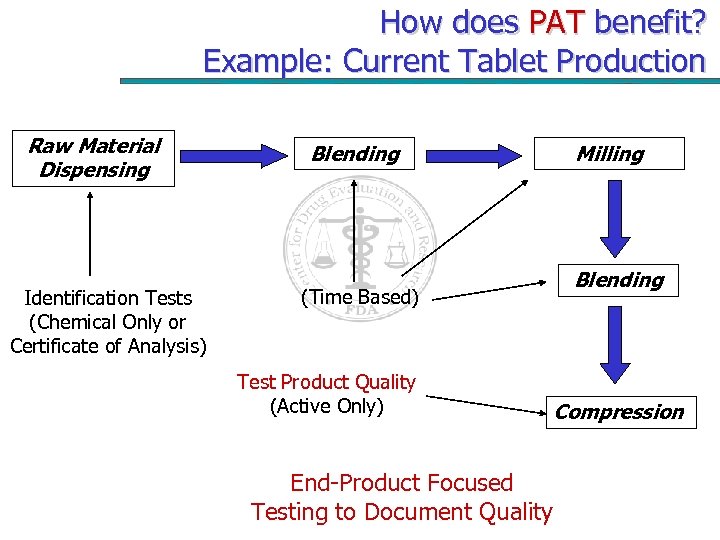 How does PAT benefit? Example: Current Tablet Production Raw Material Dispensing Identification Tests (Chemical Only or Certificate of Analysis) Blending (Time Based) Test Product Quality (Active Only) End-Product Focused Testing to Document Quality Milling Blending Compression
How does PAT benefit? Example: Current Tablet Production Raw Material Dispensing Identification Tests (Chemical Only or Certificate of Analysis) Blending (Time Based) Test Product Quality (Active Only) End-Product Focused Testing to Document Quality Milling Blending Compression
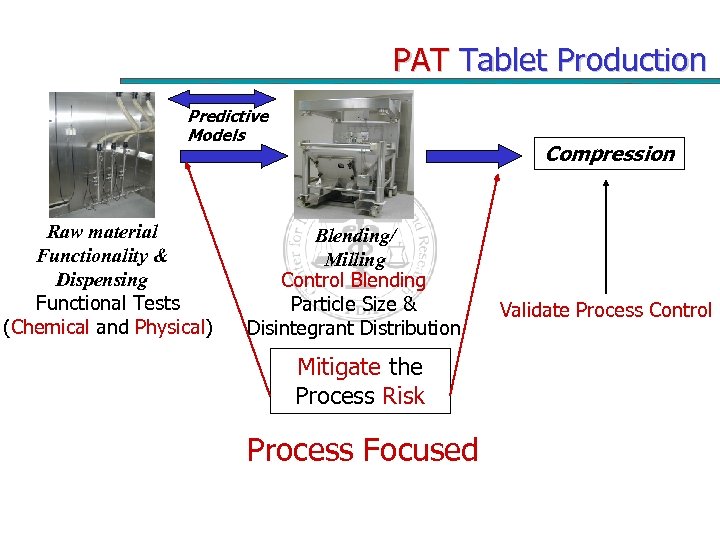 PAT Tablet Production Predictive Models Raw material Functionality & Dispensing Functional Tests (Chemical and Physical) Compression Blending/ Milling Control Blending Particle Size & Disintegrant Distribution Mitigate the Process Risk Process Focused Validate Process Control
PAT Tablet Production Predictive Models Raw material Functionality & Dispensing Functional Tests (Chemical and Physical) Compression Blending/ Milling Control Blending Particle Size & Disintegrant Distribution Mitigate the Process Risk Process Focused Validate Process Control
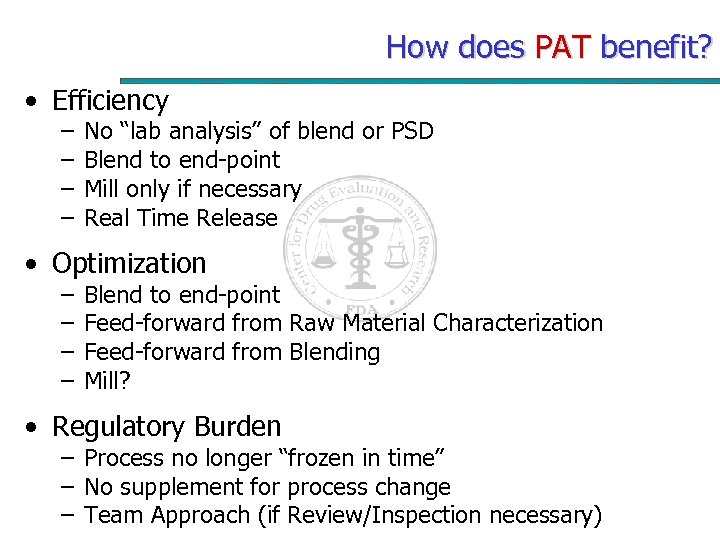 How does PAT benefit? • Efficiency – – No “lab analysis” of blend or PSD Blend to end-point Mill only if necessary Real Time Release • Optimization – – Blend to end-point Feed-forward from Raw Material Characterization Feed-forward from Blending Mill? • Regulatory Burden – Process no longer “frozen in time” – No supplement for process change – Team Approach (if Review/Inspection necessary)
How does PAT benefit? • Efficiency – – No “lab analysis” of blend or PSD Blend to end-point Mill only if necessary Real Time Release • Optimization – – Blend to end-point Feed-forward from Raw Material Characterization Feed-forward from Blending Mill? • Regulatory Burden – Process no longer “frozen in time” – No supplement for process change – Team Approach (if Review/Inspection necessary)
 Summary: Process Understanding and PAT • Inverse relationship between the level of process understanding and the risk of producing a poor quality product • Well understood process less restrictive regulatory approaches to manage change • Focus on process understanding can facilitate riskmanaged regulatory decisions and innovation • Team Approach to Review/Inspection – Several Options for Implementation
Summary: Process Understanding and PAT • Inverse relationship between the level of process understanding and the risk of producing a poor quality product • Well understood process less restrictive regulatory approaches to manage change • Focus on process understanding can facilitate riskmanaged regulatory decisions and innovation • Team Approach to Review/Inspection – Several Options for Implementation
 Next Steps for PAT • Finalize PAT Guidance • Expand the Scope of PAT – Office of Biotechnology Products • Continued Training of FDA Staff • ASTM Technical Committee • Research (Intra- and Extramural) – – Office of Testing and Research Pfizer CRADA NSF IAG Support Policy Development and Training
Next Steps for PAT • Finalize PAT Guidance • Expand the Scope of PAT – Office of Biotechnology Products • Continued Training of FDA Staff • ASTM Technical Committee • Research (Intra- and Extramural) – – Office of Testing and Research Pfizer CRADA NSF IAG Support Policy Development and Training
 Contact • Email: – PAT@cder. fda. gov – wattsc@cder. fda. gov • PAT on the Web: – http: //www. fda. gov/cder/OPS/PAT. htm • Phone: – (301)-443 -5197
Contact • Email: – PAT@cder. fda. gov – wattsc@cder. fda. gov • PAT on the Web: – http: //www. fda. gov/cder/OPS/PAT. htm • Phone: – (301)-443 -5197


