47a6299e160c69b617a2dbc4f2063eee.ppt
- Количество слайдов: 60
 Process control in Hematology section 1 M. jalalian 16 -Mar-18
Process control in Hematology section 1 M. jalalian 16 -Mar-18
 2 25 September 2013
2 25 September 2013
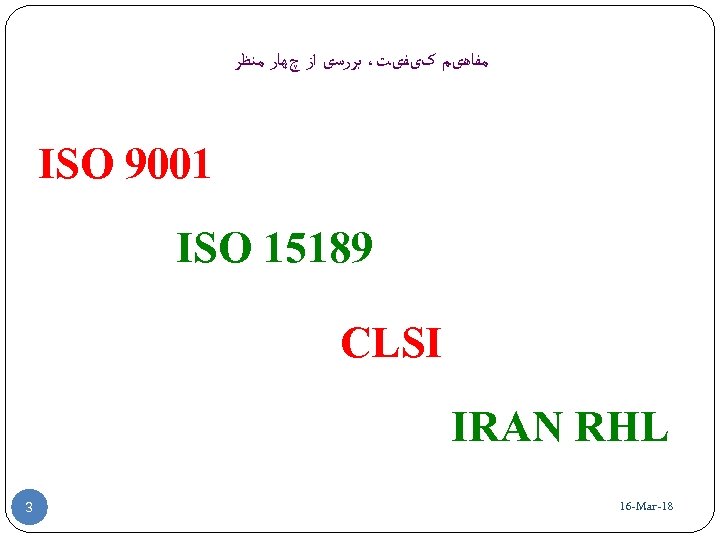 ﻣﻔﺎﻫیﻢ کیﻔیﺖ، ﺑﺮﺭﺳی ﺍﺯ چﻬﺎﺭ ﻣﻨﻈﺮ 1009 ISO 98151 ISO CLSI IRAN RHL 81 - 16 -Mar 3
ﻣﻔﺎﻫیﻢ کیﻔیﺖ، ﺑﺮﺭﺳی ﺍﺯ چﻬﺎﺭ ﻣﻨﻈﺮ 1009 ISO 98151 ISO CLSI IRAN RHL 81 - 16 -Mar 3
 9002 GP 22 A 2 CLSI ﻫﺴﺘﻪ ﻣﺮکﺰی ﻣﺪیﺮیﺖ کیﻔیﺖ ﻭ ﺗﻀﻤیﻦ کیﻔیﺖ ﻣﺤﺴﻮﺏ ﺷﺪﻩ ﻭ Run کﺎﺭی ﺭﺍ ﻣﻮﺭﺩ ﺍﻋﺘﻤﺎﺩ ﻣی ﻧﻤﺎیﺪ ﻭ ﺟﺎیگﺎﻩ آﻦ ﺩﺭ SOP ﻫﺮ آﺰﻣﻮﻥ ﻣی ﺑﺎﺷﺪ ﺍیﻦ ﻓﺮآیﻨﺪ ﺧﻄﺎی ﺳیﺴﺘﻢ، ﻣﺤیﻂ ﻭ پﺮﺳﻨﻞ ﺭﺍ ﺑﻪ ﻫﻨگﺎﻡ ﺍﻧﺠﺎﻡ آﺰﻣﺎیﺶ ﺷﻨﺎﺳﺎیی، ﺍﺭﺯیﺎﺑی ﻭ ﺗﺼﺤیﺢ ﻣی ﻧﻤﺎیﺪ، پیﺶ ﺍﺯ ﺍیﻨکﻪ ﺑﻪ ﺑیﻤﺎﺭ پﺎﺳﺦ ﺩﺍﺩﻩ ﺷﻮﺩ 3102 25 September 4
9002 GP 22 A 2 CLSI ﻫﺴﺘﻪ ﻣﺮکﺰی ﻣﺪیﺮیﺖ کیﻔیﺖ ﻭ ﺗﻀﻤیﻦ کیﻔیﺖ ﻣﺤﺴﻮﺏ ﺷﺪﻩ ﻭ Run کﺎﺭی ﺭﺍ ﻣﻮﺭﺩ ﺍﻋﺘﻤﺎﺩ ﻣی ﻧﻤﺎیﺪ ﻭ ﺟﺎیگﺎﻩ آﻦ ﺩﺭ SOP ﻫﺮ آﺰﻣﻮﻥ ﻣی ﺑﺎﺷﺪ ﺍیﻦ ﻓﺮآیﻨﺪ ﺧﻄﺎی ﺳیﺴﺘﻢ، ﻣﺤیﻂ ﻭ پﺮﺳﻨﻞ ﺭﺍ ﺑﻪ ﻫﻨگﺎﻡ ﺍﻧﺠﺎﻡ آﺰﻣﺎیﺶ ﺷﻨﺎﺳﺎیی، ﺍﺭﺯیﺎﺑی ﻭ ﺗﺼﺤیﺢ ﻣی ﻧﻤﺎیﺪ، پیﺶ ﺍﺯ ﺍیﻨکﻪ ﺑﻪ ﺑیﻤﺎﺭ پﺎﺳﺦ ﺩﺍﺩﻩ ﺷﻮﺩ 3102 25 September 4
 9002 GP 22 A 2 CLSI ﺑﺎ کﻨﺘﺮﻝ ﻭﺭﻭﺩی ﻭ ﻋﻤﻠکﺮﺩ، ﺧﺮﻭﺟی ﺭﺍ ﺗﻀﻤیﻦ ﻣی ﻧﻤﺎیﺪ 3102 25 September 5
9002 GP 22 A 2 CLSI ﺑﺎ کﻨﺘﺮﻝ ﻭﺭﻭﺩی ﻭ ﻋﻤﻠکﺮﺩ، ﺧﺮﻭﺟی ﺭﺍ ﺗﻀﻤیﻦ ﻣی ﻧﻤﺎیﺪ 3102 25 September 5
 9002 GP 22 A 2 CLSI ﺑیﻦ ﺍﻫﺪﺍﻑ کیﻔیﺖ ﻫﻤﺮﺍﺳﺘﺎیی ﺍیﺠﺎﺩ ﻣی ﻧﻤﺎیﺪ 3102 25 September 6
9002 GP 22 A 2 CLSI ﺑیﻦ ﺍﻫﺪﺍﻑ کیﻔیﺖ ﻫﻤﺮﺍﺳﺘﺎیی ﺍیﺠﺎﺩ ﻣی ﻧﻤﺎیﺪ 3102 25 September 6
 GP 22 A 2 CLSI 2009 7 25 September 2013
GP 22 A 2 CLSI 2009 7 25 September 2013
 9002 GP 22 A 2 CLSI ﺗﻌﻬﺪ ﻫﻤﻪ ﻣﺪیﺮﺍﻥ ﺩﺭ ﺗﻤﺎﻣی ﺳﻄﻮﺡ 3102 25 September 8
9002 GP 22 A 2 CLSI ﺗﻌﻬﺪ ﻫﻤﻪ ﻣﺪیﺮﺍﻥ ﺩﺭ ﺗﻤﺎﻣی ﺳﻄﻮﺡ 3102 25 September 8
 GP 22 A 2 CLSI 2009 Efficiency 9 Effectiveness Traceability 25 September 2013
GP 22 A 2 CLSI 2009 Efficiency 9 Effectiveness Traceability 25 September 2013
 WHO ﻗﺴﻤﺖ 6 ﻭ 7 ﺭﺍﻫﻨﻤﺎی چﺮﺍ کﻨﺘﺮﻝ کیﻔیﺖ ﺑﺨﺶ ﻫﻤﺎﺗﻮﻟﻮژی ﺍﺯ ﺍﻫﻤیﺖ آﻤﻮﺯﺷی ﺯیﺎﺩی ﺑﺮﺧﻮﺭﺩﺍﺭ ﺍﺳﺖ؟ QC for varying methods: Quality control processes vary, depending on whether the laboratory examinations use methods that produce quantitative, qualitative, or semi-quantitative results. These examinations differ in the following ways. 10 16 -Mar-18
WHO ﻗﺴﻤﺖ 6 ﻭ 7 ﺭﺍﻫﻨﻤﺎی چﺮﺍ کﻨﺘﺮﻝ کیﻔیﺖ ﺑﺨﺶ ﻫﻤﺎﺗﻮﻟﻮژی ﺍﺯ ﺍﻫﻤیﺖ آﻤﻮﺯﺷی ﺯیﺎﺩی ﺑﺮﺧﻮﺭﺩﺍﺭ ﺍﺳﺖ؟ QC for varying methods: Quality control processes vary, depending on whether the laboratory examinations use methods that produce quantitative, qualitative, or semi-quantitative results. These examinations differ in the following ways. 10 16 -Mar-18
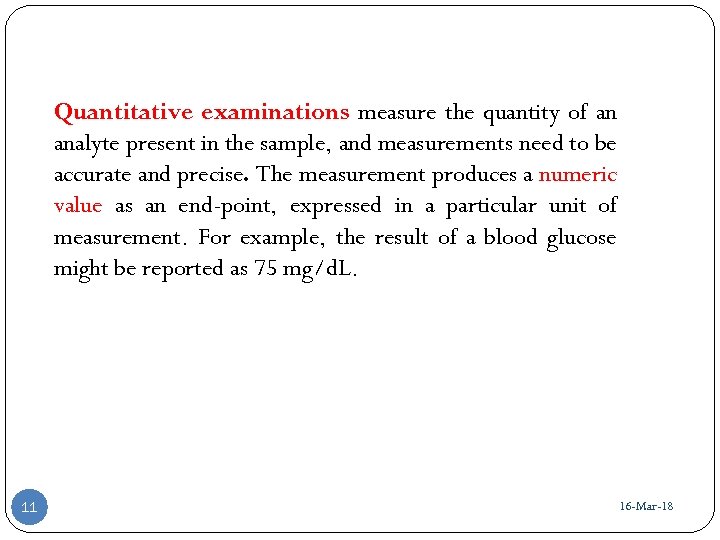 Quantitative examinations measure the quantity of an analyte present in the sample, and measurements need to be accurate and precise. The measurement produces a numeric value as an end-point, expressed in a particular unit of measurement. For example, the result of a blood glucose might be reported as 75 mg/d. L. 11 16 -Mar-18
Quantitative examinations measure the quantity of an analyte present in the sample, and measurements need to be accurate and precise. The measurement produces a numeric value as an end-point, expressed in a particular unit of measurement. For example, the result of a blood glucose might be reported as 75 mg/d. L. 11 16 -Mar-18
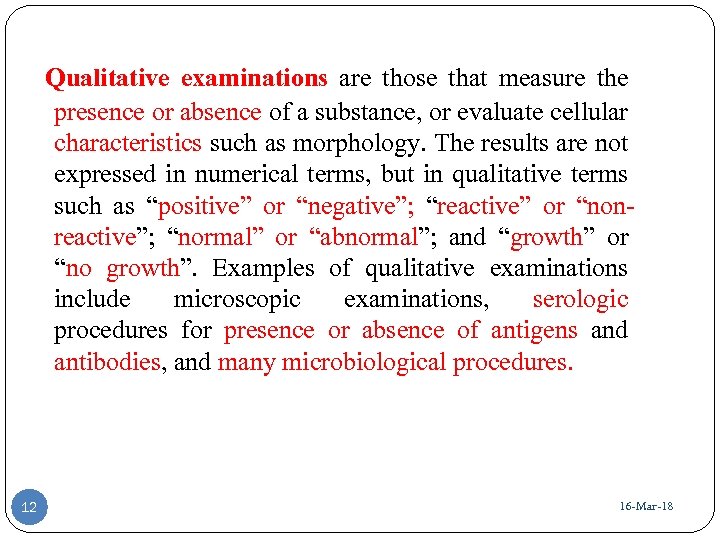 Qualitative examinations are those that measure the presence or absence of a substance, or evaluate cellular characteristics such as morphology. The results are not expressed in numerical terms, but in qualitative terms such as “positive” or “negative”; “reactive” or “nonreactive”; “normal” or “abnormal”; and “growth” or “no growth”. Examples of qualitative examinations include microscopic examinations, serologic procedures for presence or absence of antigens and antibodies, and many microbiological procedures. 12 16 -Mar-18
Qualitative examinations are those that measure the presence or absence of a substance, or evaluate cellular characteristics such as morphology. The results are not expressed in numerical terms, but in qualitative terms such as “positive” or “negative”; “reactive” or “nonreactive”; “normal” or “abnormal”; and “growth” or “no growth”. Examples of qualitative examinations include microscopic examinations, serologic procedures for presence or absence of antigens and antibodies, and many microbiological procedures. 12 16 -Mar-18
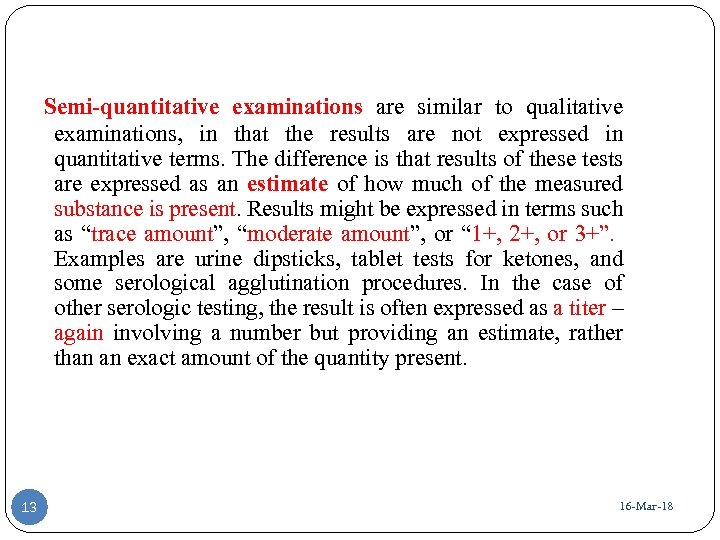 Semi-quantitative examinations are similar to qualitative examinations, in that the results are not expressed in quantitative terms. The difference is that results of these tests are expressed as an estimate of how much of the measured substance is present. Results might be expressed in terms such as “trace amount”, “moderate amount”, or “ 1+, 2+, or 3+”. Examples are urine dipsticks, tablet tests for ketones, and some serological agglutination procedures. In the case of other serologic testing, the result is often expressed as a titer – again involving a number but providing an estimate, rather than an exact amount of the quantity present. 13 16 -Mar-18
Semi-quantitative examinations are similar to qualitative examinations, in that the results are not expressed in quantitative terms. The difference is that results of these tests are expressed as an estimate of how much of the measured substance is present. Results might be expressed in terms such as “trace amount”, “moderate amount”, or “ 1+, 2+, or 3+”. Examples are urine dipsticks, tablet tests for ketones, and some serological agglutination procedures. In the case of other serologic testing, the result is often expressed as a titer – again involving a number but providing an estimate, rather than an exact amount of the quantity present. 13 16 -Mar-18
 Some microscopic examinations are considered semiquantitative because results are reported as estimates of the number of cells seen per low power field or high power field. For example, a urine microscopic examination might report 0 -5 red blood cells seen per high power field. 14 16 -Mar-18
Some microscopic examinations are considered semiquantitative because results are reported as estimates of the number of cells seen per low power field or high power field. For example, a urine microscopic examination might report 0 -5 red blood cells seen per high power field. 14 16 -Mar-18
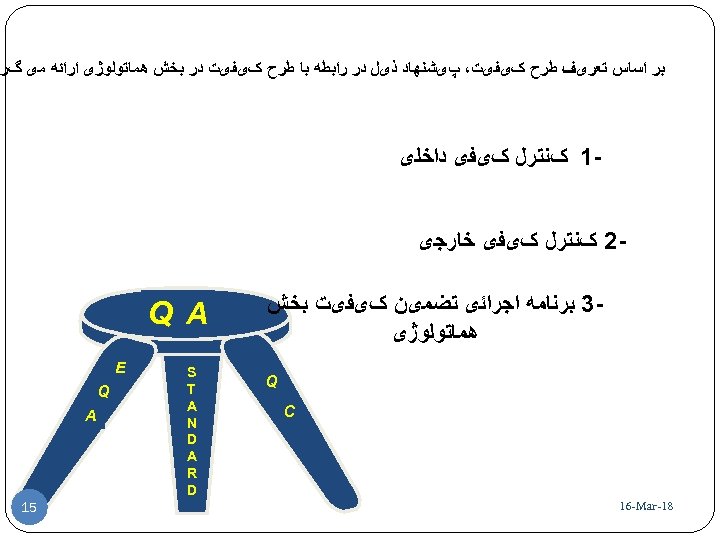 ﺑﺮ ﺍﺳﺎﺱ ﺗﻌﺮیﻒ ﻃﺮﺡ کیﻔیﺖ، پیﺸﻨﻬﺎﺩ ﺫیﻞ ﺩﺭ ﺭﺍﺑﻄﻪ ﺑﺎ ﻃﺮﺡ کیﻔیﺖ ﺩﺭ ﺑﺨﺶ ﻫﻤﺎﺗﻮﻟﻮژی ﺍﺭﺍﺋﻪ ﻣی گﺮ 1 کﻨﺘﺮﻝ کیﻔی ﺩﺍﺧﻠی 2 کﻨﺘﺮﻝ کیﻔی ﺧﺎﺭﺟی 3 ﺑﺮﻧﺎﻣﻪ ﺍﺟﺮﺍﺋی ﺗﻀﻤیﻦ کیﻔیﺖ ﺑﺨﺶ ﻫﻤﺎﺗﻮﻟﻮژی Q C 81 - 16 -Mar Q A S T A N D A R D E Q A 51
ﺑﺮ ﺍﺳﺎﺱ ﺗﻌﺮیﻒ ﻃﺮﺡ کیﻔیﺖ، پیﺸﻨﻬﺎﺩ ﺫیﻞ ﺩﺭ ﺭﺍﺑﻄﻪ ﺑﺎ ﻃﺮﺡ کیﻔیﺖ ﺩﺭ ﺑﺨﺶ ﻫﻤﺎﺗﻮﻟﻮژی ﺍﺭﺍﺋﻪ ﻣی گﺮ 1 کﻨﺘﺮﻝ کیﻔی ﺩﺍﺧﻠی 2 کﻨﺘﺮﻝ کیﻔی ﺧﺎﺭﺟی 3 ﺑﺮﻧﺎﻣﻪ ﺍﺟﺮﺍﺋی ﺗﻀﻤیﻦ کیﻔیﺖ ﺑﺨﺶ ﻫﻤﺎﺗﻮﻟﻮژی Q C 81 - 16 -Mar Q A S T A N D A R D E Q A 51
 ﻃﺮﺣﺮیﺰی ﺳیﺴﺘﻢ ﺑﻪ ﻣﻨﻈﻮﺭ ﺍﺟﺮﺍی ﺍﺛﺮﺑﺨﺶ ﻣﺪﺍﺭک ﺳﻮﺍﺑﻖ 81 - 16 -Mar 61
ﻃﺮﺣﺮیﺰی ﺳیﺴﺘﻢ ﺑﻪ ﻣﻨﻈﻮﺭ ﺍﺟﺮﺍی ﺍﺛﺮﺑﺨﺶ ﻣﺪﺍﺭک ﺳﻮﺍﺑﻖ 81 - 16 -Mar 61
 -1 کﻨﺘﺮﻝ کیﻔی ﺩﺍﺧﻠی 81 - 16 -Mar 71
-1 کﻨﺘﺮﻝ کیﻔی ﺩﺍﺧﻠی 81 - 16 -Mar 71
 1 -1 ﺗﺎییﺪ ﺻﺤﺖ ﻋﻤﻠکﺮﺩ ﺩﺳﺘگﺎﻩ ﺳﻞ کﺎﻧﺘﺮ ﺑﻪ ﻫﻨگﺎﻡ ﻧﺼﺐ، پﺲ ﺍﺯ ﺳﺮﻭیﺲ ﻭ یﺎ ﺳیکﻞ ﺯﻣﺎﻧی ﻣﺸﺨﺺ)آﺰﻣﻮﻥ ﻫﺎی ﺩﻗﺖ ﻭ ﺻﺤﺖ: ﺷﺎﻣﻞ ﺗکﺮﺍﺭ پﺬیﺮی، کﺎﻟیﺒﺮﺍﺳیﻮﻥ( ﻧکﺘﻪ: ﺗﺎییﺪ ﺻﺤﺖ ﻋﻤﻠکﺮﺩ ﺳﺎیﺮ ﺗﺠﻬیﺰﺍﺕ ﺑﺎ ﺍﻟﺰﺍﻣﺎﺕ ﻣﺮﺑﻮﻁ ﺑﻪ ﻫﺮ ﺗﺠﻬیﺰ ) ﻧﻈیﺮ آﻨچﻪ ﺑﻪ ﻋﻨﻮﺍﻥ ﻣﺜﺎﻝ ﺩﺭ ﻣﻮﺭﺩ ﺳﻞ کﺎﻧﺘﺮ ﺑیﺎﻥ ﺷﺪ ( ﺷﺎﻣﻞ: ﻓﺘﻮﻣﺘﺮ، ﺍﺳپکﺘﺮﻭﻓﻮﺗﻮﻣﺘﺮ، کﻮﺍگﻮﻟﻮﻣﺘﺮ، ﺑﻦ ﻣﺎﺭی، ﺳﻤپﻠﺮﻫﺎ، پیپﺖ ﻫﺎ ﻭ ﺳﺎیﺮ ﺷیﺸﻪ آﻼﺕ ﺣﺠﻤی، ﺗﺮﻣﻮﻣﺘﺮﻫﺎ، ﻣیکﺮﻭﺳﺎﻧﺘﺮیﻔیﻮژ ﻭ ﻏیﺮﻩ ﺑﺎیﺪ ﺍﻧﺠﺎﻡ ﺷﺪﻩ ﻭ ﻧﺘﺎیﺞ آﻦ ﺛﺒﺖ گﺮﺩﺩ. Validation ﺻﺤﻪ گﺬﺍﺭی 81 - 16 -Mar Verification ﺗﺼﺪیﻖ 81
1 -1 ﺗﺎییﺪ ﺻﺤﺖ ﻋﻤﻠکﺮﺩ ﺩﺳﺘگﺎﻩ ﺳﻞ کﺎﻧﺘﺮ ﺑﻪ ﻫﻨگﺎﻡ ﻧﺼﺐ، پﺲ ﺍﺯ ﺳﺮﻭیﺲ ﻭ یﺎ ﺳیکﻞ ﺯﻣﺎﻧی ﻣﺸﺨﺺ)آﺰﻣﻮﻥ ﻫﺎی ﺩﻗﺖ ﻭ ﺻﺤﺖ: ﺷﺎﻣﻞ ﺗکﺮﺍﺭ پﺬیﺮی، کﺎﻟیﺒﺮﺍﺳیﻮﻥ( ﻧکﺘﻪ: ﺗﺎییﺪ ﺻﺤﺖ ﻋﻤﻠکﺮﺩ ﺳﺎیﺮ ﺗﺠﻬیﺰﺍﺕ ﺑﺎ ﺍﻟﺰﺍﻣﺎﺕ ﻣﺮﺑﻮﻁ ﺑﻪ ﻫﺮ ﺗﺠﻬیﺰ ) ﻧﻈیﺮ آﻨچﻪ ﺑﻪ ﻋﻨﻮﺍﻥ ﻣﺜﺎﻝ ﺩﺭ ﻣﻮﺭﺩ ﺳﻞ کﺎﻧﺘﺮ ﺑیﺎﻥ ﺷﺪ ( ﺷﺎﻣﻞ: ﻓﺘﻮﻣﺘﺮ، ﺍﺳپکﺘﺮﻭﻓﻮﺗﻮﻣﺘﺮ، کﻮﺍگﻮﻟﻮﻣﺘﺮ، ﺑﻦ ﻣﺎﺭی، ﺳﻤپﻠﺮﻫﺎ، پیپﺖ ﻫﺎ ﻭ ﺳﺎیﺮ ﺷیﺸﻪ آﻼﺕ ﺣﺠﻤی، ﺗﺮﻣﻮﻣﺘﺮﻫﺎ، ﻣیکﺮﻭﺳﺎﻧﺘﺮیﻔیﻮژ ﻭ ﻏیﺮﻩ ﺑﺎیﺪ ﺍﻧﺠﺎﻡ ﺷﺪﻩ ﻭ ﻧﺘﺎیﺞ آﻦ ﺛﺒﺖ گﺮﺩﺩ. Validation ﺻﺤﻪ گﺬﺍﺭی 81 - 16 -Mar Verification ﺗﺼﺪیﻖ 81
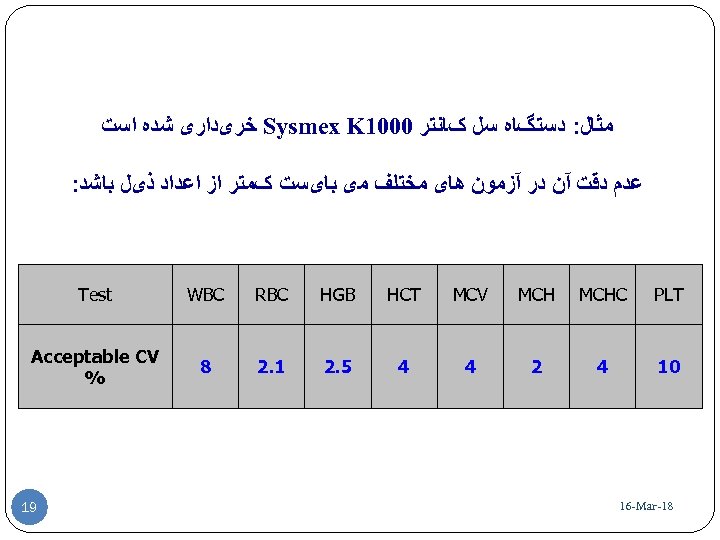 ﻣﺜﺎﻝ: ﺩﺳﺘگﺎﻩ ﺳﻞ کﺎﻧﺘﺮ 0001 Sysmex K ﺧﺮیﺪﺍﺭی ﺷﺪﻩ ﺍﺳﺖ ﻋﺪﻡ ﺩﻗﺖ آﻦ ﺩﺭ آﺰﻣﻮﻥ ﻫﺎی ﻣﺨﺘﻠﻒ ﻣی ﺑﺎیﺴﺖ کﻤﺘﺮ ﺍﺯ ﺍﻋﺪﺍﺩ ﺫیﻞ ﺑﺎﺷﺪ: PLT MCHC MCH MCV HCT HGB RBC WBC Test 01 4 2 4 4 5. 2 1. 2 8 Acceptable CV % 81 - 16 -Mar 91
ﻣﺜﺎﻝ: ﺩﺳﺘگﺎﻩ ﺳﻞ کﺎﻧﺘﺮ 0001 Sysmex K ﺧﺮیﺪﺍﺭی ﺷﺪﻩ ﺍﺳﺖ ﻋﺪﻡ ﺩﻗﺖ آﻦ ﺩﺭ آﺰﻣﻮﻥ ﻫﺎی ﻣﺨﺘﻠﻒ ﻣی ﺑﺎیﺴﺖ کﻤﺘﺮ ﺍﺯ ﺍﻋﺪﺍﺩ ﺫیﻞ ﺑﺎﺷﺪ: PLT MCHC MCH MCV HCT HGB RBC WBC Test 01 4 2 4 4 5. 2 1. 2 8 Acceptable CV % 81 - 16 -Mar 91
 1 -2 کﺎﻟیﺒﺮ کﺮﺩﻥ یﺎ ﺗﻐییﺮ ﻓﺎکﺘﻮﺭ کﺎﻟیﺒﺮﺍﺳیﻮﻥ ) ( CF ﺩﺳﺘگﺎﻩ ﺳﻞ کﺎﻧﺘﺮ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ پﻨﺞ ﻧﻤﻮﻧﻪ CBC ﻭ ﻣﻘﺎیﺴﻪ ﺭﻭﺵ ﺩﺳﺘی ﻭ ﺩﺳﺘگﺎﻫی ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﻧﺮﻡ ﺍﻓﺰﺍﺭ کﻨﺘﺮﻝ کیﻔی ﻫﺮ ﺷﺶ ﻣﺎﻩ یکﺒﺎﺭ ﻭ یﺎ ﺩﺭ ﻫﺮ ﺯﻣﺎﻧی کﻪ ﻻﺯﻡ ﺍﺳﺖ ) ﺑﻪ ﻫﻨگﺎﻡ ﺳﺮﻭیﺲ ﺩﺳﺘگﺎﻩ یﺎ ﺯﻣﺎﻧی کﻪ ﻧﺘﺎیﺞ QC ﻧیﺎﺯ ﺑﻪ کﺎﻟیﺒﺮﺍﺳیﻮﻥ ﺭﺍ ﺍﺛﺒﺎﺕ ﻧﻤﺎیﺪ ﻭ یﺎ ﺗﻐییﺮ ﺩﺭ ﻧﻮﻉ ﺍیﺰﻭﺗﻮﻥ ﻭ ﻻیﺰ (. 81 - 16 -Mar 02
1 -2 کﺎﻟیﺒﺮ کﺮﺩﻥ یﺎ ﺗﻐییﺮ ﻓﺎکﺘﻮﺭ کﺎﻟیﺒﺮﺍﺳیﻮﻥ ) ( CF ﺩﺳﺘگﺎﻩ ﺳﻞ کﺎﻧﺘﺮ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ پﻨﺞ ﻧﻤﻮﻧﻪ CBC ﻭ ﻣﻘﺎیﺴﻪ ﺭﻭﺵ ﺩﺳﺘی ﻭ ﺩﺳﺘگﺎﻫی ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﻧﺮﻡ ﺍﻓﺰﺍﺭ کﻨﺘﺮﻝ کیﻔی ﻫﺮ ﺷﺶ ﻣﺎﻩ یکﺒﺎﺭ ﻭ یﺎ ﺩﺭ ﻫﺮ ﺯﻣﺎﻧی کﻪ ﻻﺯﻡ ﺍﺳﺖ ) ﺑﻪ ﻫﻨگﺎﻡ ﺳﺮﻭیﺲ ﺩﺳﺘگﺎﻩ یﺎ ﺯﻣﺎﻧی کﻪ ﻧﺘﺎیﺞ QC ﻧیﺎﺯ ﺑﻪ کﺎﻟیﺒﺮﺍﺳیﻮﻥ ﺭﺍ ﺍﺛﺒﺎﺕ ﻧﻤﺎیﺪ ﻭ یﺎ ﺗﻐییﺮ ﺩﺭ ﻧﻮﻉ ﺍیﺰﻭﺗﻮﻥ ﻭ ﻻیﺰ (. 81 - 16 -Mar 02
 کﺎﻟیﺒﺮﺍﺳیﻮﻥ ﻣﺠﺪﺩ ﺗﺴﺖ ﻫﺎ ﺑﺮﺣﺴﺐ ﺿﺮﻭﺭﺕ ﻭ ﻧیﺎﺯ ﺩﺭ ﺣیﻦ کﺎﺭ ﺧﺼﻮﺻ ﺩﺭ ﺯﻣﺎﻥ ﺗﻌﻮیﺾ ﻣﺤﻠﻮﻝ یﺎ ﺳﺮﻭیﺲ ﺩﺳﺘگﺎﻩ یﺎ ﺗﻐییﺮﺍﺕ ﻣﺤیﻄی ﺷﺪیﺪ ﺩﺭ آﺰﻣﺎیﺸگﺎﻩ Calibration 81 - 16 -Mar Adjust 12
کﺎﻟیﺒﺮﺍﺳیﻮﻥ ﻣﺠﺪﺩ ﺗﺴﺖ ﻫﺎ ﺑﺮﺣﺴﺐ ﺿﺮﻭﺭﺕ ﻭ ﻧیﺎﺯ ﺩﺭ ﺣیﻦ کﺎﺭ ﺧﺼﻮﺻ ﺩﺭ ﺯﻣﺎﻥ ﺗﻌﻮیﺾ ﻣﺤﻠﻮﻝ یﺎ ﺳﺮﻭیﺲ ﺩﺳﺘگﺎﻩ یﺎ ﺗﻐییﺮﺍﺕ ﻣﺤیﻄی ﺷﺪیﺪ ﺩﺭ آﺰﻣﺎیﺸگﺎﻩ Calibration 81 - 16 -Mar Adjust 12
 ﺩﺭ کﺎﻟیﺒﺮﺍﺳیﻮﻥ ﻣی ﺑﺎیﺴﺖ ﺑﻪ ﻗﺎﺑﻠیﺖ ﺭﺩیﺎﺑی ﺧیﻠی ﺗﻮﺟﻪ ﺷﻮﺩ 81 - 16 -Mar 22
ﺩﺭ کﺎﻟیﺒﺮﺍﺳیﻮﻥ ﻣی ﺑﺎیﺴﺖ ﺑﻪ ﻗﺎﺑﻠیﺖ ﺭﺩیﺎﺑی ﺧیﻠی ﺗﻮﺟﻪ ﺷﻮﺩ 81 - 16 -Mar 22
 1 -3 کﻨﺘﺮﻝ ﺩﻗﺖ ﺩﺳﺘگﺎﻩ ) ﺗکﺮﺍﺭ پﺬیﺮی ( ﺑﻪ ﺻﻮﺭﺕ ﻣﺎﻫیﺎﻧﻪ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﺳﻪ ﻧﻤﻮﻧﻪ ﺑﺎ پﺎﺭﺍﻣﺘﺮﻫﺎی پﺎییﻦ، ﻧﺮﻣﺎﻝ ﻭ ﺑﺎﻻ کﻪ ﻫﺮ یک 01 ﻣﺮﺗﺒﻪ ﻭ ﻣﺠﻤﻮﻋ ﺳی ﺩﺍﺩﻩ ﺑﻪ ﺩﺳﺖ ﻣی آیﺪ ﻭ CV ﻫﺮ پﺎﺭﺍﻣﺘﺮ ﻭ ﺩﺭ ﻧﻬﺎیﺖ CV ﺗﻮﺗﺎﻝ ﻣﺤﺎﺳﺒﻪ ﻭ ﺑﺎ CV ﻣﺠﺎﺯ ﺑﺮﺍی ﻫﺮ پﺎﺭﺍﻣﺘﺮ ﻣﻘﺎیﺴﻪ گﺮﺩﺩ. 81 - 16 -Mar 32
1 -3 کﻨﺘﺮﻝ ﺩﻗﺖ ﺩﺳﺘگﺎﻩ ) ﺗکﺮﺍﺭ پﺬیﺮی ( ﺑﻪ ﺻﻮﺭﺕ ﻣﺎﻫیﺎﻧﻪ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﺳﻪ ﻧﻤﻮﻧﻪ ﺑﺎ پﺎﺭﺍﻣﺘﺮﻫﺎی پﺎییﻦ، ﻧﺮﻣﺎﻝ ﻭ ﺑﺎﻻ کﻪ ﻫﺮ یک 01 ﻣﺮﺗﺒﻪ ﻭ ﻣﺠﻤﻮﻋ ﺳی ﺩﺍﺩﻩ ﺑﻪ ﺩﺳﺖ ﻣی آیﺪ ﻭ CV ﻫﺮ پﺎﺭﺍﻣﺘﺮ ﻭ ﺩﺭ ﻧﻬﺎیﺖ CV ﺗﻮﺗﺎﻝ ﻣﺤﺎﺳﺒﻪ ﻭ ﺑﺎ CV ﻣﺠﺎﺯ ﺑﺮﺍی ﻫﺮ پﺎﺭﺍﻣﺘﺮ ﻣﻘﺎیﺴﻪ گﺮﺩﺩ. 81 - 16 -Mar 32
 -1 -4 کﻨﺘﺮﻝ ﺩﻭﺭ ﺩﺳﺘگﺎﻩ ﻣیکﺮﻭ ﻫﻤﺎﺗﻮکﺮیﺖ ﺑﻪ ﺻﻮﺭﺕ ﻫﺮ ﺳﻪ ﻣﺎﻩ ﺩﻭﺭ ﻣیکﺮﻭ ﻫﻤﺎﺗﻮکﺮیﺖ ﺑﺎیﺪ ﺣﺪﺍﻗﻞ 00001 ﺩﻭﺭ ﺩﺭ ﺩﻗیﻘﻪ ﺑﺎﺷﺪ کﻨﺘﺮﻝ ﺩﻭﺭ ﺑﺎیﺪ ﺑﺎ ﺗﺎکﻮﻣﺘﺮ کﺎﻟیﺒﺮ ﺷﺪﻩ ﺍﻧﺠﺎﻡ ﺷﻮﺩ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﺷیﻮﻩ ﻫﺎی ﺳﻨﺘی ﻣی ﺗﻮﺍﻥ ﺩﺭ ﻣﺪﺕ ﺯﻣﺎﻥ ﻫﺎی ﻣﺨﺘﻠﻒ کﻨﺘﺮﻝ ﺩﻭﺭ ﺭﺍ ﺗﺼﺪیﻖ ﻧﻤﻮﺩ ) ﺑﺮﺭﺳی Hct ﺩﺭ 3 ﻭ 5 ﻭ 01 ﺩﻗیﻘﻪ ( 81 - 16 -Mar 42
-1 -4 کﻨﺘﺮﻝ ﺩﻭﺭ ﺩﺳﺘگﺎﻩ ﻣیکﺮﻭ ﻫﻤﺎﺗﻮکﺮیﺖ ﺑﻪ ﺻﻮﺭﺕ ﻫﺮ ﺳﻪ ﻣﺎﻩ ﺩﻭﺭ ﻣیکﺮﻭ ﻫﻤﺎﺗﻮکﺮیﺖ ﺑﺎیﺪ ﺣﺪﺍﻗﻞ 00001 ﺩﻭﺭ ﺩﺭ ﺩﻗیﻘﻪ ﺑﺎﺷﺪ کﻨﺘﺮﻝ ﺩﻭﺭ ﺑﺎیﺪ ﺑﺎ ﺗﺎکﻮﻣﺘﺮ کﺎﻟیﺒﺮ ﺷﺪﻩ ﺍﻧﺠﺎﻡ ﺷﻮﺩ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﺷیﻮﻩ ﻫﺎی ﺳﻨﺘی ﻣی ﺗﻮﺍﻥ ﺩﺭ ﻣﺪﺕ ﺯﻣﺎﻥ ﻫﺎی ﻣﺨﺘﻠﻒ کﻨﺘﺮﻝ ﺩﻭﺭ ﺭﺍ ﺗﺼﺪیﻖ ﻧﻤﻮﺩ ) ﺑﺮﺭﺳی Hct ﺩﺭ 3 ﻭ 5 ﻭ 01 ﺩﻗیﻘﻪ ( 81 - 16 -Mar 42
 1 -5 کﻨﺘﺮﻝ ﺗﺎیﻤﺮ ﻣیکﺮﻭﻫﻤﺎﺗﻮکﺮیﺖ، ﺳﺪیﻤﺎﻥ ﻭ ﺳﺎیﺮ کﺮﻧﻮﻣﺘﺮﻫﺎی ﻣﻮﺟﻮﺩ ﺩﺭ ﺑﺨﺶ ﺑﻪ ﺻﻮﺭﺕ ﻫﺮ ﺷﺶ ﻣﺎﻩ کﻨﺘﺮﻝ ﺯﻣﺎﻥ ﺑﺎیﺪ ﺑﺎ کﺮﻧﻮﻣﺘﺮ یﺎ ﺳﺎﻋﺖ کﺎﻟیﺒﺮ ﺷﺪﻩ ﺍﻧﺠﺎﻡ ﺷﻮﺩ ﺯﻣﺎﻥ کﻨﺘﺮﻟی ﺑﺎیﺪ ﺑﺎ ﺯﻣﺎﻥ چﺮﺧیﺪﻥ ﻟﻮﻟﻪ ﻫﺎی ﻣیکﺮﻭﻫﻤﺎﺗﻮکﺮیﺖ یکﺴﺎﻥ ﺑﺎﺷﺪ 81 - 16 -Mar 52
1 -5 کﻨﺘﺮﻝ ﺗﺎیﻤﺮ ﻣیکﺮﻭﻫﻤﺎﺗﻮکﺮیﺖ، ﺳﺪیﻤﺎﻥ ﻭ ﺳﺎیﺮ کﺮﻧﻮﻣﺘﺮﻫﺎی ﻣﻮﺟﻮﺩ ﺩﺭ ﺑﺨﺶ ﺑﻪ ﺻﻮﺭﺕ ﻫﺮ ﺷﺶ ﻣﺎﻩ کﻨﺘﺮﻝ ﺯﻣﺎﻥ ﺑﺎیﺪ ﺑﺎ کﺮﻧﻮﻣﺘﺮ یﺎ ﺳﺎﻋﺖ کﺎﻟیﺒﺮ ﺷﺪﻩ ﺍﻧﺠﺎﻡ ﺷﻮﺩ ﺯﻣﺎﻥ کﻨﺘﺮﻟی ﺑﺎیﺪ ﺑﺎ ﺯﻣﺎﻥ چﺮﺧیﺪﻥ ﻟﻮﻟﻪ ﻫﺎی ﻣیکﺮﻭﻫﻤﺎﺗﻮکﺮیﺖ یکﺴﺎﻥ ﺑﺎﺷﺪ 81 - 16 -Mar 52
 -1 -6 ﻣﻘﺎیﺴﻪ ﺩﻗﺖ ﻧﺘﺎیﺞ ﺩﻭ ﺩﺳﺘگﺎﻩ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ آﺰﻣﻮﻥ F 1 -7 ﺍﻧﺠﺎﻡ آﺰﻣﺎیﺶ Tn ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ پﻨﺞ ﻧﻤﻮﻧﻪ CBC ﺩﺭ ﺩﻭ ﺭﻭﺯ ﻣﺘﻮﺍﻟی ﺑﻪ ﺻﻮﺭﺕ ﻫﻔﺘگی ﻭ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﻧﺮﻡ ﺍﻓﺰﺍﺭ کﻨﺘﺮﻝ کیﻔی. -1 -8 ﺍﻧﺠﺎﻡ ﺗﺴﺖ ﻣﻀﺎﻋﻒ ﺑﻪ ﺻﻮﺭﺕ ﺭﻭﺯﺍﻧﻪ 1 -9 ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﺧﻮﻥ کﻨﺘﺮﻝ ﺑﻪ ﺻﻮﺭﺕ ﺭﻭﺯﺍﻧﻪ ﻭ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﺑﺮﻧﺎﻣﻪ کﻨﺘﺮﻝ کیﻔی ﺑﺎ ﻣﻨﺤﻨی ﻫﺎی پﺎیﻪ ﻟﻮی ﺟﻨیﻨگ ﻭ ﻗﻮﺍﻧیﻦ ﻭﺳﺘگﺎﺭﺩ 81 - 16 -Mar 62
-1 -6 ﻣﻘﺎیﺴﻪ ﺩﻗﺖ ﻧﺘﺎیﺞ ﺩﻭ ﺩﺳﺘگﺎﻩ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ آﺰﻣﻮﻥ F 1 -7 ﺍﻧﺠﺎﻡ آﺰﻣﺎیﺶ Tn ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ پﻨﺞ ﻧﻤﻮﻧﻪ CBC ﺩﺭ ﺩﻭ ﺭﻭﺯ ﻣﺘﻮﺍﻟی ﺑﻪ ﺻﻮﺭﺕ ﻫﻔﺘگی ﻭ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﻧﺮﻡ ﺍﻓﺰﺍﺭ کﻨﺘﺮﻝ کیﻔی. -1 -8 ﺍﻧﺠﺎﻡ ﺗﺴﺖ ﻣﻀﺎﻋﻒ ﺑﻪ ﺻﻮﺭﺕ ﺭﻭﺯﺍﻧﻪ 1 -9 ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﺧﻮﻥ کﻨﺘﺮﻝ ﺑﻪ ﺻﻮﺭﺕ ﺭﻭﺯﺍﻧﻪ ﻭ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ ﺑﺮﻧﺎﻣﻪ کﻨﺘﺮﻝ کیﻔی ﺑﺎ ﻣﻨﺤﻨی ﻫﺎی پﺎیﻪ ﻟﻮی ﺟﻨیﻨگ ﻭ ﻗﻮﺍﻧیﻦ ﻭﺳﺘگﺎﺭﺩ 81 - 16 -Mar 62
 ﺍﻃﻼﻋﺎﺕ آﻤﺎﺭی ﺩﺭ ﺍیﻦ ﺭﺍﺑﻄﻪ ﺑﺴیﺎﺭ ﺣﺎﺋﺰ ﺍﻫﻤیﺖ ﻣی ﺑﺎﺷﺪ -Mean -Median -Mode -SD -CV 81 - 16 -Mar Accurate Precise 72
ﺍﻃﻼﻋﺎﺕ آﻤﺎﺭی ﺩﺭ ﺍیﻦ ﺭﺍﺑﻄﻪ ﺑﺴیﺎﺭ ﺣﺎﺋﺰ ﺍﻫﻤیﺖ ﻣی ﺑﺎﺷﺪ -Mean -Median -Mode -SD -CV 81 - 16 -Mar Accurate Precise 72
 ﺗﻌﺮیﻒ ﻣﻔﺎﻫیﻢ ﺩﻗﺖ ﻭﺻﺤﺖ (: ﻧﺰﺩیکی ﻧﺘﺎیﺞ ﺍﻧﺪﺍﺯﻩ گیﺮی ﺷﺪﻩ ﺑﻪ ﻋﺪﺩ ﻭﺍﻗﻌی Accuracy ) ﺻﺤﺖ the closeness of measurements to the true value. (: ﻣیﺰﺍﻥ ﺗﻐییﺮﺍﺕ ﺩﺭ ﻧﺘﺎیﺞ ﺍﻧﺪﺍﺯﻩ گیﺮی Precision ) ﺩﻗﺖ the amount of variation in the measurements. 28
ﺗﻌﺮیﻒ ﻣﻔﺎﻫیﻢ ﺩﻗﺖ ﻭﺻﺤﺖ (: ﻧﺰﺩیکی ﻧﺘﺎیﺞ ﺍﻧﺪﺍﺯﻩ گیﺮی ﺷﺪﻩ ﺑﻪ ﻋﺪﺩ ﻭﺍﻗﻌی Accuracy ) ﺻﺤﺖ the closeness of measurements to the true value. (: ﻣیﺰﺍﻥ ﺗﻐییﺮﺍﺕ ﺩﺭ ﻧﺘﺎیﺞ ﺍﻧﺪﺍﺯﻩ گیﺮی Precision ) ﺩﻗﺖ the amount of variation in the measurements. 28
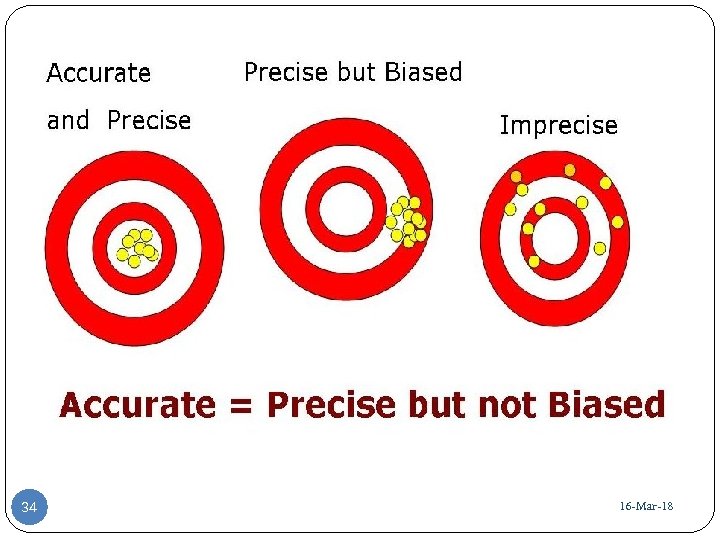
 ﺗﺴﺖ ﺑﺎ ﺩﻗﺖ ﻭ ﺻﺤﺖ ﻣﻨﺎﺳﺐ Precise and Accurate 29 16 -Mar-18
ﺗﺴﺖ ﺑﺎ ﺩﻗﺖ ﻭ ﺻﺤﺖ ﻣﻨﺎﺳﺐ Precise and Accurate 29 16 -Mar-18
 ﺧﻄﺎی ﺳیﺴﺘﻤﺎﺗیک 81 - 16 -Mar 03
ﺧﻄﺎی ﺳیﺴﺘﻤﺎﺗیک 81 - 16 -Mar 03
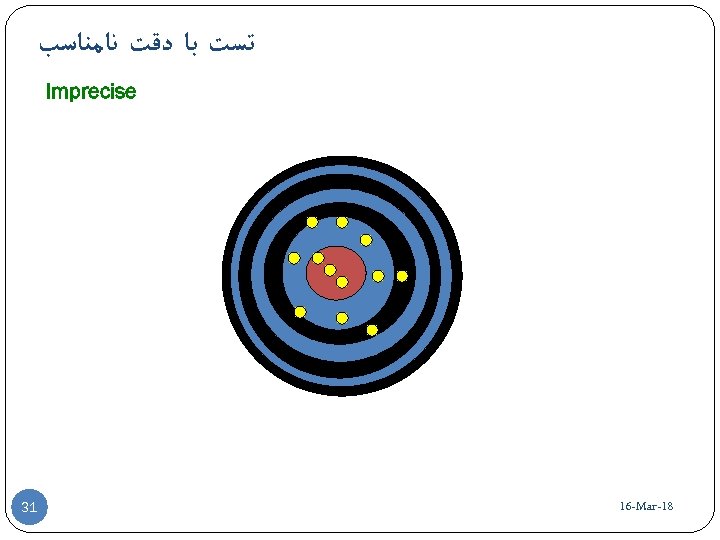 ﺗﺴﺖ ﺑﺎ ﺩﻗﺖ ﻧﺎﻣﻨﺎﺳﺐ Imprecise 81 - 16 -Mar 13
ﺗﺴﺖ ﺑﺎ ﺩﻗﺖ ﻧﺎﻣﻨﺎﺳﺐ Imprecise 81 - 16 -Mar 13
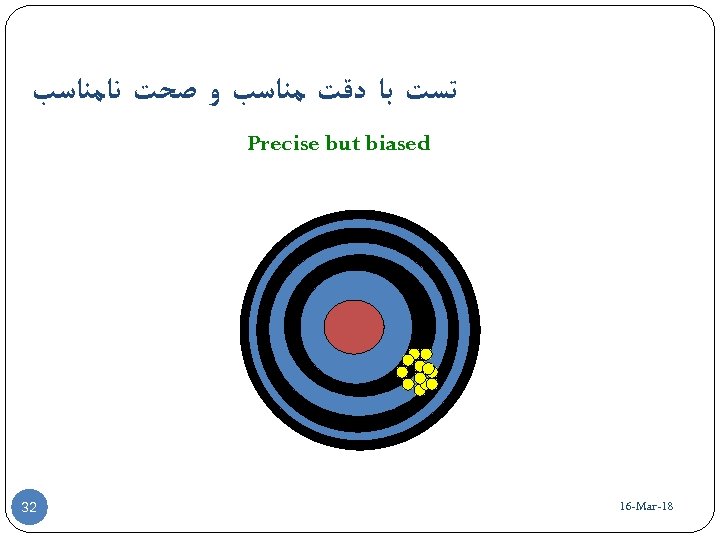 ﺗﺴﺖ ﺑﺎ ﺩﻗﺖ ﻣﻨﺎﺳﺐ ﻭ ﺻﺤﺖ ﻧﺎﻣﻨﺎﺳﺐ Precise but biased 81 - 16 -Mar 23
ﺗﺴﺖ ﺑﺎ ﺩﻗﺖ ﻣﻨﺎﺳﺐ ﻭ ﺻﺤﺖ ﻧﺎﻣﻨﺎﺳﺐ Precise but biased 81 - 16 -Mar 23
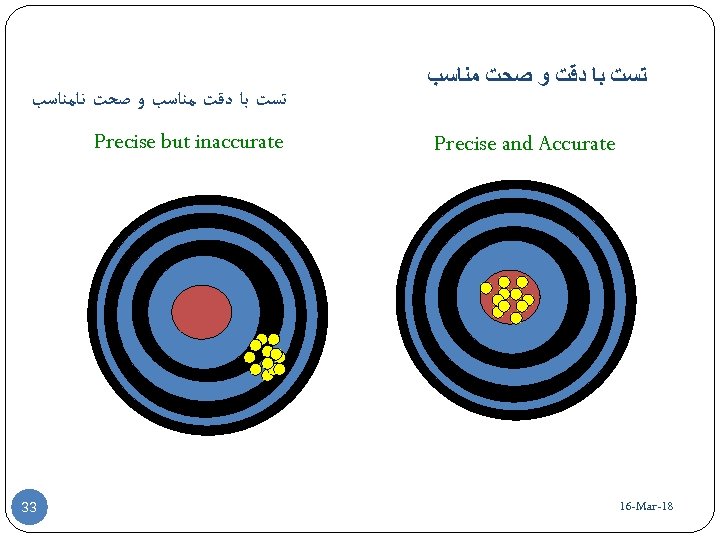 ﺗﺴﺖ ﺑﺎ ﺩﻗﺖ ﻣﻨﺎﺳﺐ ﻭ ﺻﺤﺖ ﻧﺎﻣﻨﺎﺳﺐ Precise but inaccurate 33 ﺗﺴﺖ ﺑﺎ ﺩﻗﺖ ﻭ ﺻﺤﺖ ﻣﻨﺎﺳﺐ Precise and Accurate 16 -Mar-18
ﺗﺴﺖ ﺑﺎ ﺩﻗﺖ ﻣﻨﺎﺳﺐ ﻭ ﺻﺤﺖ ﻧﺎﻣﻨﺎﺳﺐ Precise but inaccurate 33 ﺗﺴﺖ ﺑﺎ ﺩﻗﺖ ﻭ ﺻﺤﺖ ﻣﻨﺎﺳﺐ Precise and Accurate 16 -Mar-18
 34 16 -Mar-18
34 16 -Mar-18
 ﻣﻔﺎﻫیﻢ ﺍﺳﺎﺳی ﺩﺭ ﺣﻮﺯﻩ کﻨﺘﺮﻝ کیﻔیﺖ آﻤﺎﺭی 81 - 16 -Mar 53
ﻣﻔﺎﻫیﻢ ﺍﺳﺎﺳی ﺩﺭ ﺣﻮﺯﻩ کﻨﺘﺮﻝ کیﻔیﺖ آﻤﺎﺭی 81 - 16 -Mar 53
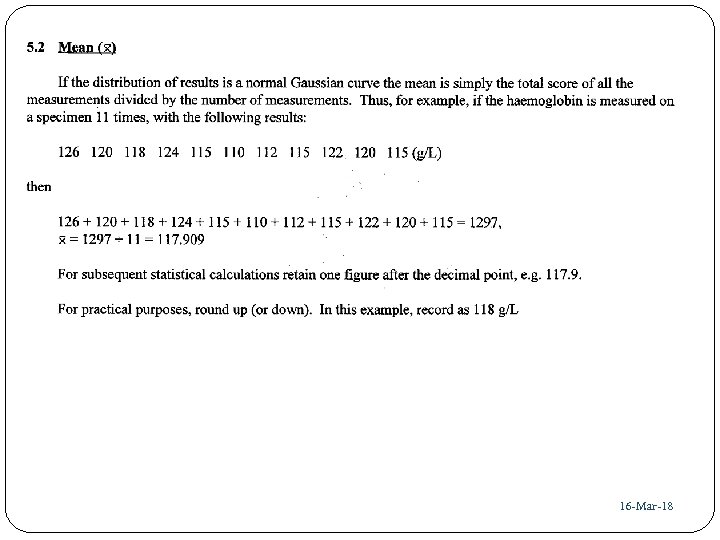 36 16 -Mar-18
36 16 -Mar-18
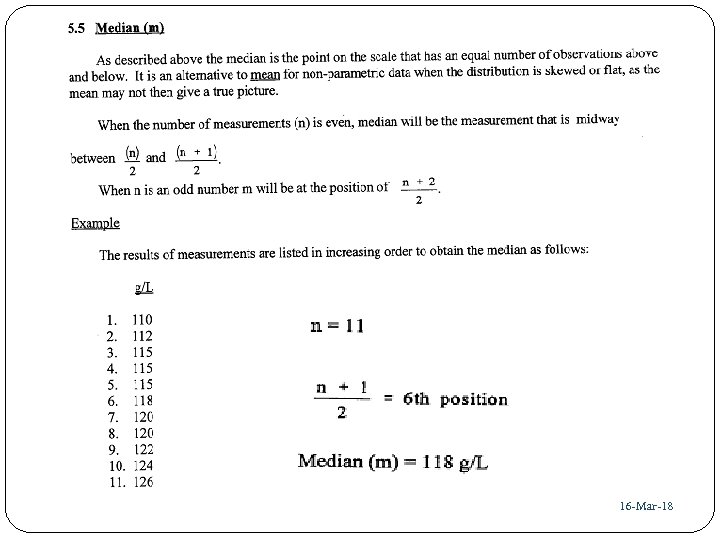 37 16 -Mar-18
37 16 -Mar-18
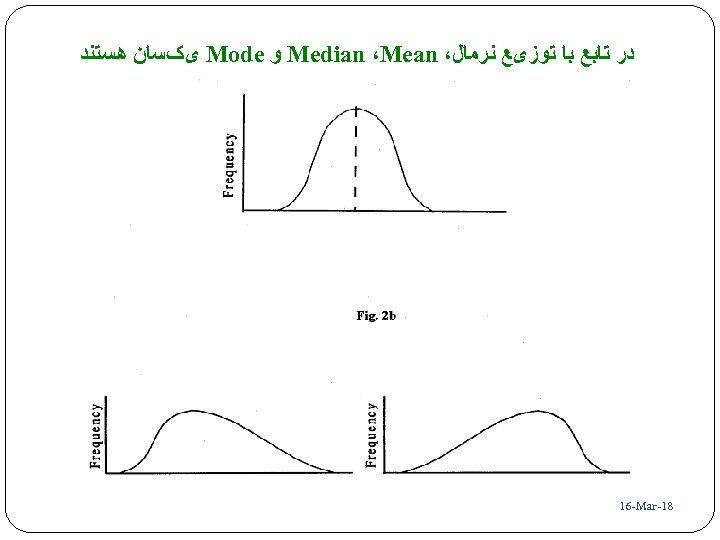 ﺩﺭ ﺗﺎﺑﻊ ﺑﺎ ﺗﻮﺯیﻊ ﻧﺮﻣﺎﻝ، Median ،Mean ﻭ Mode یکﺴﺎﻥ ﻫﺴﺘﻨﺪ 81 - 16 -Mar 83
ﺩﺭ ﺗﺎﺑﻊ ﺑﺎ ﺗﻮﺯیﻊ ﻧﺮﻣﺎﻝ، Median ،Mean ﻭ Mode یکﺴﺎﻥ ﻫﺴﺘﻨﺪ 81 - 16 -Mar 83
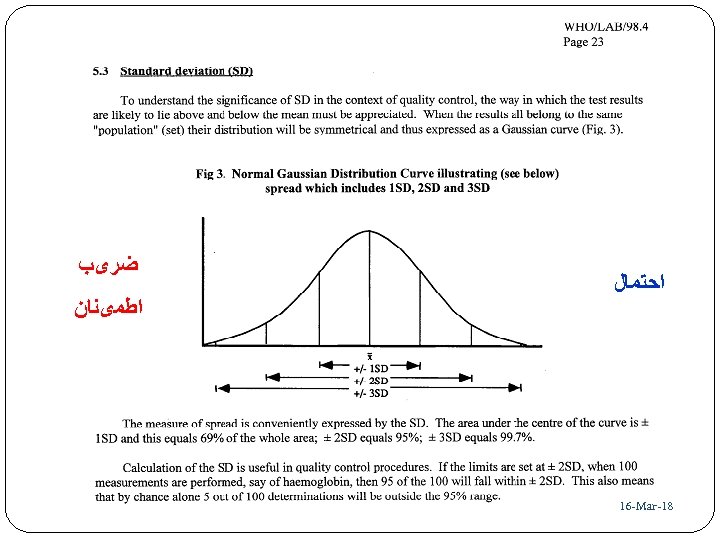 ﺍﺣﺘﻤﺎﻝ 81 - 16 -Mar ﺿﺮیﺐ ﺍﻃﻤیﻨﺎﻥ 93
ﺍﺣﺘﻤﺎﻝ 81 - 16 -Mar ﺿﺮیﺐ ﺍﻃﻤیﻨﺎﻥ 93
 40 16 -Mar-18
40 16 -Mar-18
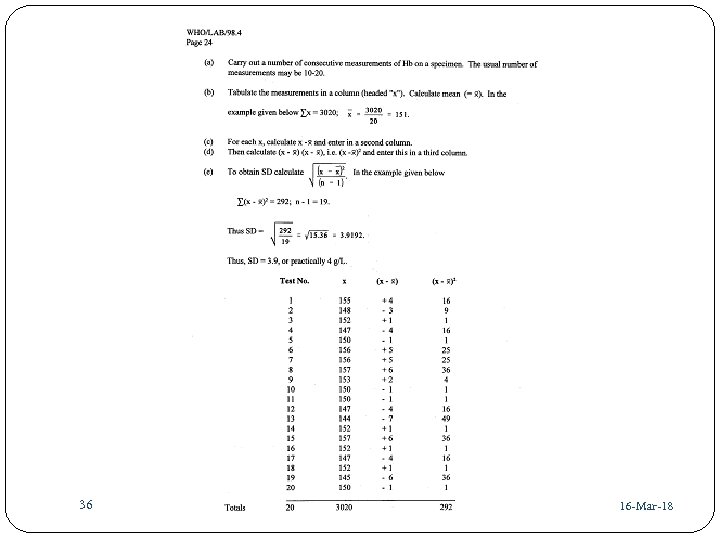 36 41 16 -Mar-18
36 41 16 -Mar-18
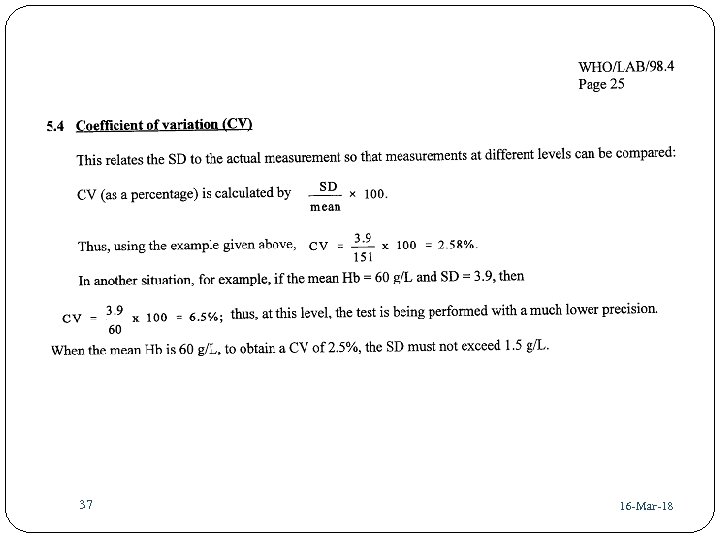 37 42 16 -Mar-18
37 42 16 -Mar-18
 آیﺎ ﻣﺤﺪﻭﺩﻩ کﻨﺘﺮﻝ ﻫﺎ ﻣی ﺗﻮﺍﻧﺪ ﻣﺒﻨﺎی ﺗﺮﺳیﻢ ﻣﻨﺤﻨی ﻫﺎی پﺎیﻪ ﺑﺎﺷﺪ؟ ﻣﺤﺪﻭﺩﻩ ﺍﻱ کﻪ ﺩﺭ ﺑﺮﻭﺷﻮﺭﻫﺎﻱ کﻨﺘﺮﻝ ﻫﺎﻱ ﺗﺠﺎﺭﻱ ﺩﺭﺝ ﺷﺪﻩ ﻧﺒﺎﻳﺪ ﺑﺮﺍﻱ ﺗﺮﺳیﻢ چﺎﺭﺕ کﻨﺘﺮﻝ کیﻔیﺖ ﺍﺳﺘﻔﺎﺩﻩ ﺷﻮﺩ. ﺍﻳﻦ ﻣﺤﺪﻭﺩﻩ ﻋﻤﺪﺗ ﺑﺮﺍﺳﺎﺱ آﺰﻣﺎﻳﺶ ﺧﻮﻥ کﻨﺘﺮﻝ ﻫﺎ ﺩﺭآﺰﻣﺎﻳﺸگﺎﻩ ﻫﺎﻱ ﻣﺨﺘﻠﻒ ﺗﻌییﻦ ﻣﻲ ﺷﻮﺩ ﻭ ﻣﺘﻐیﺮﻫﺎﻱ ﻣﺘﻌﺪﺩﻱ ﻣﺎﻧﻨﺪ ﺍﺧﺘﻼ ﻑ ﺩﺳﺘگﺎﻩ ﻫﺎ، ﺷﻤﺎﺭﻩ ﺳﺎﺧﺖ ﻫﺎﻱ ﻣﺨﺘﻠﻒ ﻣﺤﻠﻮﻝ ﻫﺎ ﻭ کﺎﻟیﺒﺮﺍﺗﻮﺭ ﺑﺮ ﺭﻭﻱ آﻦ ﺗﺎﺛیﺮ ﻣﻲ گﺬﺍﺭﻧﺪ. ﺩﺭ ﻧﺘیﺠﻪ ﻣﺤﺪﻭﺩﻩ ﻣﻨﺪﺭﺝ ﺩﺭ ﺑﺮﻭﺷﻮﺭ ﺑﺴیﺎﺭ ﺑﺰﺭگﺘﺮ ﺍﺯ ﻣﺤﺪﻭﺩﻩ ﺣﺎﺻﻞ ﺍﺯ ﻋﻤﻠکﺮﺩ ﻳک آﺰﻣﺎﻳﺸگﺎﻩ ﻣﻲ ﺑﺎﺷﺪ. ﺍﻟﺒﺘﻪ ﺩﺭ ﺷﺮﻭﻉ کﺎﺭ، ﺑﺎیﺪ ﺑﻪ یﺎﺩ ﺩﺍﺷﺖ کﻪ ﺗﺎ ﺯﻣﺎﻧﻲ کﻪ ﺗﻌﺪﺍﺩ ﻧﺘﺎﻳﺞ ﺑﻪ ﺣﺪ ﻣﻄﻠﻮﺏ ﻧﺮﺳیﺪﻩ، ﻣﺤﺪﻭﺩﻩ ﺳﺮﻡ کﻨﺘﺮﻝ ﻗﺎﺑﻞ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺳﺖ ﻻﺯﻡ ﺑﻪ ﺫکﺮ ﺍﺳﺖ ﺣﺘﻲ ﺩﺭ ﺍﺑﺘﺪﺍﻱ کﺎﺭ، ﺗﻨﻬﺎ ﺩﺭ ﺻﻮﺭﺗی ﻣﻲ ﺗﻮﺍﻥ ﺍﺯ ﻣﺤﺪﻭﺩﻩ ﻣﻨﺪﺭﺝ ﺩﺭ ﺑﺮﻭﺷﻮﺭ کﻨﺘﺮﻝ ﺍﺳﺘﻔﺎﺩﻩ ﻧﻤﻮﺩ کﻪ کﻨﺘﺮﻝ ﺑﺎ ﺩﺳﺘگﺎﻩ ﻣﻮﺭﺩ ﺍﺳﺘﻔﺎﺩﻩ ﻫﻤﺨﻮﺍﻧﻲ ﺩﺍﺷﺘﻪ ﻭ ﺍﻳﻦ ﻣﻮﺿﻮﻉ ﻣﻮﺭﺩ ﺗﺎﺋیﺪ ﺳﺎﺯﻧﺪﻩ ﺩﺳﺘگﺎﻩ ﻗﺮﺍﺭ ﺩﺍﺷﺘﻪ ﺑﺎﺷﺪ. 81 - 16 -Mar 34
آیﺎ ﻣﺤﺪﻭﺩﻩ کﻨﺘﺮﻝ ﻫﺎ ﻣی ﺗﻮﺍﻧﺪ ﻣﺒﻨﺎی ﺗﺮﺳیﻢ ﻣﻨﺤﻨی ﻫﺎی پﺎیﻪ ﺑﺎﺷﺪ؟ ﻣﺤﺪﻭﺩﻩ ﺍﻱ کﻪ ﺩﺭ ﺑﺮﻭﺷﻮﺭﻫﺎﻱ کﻨﺘﺮﻝ ﻫﺎﻱ ﺗﺠﺎﺭﻱ ﺩﺭﺝ ﺷﺪﻩ ﻧﺒﺎﻳﺪ ﺑﺮﺍﻱ ﺗﺮﺳیﻢ چﺎﺭﺕ کﻨﺘﺮﻝ کیﻔیﺖ ﺍﺳﺘﻔﺎﺩﻩ ﺷﻮﺩ. ﺍﻳﻦ ﻣﺤﺪﻭﺩﻩ ﻋﻤﺪﺗ ﺑﺮﺍﺳﺎﺱ آﺰﻣﺎﻳﺶ ﺧﻮﻥ کﻨﺘﺮﻝ ﻫﺎ ﺩﺭآﺰﻣﺎﻳﺸگﺎﻩ ﻫﺎﻱ ﻣﺨﺘﻠﻒ ﺗﻌییﻦ ﻣﻲ ﺷﻮﺩ ﻭ ﻣﺘﻐیﺮﻫﺎﻱ ﻣﺘﻌﺪﺩﻱ ﻣﺎﻧﻨﺪ ﺍﺧﺘﻼ ﻑ ﺩﺳﺘگﺎﻩ ﻫﺎ، ﺷﻤﺎﺭﻩ ﺳﺎﺧﺖ ﻫﺎﻱ ﻣﺨﺘﻠﻒ ﻣﺤﻠﻮﻝ ﻫﺎ ﻭ کﺎﻟیﺒﺮﺍﺗﻮﺭ ﺑﺮ ﺭﻭﻱ آﻦ ﺗﺎﺛیﺮ ﻣﻲ گﺬﺍﺭﻧﺪ. ﺩﺭ ﻧﺘیﺠﻪ ﻣﺤﺪﻭﺩﻩ ﻣﻨﺪﺭﺝ ﺩﺭ ﺑﺮﻭﺷﻮﺭ ﺑﺴیﺎﺭ ﺑﺰﺭگﺘﺮ ﺍﺯ ﻣﺤﺪﻭﺩﻩ ﺣﺎﺻﻞ ﺍﺯ ﻋﻤﻠکﺮﺩ ﻳک آﺰﻣﺎﻳﺸگﺎﻩ ﻣﻲ ﺑﺎﺷﺪ. ﺍﻟﺒﺘﻪ ﺩﺭ ﺷﺮﻭﻉ کﺎﺭ، ﺑﺎیﺪ ﺑﻪ یﺎﺩ ﺩﺍﺷﺖ کﻪ ﺗﺎ ﺯﻣﺎﻧﻲ کﻪ ﺗﻌﺪﺍﺩ ﻧﺘﺎﻳﺞ ﺑﻪ ﺣﺪ ﻣﻄﻠﻮﺏ ﻧﺮﺳیﺪﻩ، ﻣﺤﺪﻭﺩﻩ ﺳﺮﻡ کﻨﺘﺮﻝ ﻗﺎﺑﻞ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺳﺖ ﻻﺯﻡ ﺑﻪ ﺫکﺮ ﺍﺳﺖ ﺣﺘﻲ ﺩﺭ ﺍﺑﺘﺪﺍﻱ کﺎﺭ، ﺗﻨﻬﺎ ﺩﺭ ﺻﻮﺭﺗی ﻣﻲ ﺗﻮﺍﻥ ﺍﺯ ﻣﺤﺪﻭﺩﻩ ﻣﻨﺪﺭﺝ ﺩﺭ ﺑﺮﻭﺷﻮﺭ کﻨﺘﺮﻝ ﺍﺳﺘﻔﺎﺩﻩ ﻧﻤﻮﺩ کﻪ کﻨﺘﺮﻝ ﺑﺎ ﺩﺳﺘگﺎﻩ ﻣﻮﺭﺩ ﺍﺳﺘﻔﺎﺩﻩ ﻫﻤﺨﻮﺍﻧﻲ ﺩﺍﺷﺘﻪ ﻭ ﺍﻳﻦ ﻣﻮﺿﻮﻉ ﻣﻮﺭﺩ ﺗﺎﺋیﺪ ﺳﺎﺯﻧﺪﻩ ﺩﺳﺘگﺎﻩ ﻗﺮﺍﺭ ﺩﺍﺷﺘﻪ ﺑﺎﺷﺪ. 81 - 16 -Mar 34
 ﻫﺪﻑ ﺍﺯ ﺍﺟﺮﺍی ﻋﺪﻡ ﻗﻄﻌیﺖ: ﺑﺎ ﺗﻮﺟﻪ ﺑﻪ ﺍﻫﻤیﺖ ﺍﻧﺪﺍﺯﻩ گیﺮیﻬﺎ ﻭ ﺗﺼﻤیﻢ گیﺮی ﺑﺮﺍی آﻨﺎﻟیﺖ ﻣﻮﺭﺩ ﻧﻈﺮ ﻭ ﺍیﻨکﻪ ﺗﻤﺎﻡ ﺍﻧﺪﺍﺯﻩ گیﺮیﻬﺎ ﺗﺎﺑﻊ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺑﻮﺩﻩ ﻭ ﺯﻣﺎﻧی ﺍﻧﺪﺍﺯﻩ گیﺮی کﺎﻣﻞ ﻣیﺸﻮﺩ کﻪ ﺑﺎ ﺗﻮﺻیﻒ ﻋﺪﻡ ﻗﻄﻌیﺖ ﻫﻤﺮﺍﻩ ﺑﺎﺷﺪ ﺷک ﻭ ﺗﺮﺩیﺪ )ﻋﺪﻡ ﻗﻄﻌیﺖ(ﺩﺭ ﻫﺮ ﺍﻧﺪﺍﺯﻩ گیﺮی ﻭﺟﻮﺩ ﺩﺍﺭﺩ ﻭ ﻣیﺰﺍﻥ آﻦ چﻘﺪﺭ ﺑﺎیﺪ ﺑﺎﺷﺪ ﻣﺜﻼ“ 59ﺩﺭﺻﺪ ﺍﻃﻤیﻨﺎﻥ 8/99 ﺩﺭﺻﺪ ﺍﻃﻤیﻨﺎﻥ ﺗﻔﺎﻭﺕ ﺧﻄﺎ ﺑﺎ ﻋﺪﻡ ﻗﻄﻌیﺖ: ﺧﻄﺎ ﺗﻔﺎﻭﺕ ﺑیﻦ ﺍﺭﺯﺵ ﺍﻧﺪﺍﺯﻩ گیﺮی ﺷﺪﻩ ﻭ ﺍﺭﺯﺵ ﻭﺍﻗﻌی ﻣﺎﺩﻩ ﻣﻮﺭﺩ ﺍﻧﺪﺍﺯﻩ گیﺮی ﺍﺳﺖ. ﻋﺪﻡ ﻗﻄﻌیﺖ: ﺗﻌییﻦ ﺧﺎﺻیﺖ ﻋﺪﻡ ﻗﻄﻌیﺖ )ﺷک ﻭ گﻤﺎﻥ(ﻧﺎﺷی ﺍﺯ ﻧﺘیﺠﻪ ﺍﻧﺪﺍﺯﻩ گیﺮی ﺍﺳﺖ. چﺮﺍ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﻧﺪﺍﺯﻩ گیﺮی ﻣﻬﻢ ﺍﺳﺖ: ﺑﺮﺍی ﺑﺪﺳﺖ آﻮﺭﺩﻥ ﺍﻧﺪﺍﺯﻩ گیﺮی ﺑﺎ کیﻔیﺖ ﺑﺎﻻ ﺑﻪ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﻧﺪﺍﺯﻩ گیﺮی ﻫﺎ ﻧیﺎﺯ ﻣﻨﺪیﻢ. ﻋﻠﺖ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﻧﺪﺍﺯﻩ گیﺮیﻬﺎ: 1 -ﻋﺪﻡ کﺎﻟیﺒﺮﺍﺳیﻮﻥ 2 -ﻋﺪﻡ ﺩﻗﺖ ﺗﺠﻬیﺰﺍﺕ 3 -ﻋﺪﻡ ﺻﺤﺖ ﻭ. . . .
ﻫﺪﻑ ﺍﺯ ﺍﺟﺮﺍی ﻋﺪﻡ ﻗﻄﻌیﺖ: ﺑﺎ ﺗﻮﺟﻪ ﺑﻪ ﺍﻫﻤیﺖ ﺍﻧﺪﺍﺯﻩ گیﺮیﻬﺎ ﻭ ﺗﺼﻤیﻢ گیﺮی ﺑﺮﺍی آﻨﺎﻟیﺖ ﻣﻮﺭﺩ ﻧﻈﺮ ﻭ ﺍیﻨکﻪ ﺗﻤﺎﻡ ﺍﻧﺪﺍﺯﻩ گیﺮیﻬﺎ ﺗﺎﺑﻊ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺑﻮﺩﻩ ﻭ ﺯﻣﺎﻧی ﺍﻧﺪﺍﺯﻩ گیﺮی کﺎﻣﻞ ﻣیﺸﻮﺩ کﻪ ﺑﺎ ﺗﻮﺻیﻒ ﻋﺪﻡ ﻗﻄﻌیﺖ ﻫﻤﺮﺍﻩ ﺑﺎﺷﺪ ﺷک ﻭ ﺗﺮﺩیﺪ )ﻋﺪﻡ ﻗﻄﻌیﺖ(ﺩﺭ ﻫﺮ ﺍﻧﺪﺍﺯﻩ گیﺮی ﻭﺟﻮﺩ ﺩﺍﺭﺩ ﻭ ﻣیﺰﺍﻥ آﻦ چﻘﺪﺭ ﺑﺎیﺪ ﺑﺎﺷﺪ ﻣﺜﻼ“ 59ﺩﺭﺻﺪ ﺍﻃﻤیﻨﺎﻥ 8/99 ﺩﺭﺻﺪ ﺍﻃﻤیﻨﺎﻥ ﺗﻔﺎﻭﺕ ﺧﻄﺎ ﺑﺎ ﻋﺪﻡ ﻗﻄﻌیﺖ: ﺧﻄﺎ ﺗﻔﺎﻭﺕ ﺑیﻦ ﺍﺭﺯﺵ ﺍﻧﺪﺍﺯﻩ گیﺮی ﺷﺪﻩ ﻭ ﺍﺭﺯﺵ ﻭﺍﻗﻌی ﻣﺎﺩﻩ ﻣﻮﺭﺩ ﺍﻧﺪﺍﺯﻩ گیﺮی ﺍﺳﺖ. ﻋﺪﻡ ﻗﻄﻌیﺖ: ﺗﻌییﻦ ﺧﺎﺻیﺖ ﻋﺪﻡ ﻗﻄﻌیﺖ )ﺷک ﻭ گﻤﺎﻥ(ﻧﺎﺷی ﺍﺯ ﻧﺘیﺠﻪ ﺍﻧﺪﺍﺯﻩ گیﺮی ﺍﺳﺖ. چﺮﺍ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﻧﺪﺍﺯﻩ گیﺮی ﻣﻬﻢ ﺍﺳﺖ: ﺑﺮﺍی ﺑﺪﺳﺖ آﻮﺭﺩﻥ ﺍﻧﺪﺍﺯﻩ گیﺮی ﺑﺎ کیﻔیﺖ ﺑﺎﻻ ﺑﻪ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﻧﺪﺍﺯﻩ گیﺮی ﻫﺎ ﻧیﺎﺯ ﻣﻨﺪیﻢ. ﻋﻠﺖ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﻧﺪﺍﺯﻩ گیﺮیﻬﺎ: 1 -ﻋﺪﻡ کﺎﻟیﺒﺮﺍﺳیﻮﻥ 2 -ﻋﺪﻡ ﺩﻗﺖ ﺗﺠﻬیﺰﺍﺕ 3 -ﻋﺪﻡ ﺻﺤﺖ ﻭ. . . .
 ﻋﺪﻡ ﻗﻄﻌیﺖ آﺰﻣﺎیﺸﺎﺕ ﺍﺯ کﺠﺎ ﻧﺎﺷی ﻣیﺸﻮﺩ؟ 1 -ﻗﺒﻞ ﺍﺯ آﺰﻣﺎیﺶ)پﺬیﺮﺵ آﺰﻣﺎیﺸگﺎﻩ , ﺑﺨﺶ ﻧﻤﻮﻧﻪ ﺑﺮﺩﺍﺭی , ﺑﺨﺶ آﻤﺎﺩﻩ ﺳﺎﺯی ﻧﻤﻮﻧﻪ 2 -ﺯﻣﺎﻥ آﺰﻣﺎیﺶ )ﺩﺳﺘگﺎﻫﻬﺎ-پﺮﺳﻨﻞ آﺰﻣﺎیﺸگﺎﻩ – کﺎﻟیﺒﺮﺍﺗﻮﺭﻫﺎ ﺭﻭﺵ آﺰﻣﺎیﺶ , ﺩﻣﺎی ﻣﺤیﻂ ﻭ. . . 3 - پﺲ ﺍﺯ آﺰﻣﺎیﺶ )ﺧﻄﺎی گﺰﺍﺭﺵ ﺩﻫی , ﻋﺪﻡ ﺗﻔﺴیﺮ آﺰﻣﺎیﺶ , ﻋﺪﻡ ﺍﺭﺍﺋﻪ ﺗﺴﺖ ﻫﺎی ﺗﺎییﺪی ﻭ ﺗکﻤیﻠی ﻭ. . . ﺍﻧﻮﺍﻉ ﻋﺪﻡ ﻗﻄﻌیﺖ: 1ﻋﺪﻡ ﻗﻄﻌیﺖ ﺗﺼﺎﺩﻓی 2 -ﻋﺪﻡ ﻗﻄﻌیﺖ ﺩﺳﺘگﺎﻫی ﺟﻬﺖ ﺷﻨﺎﺳﺎیی ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﻧﺪﺍﺯﻩ گیﺮیﻬﺎ ﺍﻭﻝ ﺑﺎیﺪ ﻣﻨﺎﺑﻊ ﻋﺪﻡ ﻗﻄﻌیﺖ یک ﺍﻧﺪﺍﺯﻩ گیﺮی ﺭﺍ ﻣﺸﺨﺺ کﺮﺩ. ﺩﻭ ﺭﻭﺵ ﺑﺮﺍی ﺗﺨﻤیﻦ ﻋﺪﻡ ﻗﻄﻌیﺖ ﻭﺟﻮﺩ ﺩﺍﺭﺩ 1ﺗیپ : A ﺳﻨﺠﺶ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ آﻨﺎﻟیﺰ آﻤﺎﺭی ﺳﺮﻡ کﻨﺘﺮﻝ -2ﺗیپ : B ﺳﻨﺠﺶ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ کﺎﻟیﺒﺮﺍﺗﻮﺭ, کﻨﺘﺮﻝ کیﻔی ﺧﺎﺭﺟی ﻭ. .
ﻋﺪﻡ ﻗﻄﻌیﺖ آﺰﻣﺎیﺸﺎﺕ ﺍﺯ کﺠﺎ ﻧﺎﺷی ﻣیﺸﻮﺩ؟ 1 -ﻗﺒﻞ ﺍﺯ آﺰﻣﺎیﺶ)پﺬیﺮﺵ آﺰﻣﺎیﺸگﺎﻩ , ﺑﺨﺶ ﻧﻤﻮﻧﻪ ﺑﺮﺩﺍﺭی , ﺑﺨﺶ آﻤﺎﺩﻩ ﺳﺎﺯی ﻧﻤﻮﻧﻪ 2 -ﺯﻣﺎﻥ آﺰﻣﺎیﺶ )ﺩﺳﺘگﺎﻫﻬﺎ-پﺮﺳﻨﻞ آﺰﻣﺎیﺸگﺎﻩ – کﺎﻟیﺒﺮﺍﺗﻮﺭﻫﺎ ﺭﻭﺵ آﺰﻣﺎیﺶ , ﺩﻣﺎی ﻣﺤیﻂ ﻭ. . . 3 - پﺲ ﺍﺯ آﺰﻣﺎیﺶ )ﺧﻄﺎی گﺰﺍﺭﺵ ﺩﻫی , ﻋﺪﻡ ﺗﻔﺴیﺮ آﺰﻣﺎیﺶ , ﻋﺪﻡ ﺍﺭﺍﺋﻪ ﺗﺴﺖ ﻫﺎی ﺗﺎییﺪی ﻭ ﺗکﻤیﻠی ﻭ. . . ﺍﻧﻮﺍﻉ ﻋﺪﻡ ﻗﻄﻌیﺖ: 1ﻋﺪﻡ ﻗﻄﻌیﺖ ﺗﺼﺎﺩﻓی 2 -ﻋﺪﻡ ﻗﻄﻌیﺖ ﺩﺳﺘگﺎﻫی ﺟﻬﺖ ﺷﻨﺎﺳﺎیی ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﻧﺪﺍﺯﻩ گیﺮیﻬﺎ ﺍﻭﻝ ﺑﺎیﺪ ﻣﻨﺎﺑﻊ ﻋﺪﻡ ﻗﻄﻌیﺖ یک ﺍﻧﺪﺍﺯﻩ گیﺮی ﺭﺍ ﻣﺸﺨﺺ کﺮﺩ. ﺩﻭ ﺭﻭﺵ ﺑﺮﺍی ﺗﺨﻤیﻦ ﻋﺪﻡ ﻗﻄﻌیﺖ ﻭﺟﻮﺩ ﺩﺍﺭﺩ 1ﺗیپ : A ﺳﻨﺠﺶ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ آﻨﺎﻟیﺰ آﻤﺎﺭی ﺳﺮﻡ کﻨﺘﺮﻝ -2ﺗیپ : B ﺳﻨﺠﺶ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺑﺎ ﺍﺳﺘﻔﺎﺩﻩ ﺍﺯ کﺎﻟیﺒﺮﺍﺗﻮﺭ, کﻨﺘﺮﻝ کیﻔی ﺧﺎﺭﺟی ﻭ. .
 ﻣﺤﺎﺳﺒﻪ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺑﺮﺍی ﺍﺭﺯیﺎﺑی ﺗیپ A ﺟﻬﺖ ﻣﺤﺎﺳﺒﻪ ﻋﺪﻡ ﻗﻄﻌیﺖ ﻧیﺎﺯ ﺑﻪ 03 ﺍﻧﺪﺍﺯﻩ گیﺮی ﻣیﺒﺎﺷﺪ )03= ( n ﻣیﺎﻧگیﻦ X ﺩﺍﺩﻩ ﻫﺎ ﺍﻧﺤﺮﺍﻑ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ) ( SD ﻋﺪﻡ ﻗﻄﻌیﺖ ﻣیﺎﻧگیﻦ یﺎ ﺍﻧﺤﺮﺍﻑ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﻣیﺎﻧگیﻦ ) U=SD/√n ﻣﺤﺎﺳﺒﻪ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺑﺮﺍی ﺍﺭﺯیﺎﺑی ﺗیپ B ﺑﺎ ﺗﻌییﻦ ﻣیﺰﺍﻥ ﻋﺪﻡ ﺻﺤﺖ کﺎﻟیﺒﺮﺍﺗﻮﺭ, ﻋﺪﻡ ﺻﺤﺖ کﻨﺘﺮﻝ کیﻔی ﺧﺎﺭﺟی
ﻣﺤﺎﺳﺒﻪ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺑﺮﺍی ﺍﺭﺯیﺎﺑی ﺗیپ A ﺟﻬﺖ ﻣﺤﺎﺳﺒﻪ ﻋﺪﻡ ﻗﻄﻌیﺖ ﻧیﺎﺯ ﺑﻪ 03 ﺍﻧﺪﺍﺯﻩ گیﺮی ﻣیﺒﺎﺷﺪ )03= ( n ﻣیﺎﻧگیﻦ X ﺩﺍﺩﻩ ﻫﺎ ﺍﻧﺤﺮﺍﻑ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ) ( SD ﻋﺪﻡ ﻗﻄﻌیﺖ ﻣیﺎﻧگیﻦ یﺎ ﺍﻧﺤﺮﺍﻑ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﻣیﺎﻧگیﻦ ) U=SD/√n ﻣﺤﺎﺳﺒﻪ ﻋﺪﻡ ﻗﻄﻌیﺖ ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺑﺮﺍی ﺍﺭﺯیﺎﺑی ﺗیپ B ﺑﺎ ﺗﻌییﻦ ﻣیﺰﺍﻥ ﻋﺪﻡ ﺻﺤﺖ کﺎﻟیﺒﺮﺍﺗﻮﺭ, ﻋﺪﻡ ﺻﺤﺖ کﻨﺘﺮﻝ کیﻔی ﺧﺎﺭﺟی
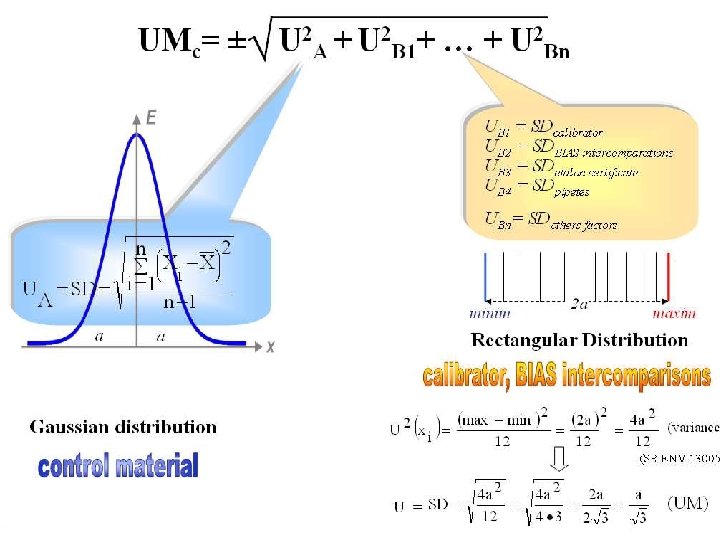
 ﺟﻬﺖ ﺑﺮﺭﺳی ﻋﺪﻡ ﻗﻄﻌیﺖ ﻫﺎی ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺍﺯ ﻃﺮیﻖ ﻭ ﻣﺤﺎﺳﺒﻪ آﻦ ﻋﺪﻡ ﻗﻄﻌیﺖ B ﻭ ﺗیپ A ﺍﺭﺯیﺎﺑی ﺗیپ . Uc ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺑﺎ ﻧﻤﺎﺩ UMc = ± UA = U measurement procedure (from internal quality control), UB 1 = Ucalibrator (from manufacturer specifications), UB 2 = UBias (from external quality control), Uother factors (from speciality literature data) (4). Expanded uncertainty, usually shown by the symbol U, is obtained by multiplying the UMc by a coverage factor, denoted by symbol k = 2, for a level of confidence of approximately 95% (2). UM expanded = UMc ・ k [9]. UMc = ± UMc=±
ﺟﻬﺖ ﺑﺮﺭﺳی ﻋﺪﻡ ﻗﻄﻌیﺖ ﻫﺎی ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺍﺯ ﻃﺮیﻖ ﻭ ﻣﺤﺎﺳﺒﻪ آﻦ ﻋﺪﻡ ﻗﻄﻌیﺖ B ﻭ ﺗیپ A ﺍﺭﺯیﺎﺑی ﺗیپ . Uc ﺍﺳﺘﺎﻧﺪﺍﺭﺩ ﺑﺎ ﻧﻤﺎﺩ UMc = ± UA = U measurement procedure (from internal quality control), UB 1 = Ucalibrator (from manufacturer specifications), UB 2 = UBias (from external quality control), Uother factors (from speciality literature data) (4). Expanded uncertainty, usually shown by the symbol U, is obtained by multiplying the UMc by a coverage factor, denoted by symbol k = 2, for a level of confidence of approximately 95% (2). UM expanded = UMc ・ k [9]. UMc = ± UMc=±
 Shewhart Control Charts A Shewhart Control Chart depend on the use of IQC specimens and is developed in the following manner 49 25 September 2013
Shewhart Control Charts A Shewhart Control Chart depend on the use of IQC specimens and is developed in the following manner 49 25 September 2013
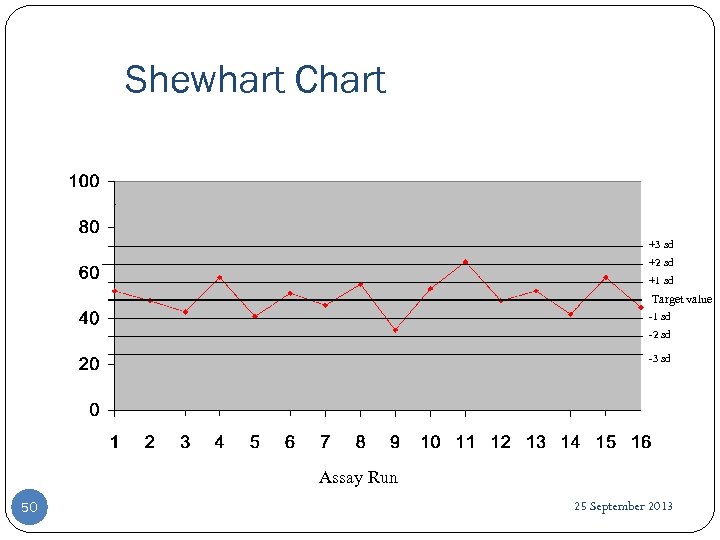 Shewhart Chart +3 sd +2 sd +1 sd Target value -1 sd -2 sd -3 sd Assay Run 50 25 September 2013
Shewhart Chart +3 sd +2 sd +1 sd Target value -1 sd -2 sd -3 sd Assay Run 50 25 September 2013
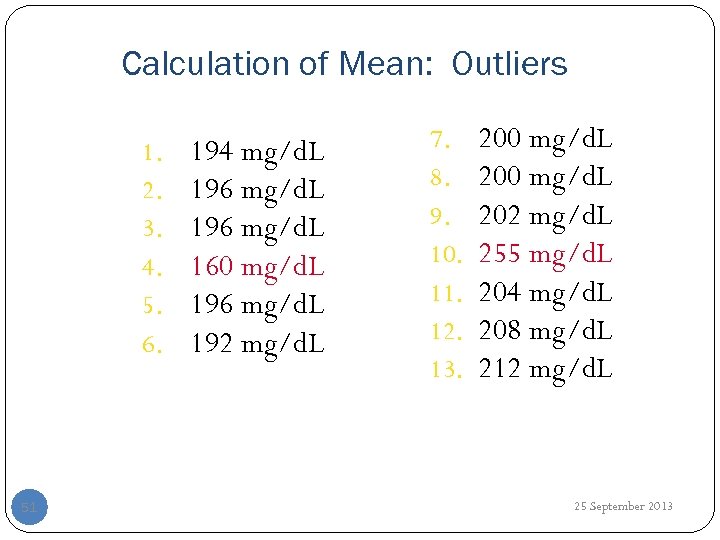 Calculation of Mean: Outliers 1. 2. 3. 4. 5. 6. 51 194 mg/d. L 196 mg/d. L 160 mg/d. L 196 mg/d. L 192 mg/d. L 7. 8. 9. 10. 11. 12. 13. 200 mg/d. L 202 mg/d. L 255 mg/d. L 204 mg/d. L 208 mg/d. L 212 mg/d. L 25 September 2013
Calculation of Mean: Outliers 1. 2. 3. 4. 5. 6. 51 194 mg/d. L 196 mg/d. L 160 mg/d. L 196 mg/d. L 192 mg/d. L 7. 8. 9. 10. 11. 12. 13. 200 mg/d. L 202 mg/d. L 255 mg/d. L 204 mg/d. L 208 mg/d. L 212 mg/d. L 25 September 2013
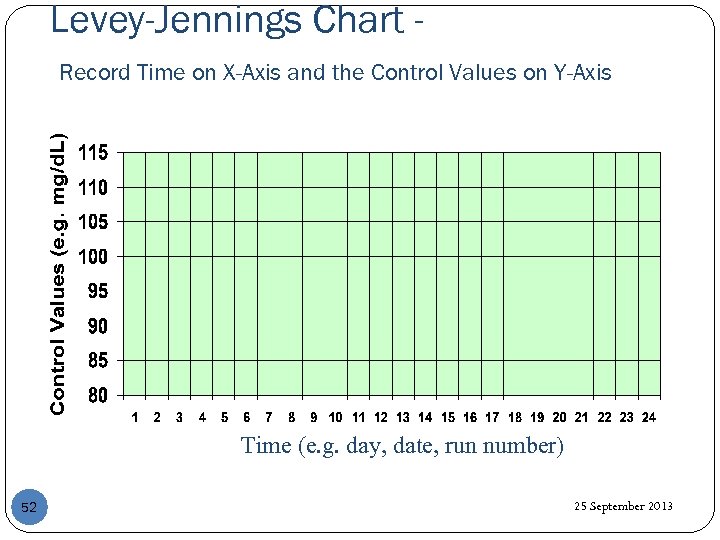 Levey-Jennings Chart Record Time on X-Axis and the Control Values on Y-Axis Time (e. g. day, date, run number) 52 25 September 2013
Levey-Jennings Chart Record Time on X-Axis and the Control Values on Y-Axis Time (e. g. day, date, run number) 52 25 September 2013
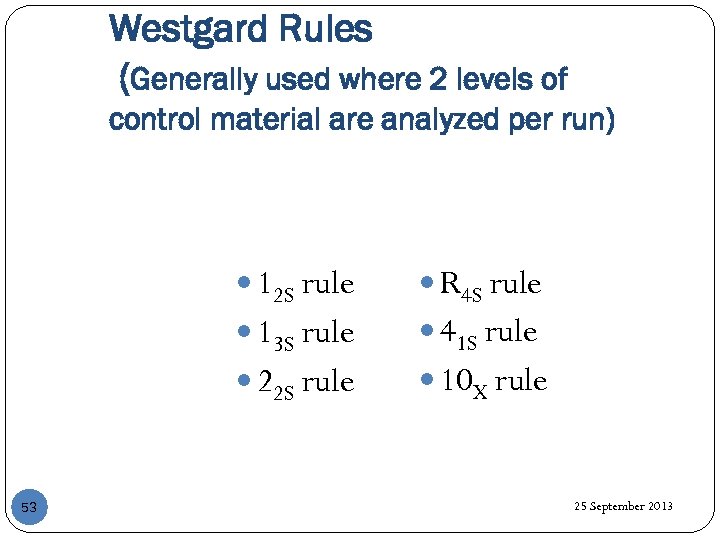 Westgard Rules (Generally used where 2 levels of control material are analyzed per run) 12 S rule 13 S rule 41 S rule 22 S rule 53 R 4 S rule 10 X rule 25 September 2013
Westgard Rules (Generally used where 2 levels of control material are analyzed per run) 12 S rule 13 S rule 41 S rule 22 S rule 53 R 4 S rule 10 X rule 25 September 2013
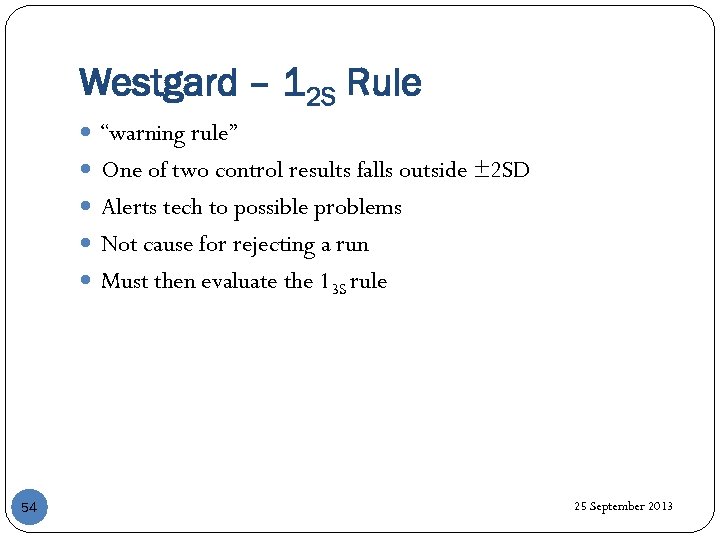 Westgard – 12 S Rule “warning rule” One of two control results falls outside ± 2 SD Alerts tech to possible problems Not cause for rejecting a run Must then evaluate the 13 S rule 54 25 September 2013
Westgard – 12 S Rule “warning rule” One of two control results falls outside ± 2 SD Alerts tech to possible problems Not cause for rejecting a run Must then evaluate the 13 S rule 54 25 September 2013
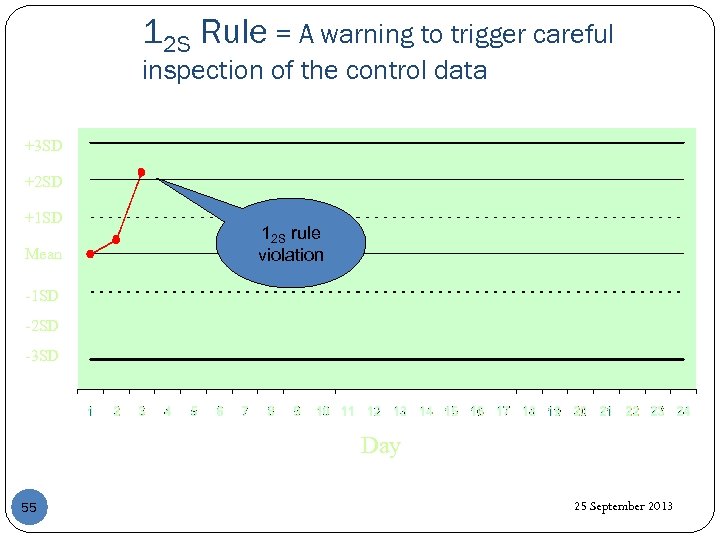 12 S Rule = A warning to trigger careful inspection of the control data +3 SD +2 SD +1 SD Mean 12 S rule violation -1 SD -2 SD -3 SD Day 55 25 September 2013
12 S Rule = A warning to trigger careful inspection of the control data +3 SD +2 SD +1 SD Mean 12 S rule violation -1 SD -2 SD -3 SD Day 55 25 September 2013
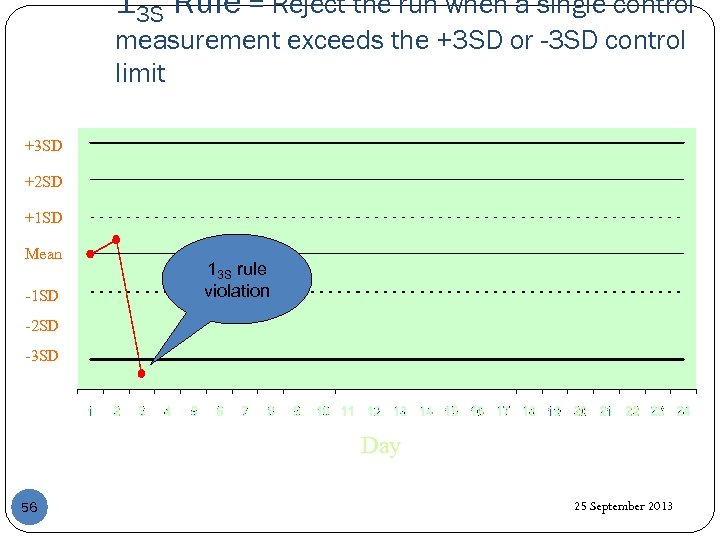 13 S Rule = Reject the run when a single control measurement exceeds the +3 SD or -3 SD control limit +3 SD +2 SD +1 SD Mean -1 SD 13 S rule violation -2 SD -3 SD Day 56 25 September 2013
13 S Rule = Reject the run when a single control measurement exceeds the +3 SD or -3 SD control limit +3 SD +2 SD +1 SD Mean -1 SD 13 S rule violation -2 SD -3 SD Day 56 25 September 2013
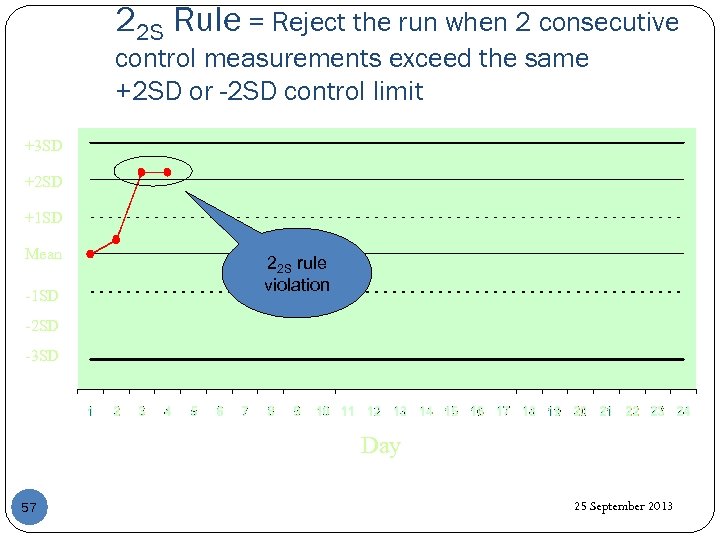 22 S Rule = Reject the run when 2 consecutive control measurements exceed the same +2 SD or -2 SD control limit +3 SD +2 SD +1 SD Mean -1 SD 22 S rule violation -2 SD -3 SD Day 57 25 September 2013
22 S Rule = Reject the run when 2 consecutive control measurements exceed the same +2 SD or -2 SD control limit +3 SD +2 SD +1 SD Mean -1 SD 22 S rule violation -2 SD -3 SD Day 57 25 September 2013
 When a rule is violated Warning rule = use other rules to inspect the control points Rejection rule = “out of control” 58 Stop testing Identify and correct problem Repeat testing on patient samples and controls Do not report patient results until problem is solved and controls indicate proper performance 25 September 2013
When a rule is violated Warning rule = use other rules to inspect the control points Rejection rule = “out of control” 58 Stop testing Identify and correct problem Repeat testing on patient samples and controls Do not report patient results until problem is solved and controls indicate proper performance 25 September 2013
 Westgard – 22 S Rule 2 consecutive control values for the same level fall outside of ± 2 SD in the same direction, or Both controls in the same run exceed ± 2 SD Patient results cannot be reported Requires corrective action 59 25 September 2013
Westgard – 22 S Rule 2 consecutive control values for the same level fall outside of ± 2 SD in the same direction, or Both controls in the same run exceed ± 2 SD Patient results cannot be reported Requires corrective action 59 25 September 2013
 When a rule is violated Warning rule = use other rules to inspect the control points Rejection rule = “out of control” 60 Stop testing Identify and correct problem Repeat testing on patient samples and controls Do not report patient results until problem is solved and controls indicate proper performance 25 September 2013
When a rule is violated Warning rule = use other rules to inspect the control points Rejection rule = “out of control” 60 Stop testing Identify and correct problem Repeat testing on patient samples and controls Do not report patient results until problem is solved and controls indicate proper performance 25 September 2013


