RDBVMAH Transgenic animals.pptx
- Количество слайдов: 30
Problems of genetic engineering in the creation of transgenic animals 1. The methods of introduction of foreign DNA into the host cells; 2. Status and prospects of obtaining and using transgenic animals; 3. Problems in creating of transgenic animals

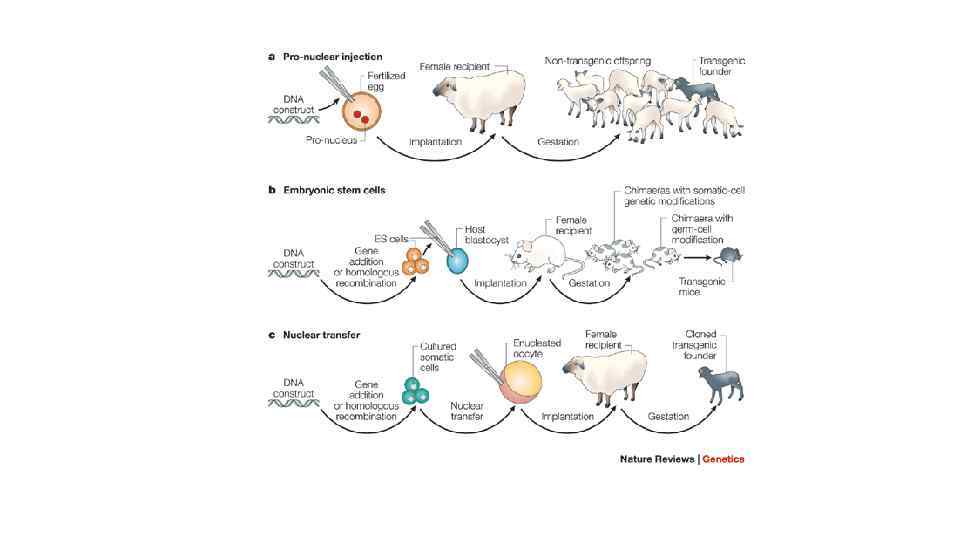
A transgenic animal is one that carries a foreign gene that has been deliberately inserted into its genome. Transgenic technology developed and refined on laboratory mice. Since the early 1980's hundreds of genes were introduced to different strains of mice. The introduction of foreign DNA into mice was carried out by different methods: - by using retroviral vectors to infect embryo cells in the early stages of its development before implantation into a female recipient, - by microinjection DNA into the sperm nucleus (male pronucleus) of fertilized egg, - by introduction of genetically modified embryonic stem cells in preimplanted embryo in its early stages of development.
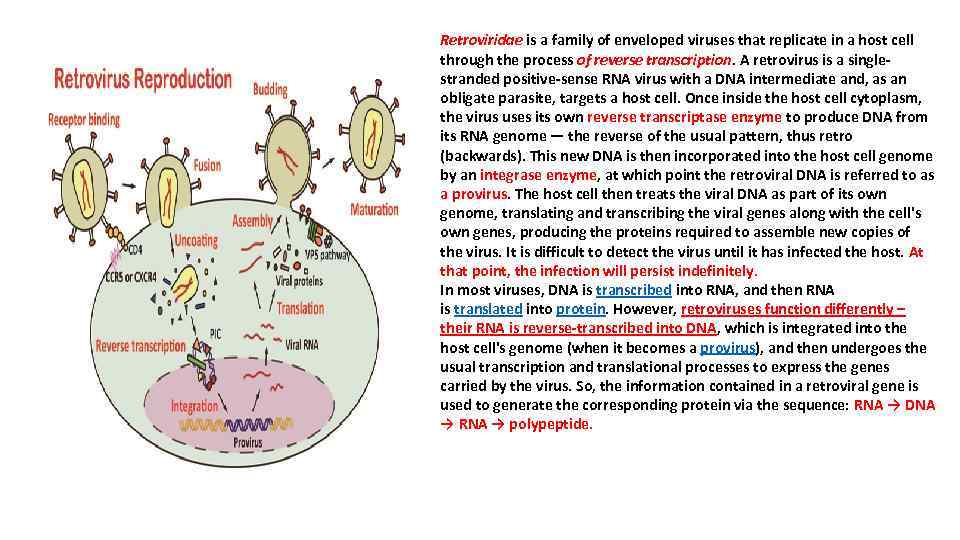
Retroviridae is a family of enveloped viruses that replicate in a host cell through the process of reverse transcription. A retrovirus is a singlestranded positive-sense RNA virus with a DNA intermediate and, as an obligate parasite, targets a host cell. Once inside the host cell cytoplasm, the virus uses its own reverse transcriptase enzyme to produce DNA from its RNA genome — the reverse of the usual pattern, thus retro (backwards). This new DNA is then incorporated into the host cell genome by an integrase enzyme, at which point the retroviral DNA is referred to as a provirus. The host cell then treats the viral DNA as part of its own genome, translating and transcribing the viral genes along with the cell's own genes, producing the proteins required to assemble new copies of the virus. It is difficult to detect the virus until it has infected the host. At that point, the infection will persist indefinitely. In most viruses, DNA is transcribed into RNA, and then RNA is translated into protein. However, retroviruses function differently – their RNA is reverse-transcribed into DNA, which is integrated into the host cell's genome (when it becomes a provirus), and then undergoes the usual transcription and translational processes to express the genes carried by the virus. So, the information contained in a retroviral gene is used to generate the corresponding protein via the sequence: RNA → DNA → RNA → polypeptide.
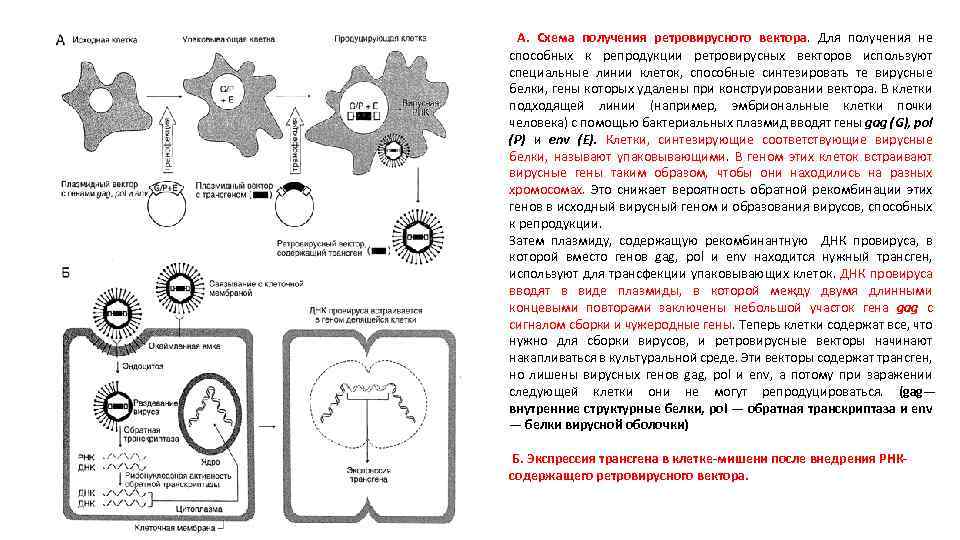
А. Схема получения ретровирусного вектора. Для получения не способных к репродукции ретровирусных векторов используют специальные линии клеток, способные синтезировать те вирусные белки, гены которых удалены при конструировании вектора. В клетки подходящей линии (например, эмбриональные клетки почки человека) с помощью бактериальных плазмид вводят гены gag (G), pol (Р) и env (Е). Клетки, синтезирующие соответствующие вирусные белки, называют упаковывающими. В геном этих клеток встраивают вирусные гены таким образом, чтобы они находились на разных хромосомах. Это снижает вероятность обратной рекомбинации этих генов в исходный вирусный геном и образования вирусов, способных к репродукции. Затем плазмиду, содержащую рекомбинантную ДНК провируса, в которой вместо генов gag, pol и env находится нужный трансген, используют для трансфекции упаковывающих клеток. ДНК провируса вводят в виде плазмиды, в которой между двумя длинными концевыми повторами заключены небольшой участок гена gag с сигналом сборки и чужеродные гены. Теперь клетки содержат все, что нужно для сборки вирусов, и ретровирусные векторы начинают накапливаться в культуральной среде. Эти векторы содержат трансген, но лишены вирусных генов gag, pol и env, а потому при заражении следующей клетки они не могут репродуцироваться. (gag— внутренние структурные белки, pol — обратная транскриптаза и env — белки вирусной оболочки) Б. Экспрессия трансгена в клетке-мишени после внедрения РНКсодержащего ретровирусного вектора.
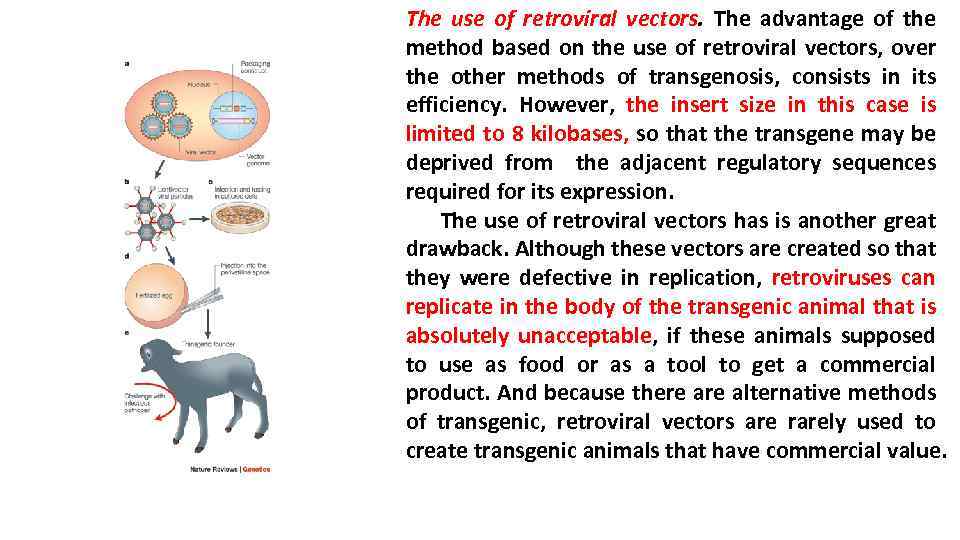
The use of retroviral vectors. The advantage of the method based on the use of retroviral vectors, over the other methods of transgenosis, consists in its efficiency. However, the insert size in this case is limited to 8 kilobases, so that the transgene may be deprived from the adjacent regulatory sequences required for its expression. The use of retroviral vectors has is another great drawback. Although these vectors are created so that they were defective in replication, retroviruses can replicate in the body of the transgenic animal that is absolutely unacceptable, if these animals supposed to use as food or as a tool to get a commercial product. And because there alternative methods of transgenic, retroviral vectors are rarely used to create transgenic animals that have commercial value.
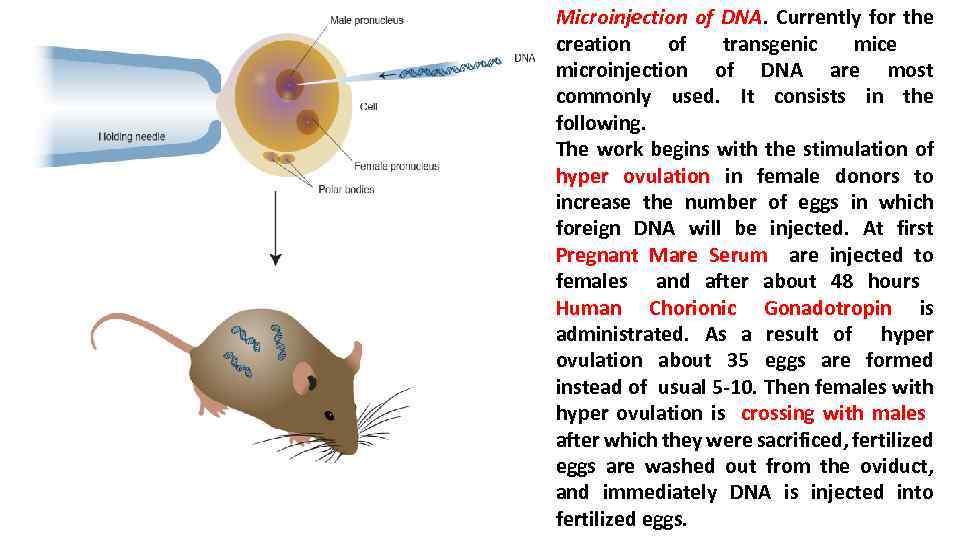
Microinjection of DNA. Currently for the creation of transgenic mice microinjection of DNA are most commonly used. It consists in the following. The work begins with the stimulation of hyper ovulation in female donors to increase the number of eggs in which foreign DNA will be injected. At first Pregnant Mare Serum are injected to females and after about 48 hours Human Chorionic Gonadotropin is administrated. As a result of hyper ovulation about 35 eggs are formed instead of usual 5 -10. Then females with hyper ovulation is crossing with males after which they were sacrificed, fertilized eggs are washed out from the oviduct, and immediately DNA is injected into fertilized eggs.
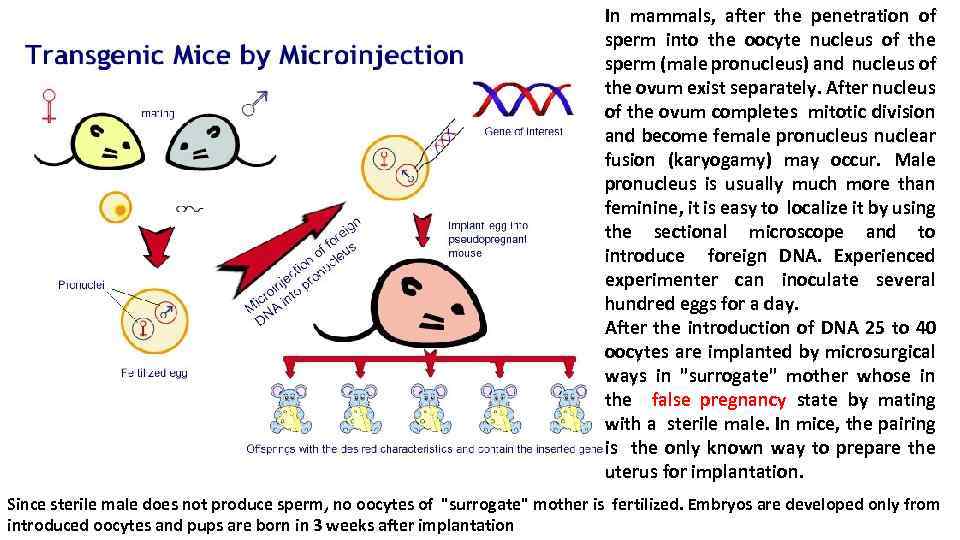
In mammals, after the penetration of sperm into the oocyte nucleus of the sperm (male pronucleus) and nucleus of the ovum exist separately. After nucleus of the ovum completes mitotic division and become female pronucleus nuclear fusion (karyogamy) may occur. Male pronucleus is usually much more than feminine, it is easy to localize it by using the sectional microscope and to introduce foreign DNA. Experienced experimenter can inoculate several hundred eggs for a day. After the introduction of DNA 25 to 40 oocytes are implanted by microsurgical ways in "surrogate" mother whose in the false pregnancy state by mating with a sterile male. In mice, the pairing is the only known way to prepare the uterus for implantation. Since sterile male does not produce sperm, no oocytes of "surrogate" mother is fertilized. Embryos are developed only from introduced oocytes and pups are born in 3 weeks after implantation

Sterilization (vasoectomy) of males Vasectomy (lat. Vas - a vessel, duct + ectomy) - a surgical procedure in which the ligation or deleting part of the vas deferens of male is carried out (lat. Ductus deferens). This operation leads to sterility (inability to have offspring) while preserving sexual function. After vasectomy the male saved sexual behavior: libido, erection, ejaculation. But the obstruction of the vas deferens results in the absence of sperm in the ejaculate (azoospermia).
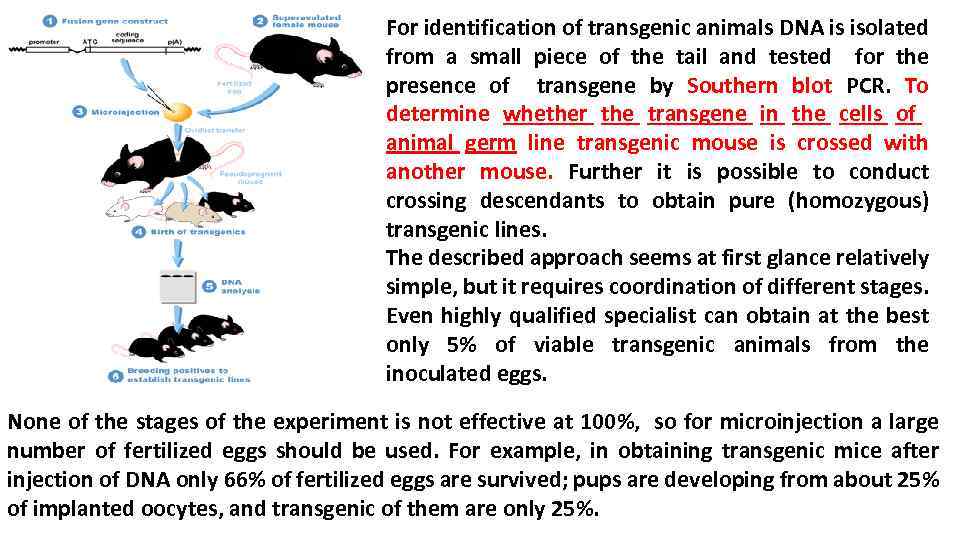
For identification of transgenic animals DNA is isolated from a small piece of the tail and tested for the presence of transgene by Southern blot PCR. To determine whether the transgene in the cells of animal germ line transgenic mouse is crossed with another mouse. Further it is possible to conduct crossing descendants to obtain pure (homozygous) transgenic lines. The described approach seems at first glance relatively simple, but it requires coordination of different stages. Even highly qualified specialist can obtain at the best only 5% of viable transgenic animals from the inoculated eggs. None of the stages of the experiment is not effective at 100%, so for microinjection a large number of fertilized eggs should be used. For example, in obtaining transgenic mice after injection of DNA only 66% of fertilized eggs are survived; pups are developing from about 25% of implanted oocytes, and transgenic of them are only 25%.
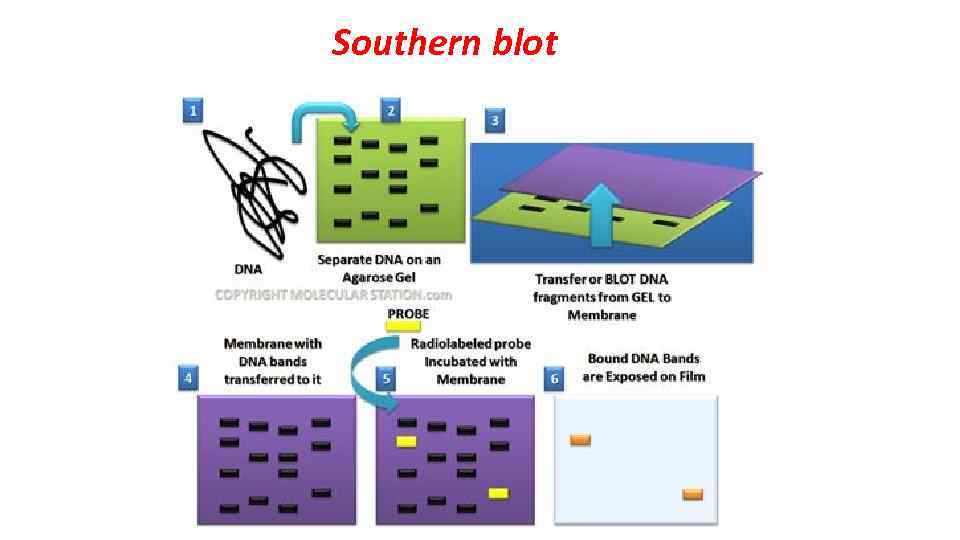
Southern blot
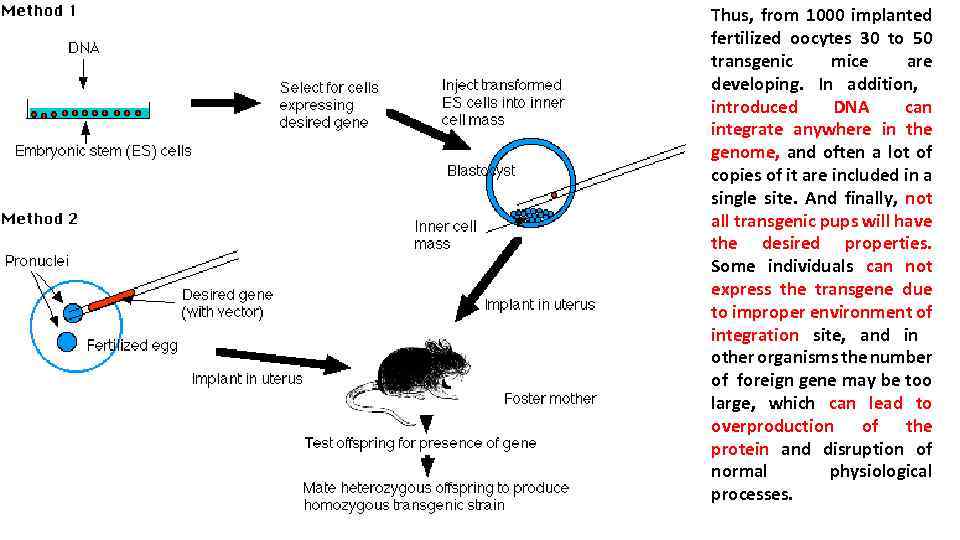
Thus, from 1000 implanted fertilized oocytes 30 to 50 transgenic mice are developing. In addition, introduced DNA can integrate anywhere in the genome, and often a lot of copies of it are included in a single site. And finally, not all transgenic pups will have the desired properties. Some individuals can not express the transgene due to improper environment of integration site, and in other organisms the number of foreign gene may be too large, which can lead to overproduction of the protein and disruption of normal physiological processes.
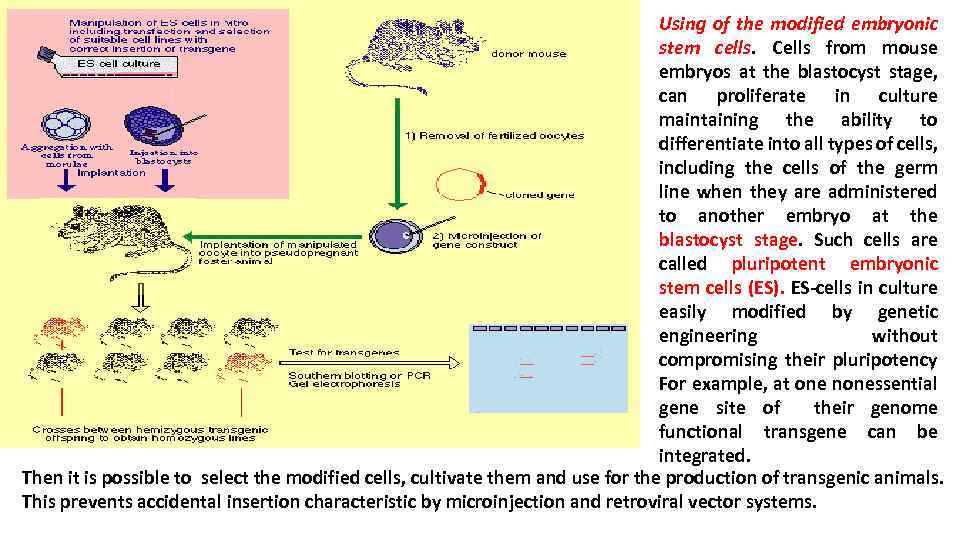
Using of the modified embryonic stem cells. Cells from mouse embryos at the blastocyst stage, can proliferate in culture maintaining the ability to differentiate into all types of cells, including the cells of the germ line when they are administered to another embryo at the blastocyst stage. Such cells are called pluripotent embryonic stem cells (ES). ES-cells in culture easily modified by genetic engineering without compromising their pluripotency For example, at one nonessential gene site of their genome functional transgene can be integrated. Then it is possible to select the modified cells, cultivate them and use for the production of transgenic animals. This prevents accidental insertion characteristic by microinjection and retroviral vector systems.
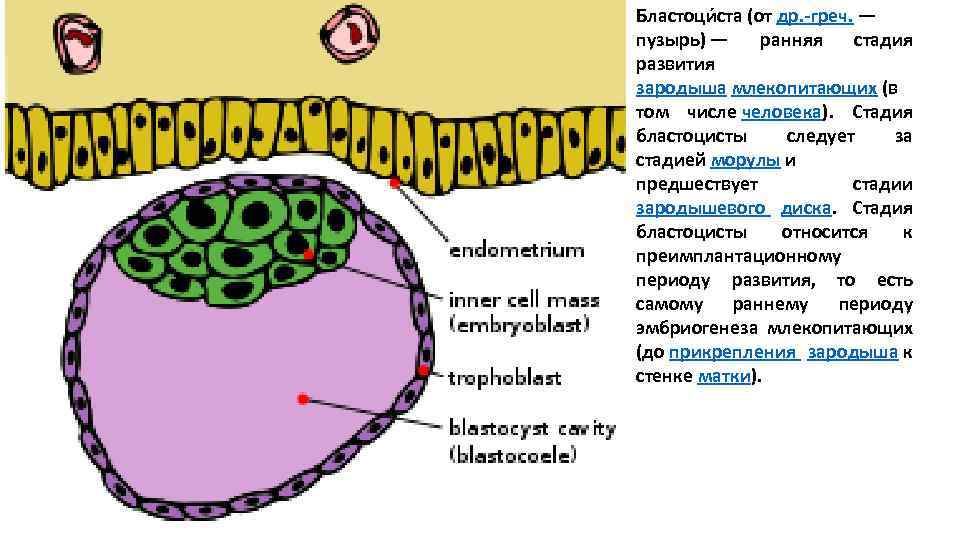
Бластоци ста (от др. -греч. — пузырь) — ранняя стадия развития зародыша млекопитающих (в том числе человека). Стадия бластоцисты следует за стадией морулы и предшествует стадии зародышевого диска. Стадия бластоцисты относится к преимплантационному периоду развития, то есть самому раннему периоду эмбриогенеза млекопитающих (до прикрепления зародыша к стенке матки).
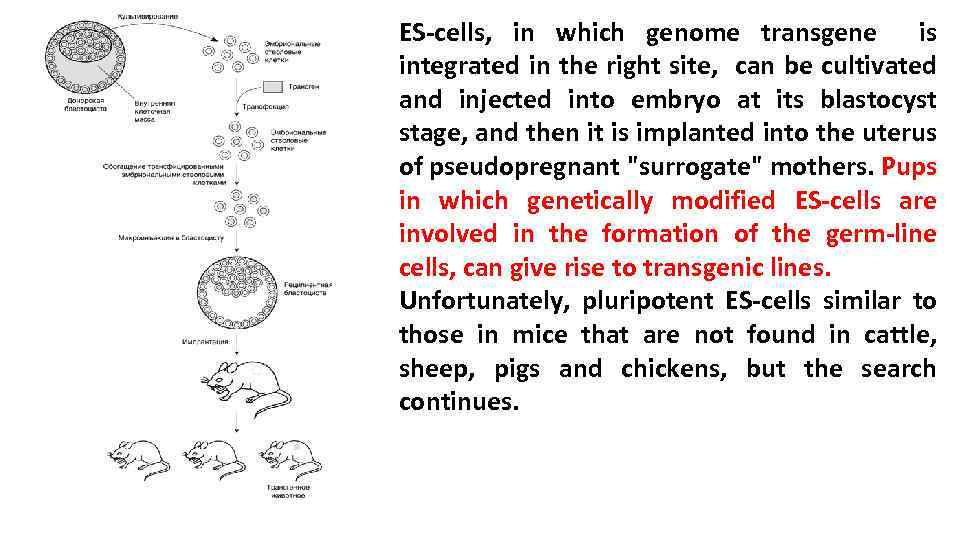
ES-cells, in which genome transgene is integrated in the right site, can be cultivated and injected into embryo at its blastocyst stage, and then it is implanted into the uterus of pseudopregnant "surrogate" mothers. Pups in which genetically modified ES-cells are involved in the formation of the germ-line cells, can give rise to transgenic lines. Unfortunately, pluripotent ES-cells similar to those in mice that are not found in cattle, sheep, pigs and chickens, but the search continues.
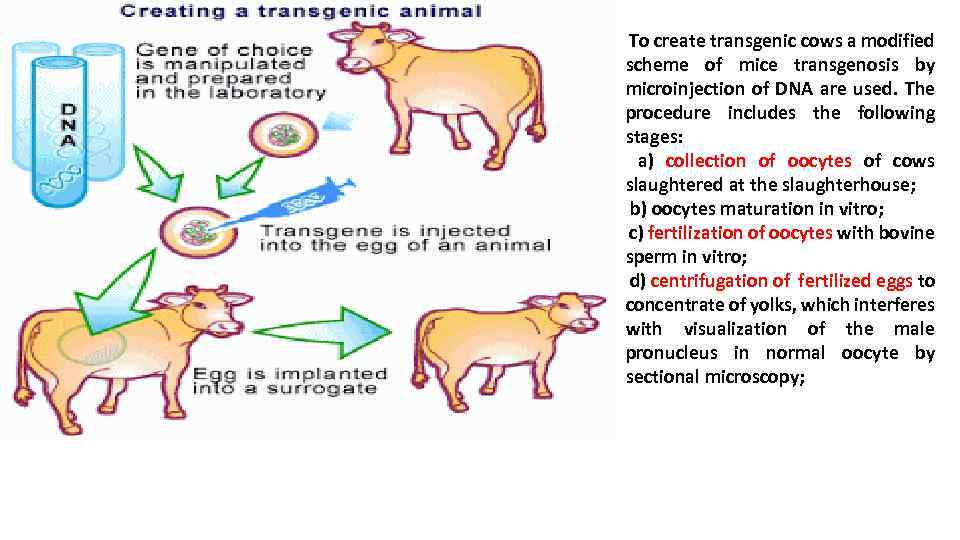
To create transgenic cows a modified scheme of mice transgenosis by microinjection of DNA are used. The procedure includes the following stages: a) collection of oocytes of cows slaughtered at the slaughterhouse; b) oocytes maturation in vitro; c) fertilization of oocytes with bovine sperm in vitro; d) centrifugation of fertilized eggs to concentrate of yolks, which interferes with visualization of the male pronucleus in normal oocyte by sectional microscopy;
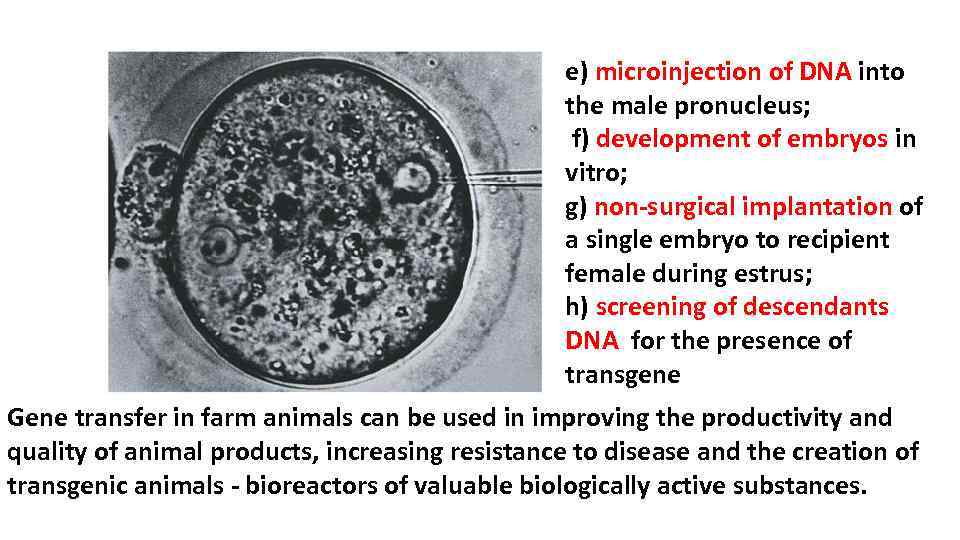
e) microinjection of DNA into the male pronucleus; f) development of embryos in vitro; g) non-surgical implantation of a single embryo to recipient female during estrus; h) screening of descendants DNA for the presence of transgene Gene transfer in farm animals can be used in improving the productivity and quality of animal products, increasing resistance to disease and the creation of transgenic animals - bioreactors of valuable biologically active substances.
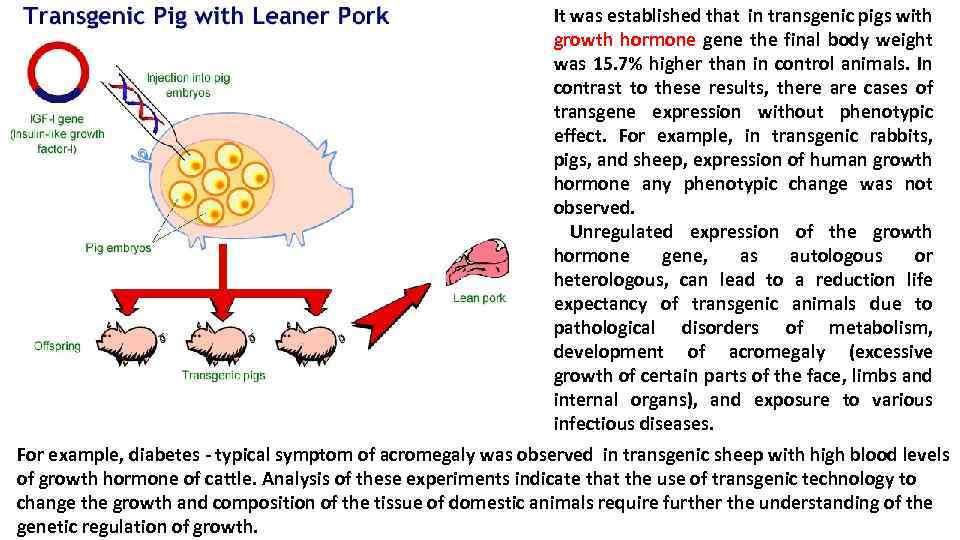
It was established that in transgenic pigs with growth hormone gene the final body weight was 15. 7% higher than in control animals. In contrast to these results, there are cases of transgene expression without phenotypic effect. For example, in transgenic rabbits, pigs, and sheep, expression of human growth hormone any phenotypic change was not observed. Unregulated expression of the growth hormone gene, as autologous or heterologous, can lead to a reduction life expectancy of transgenic animals due to pathological disorders of metabolism, development of acromegaly (excessive growth of certain parts of the face, limbs and internal organs), and exposure to various infectious diseases. For example, diabetes - typical symptom of acromegaly was observed in transgenic sheep with high blood levels of growth hormone of cattle. Analysis of these experiments indicate that the use of transgenic technology to change the growth and composition of the tissue of domestic animals require further the understanding of the genetic regulation of growth.

Creation of transgenic animals opens real prospects for improving the quality or composition of animal products. For example, it is possible to reduce lactose in milk by creating transgenic cows and sheep, which have specific for mammary gland promoter, linked to the gene lactase. Thus in cow (sheep) milk lactose can be cleaved into glucose and galactose. Such milk could be used in nutrition of newborn children suffering from hereditary lactose intolerance. For these children during infancy milk should be given only after processing by enzyme. In addition, milk would be useful in a variety of gastrointestinal human diseases associated with decreased activity of lactase (beta-galactosidase). (Promoter - region of DNA to which RNA polymerase binds to start the synthesis of m. RNA)

The presence in the milk of various microflora caused problems associated with the storage, processing, consumption of milk and animal health. In this regard, the genes which are responsible for the production of antibodies against specific pathogens are great interest. An important task is getting milk and dairy products containing thermostable enzyme lysozyme are constructed. During pasteurization of milk this enzyme, which has strong antibacterial property does not lose its activity, which will significantly increase shelf life of milk and milkproducts. The possibility of the introduction of genes encoding antibodies with protective effects against agents of cows mastitis are considered.
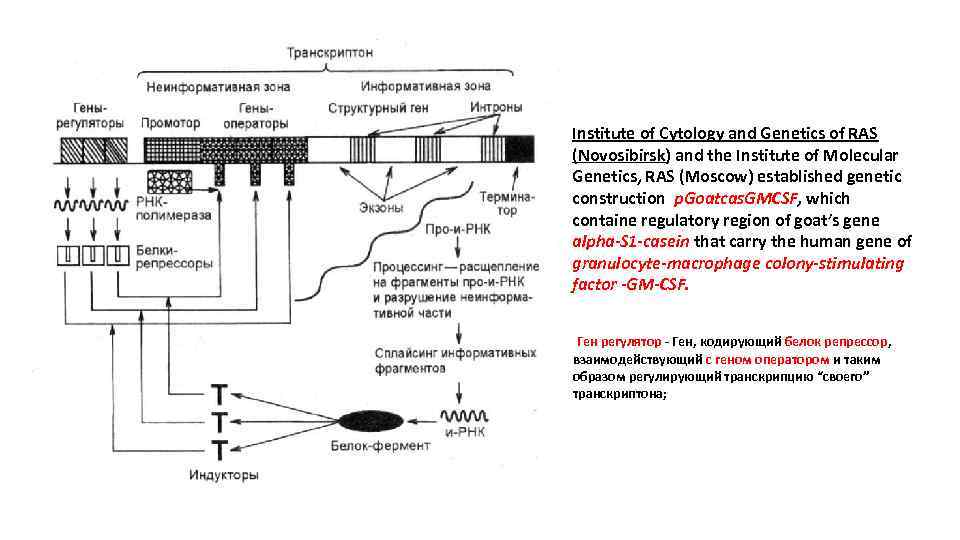
Institute of Cytology and Genetics of RAS (Novosibirsk) and the Institute of Molecular Genetics, RAS (Moscow) established genetic construction p. Goatcas. GMCSF, which containe regulatory region of goat’s gene alpha-S 1 -casein that carry the human gene of granulocyte-macrophage colony-stimulating factor -GM-CSF. Ген регулятор - Ген, кодирующий белок репрессор, взаимодействующий с геном оператором и таким образом регулирующий транскрипцию “своего” транскриптона;
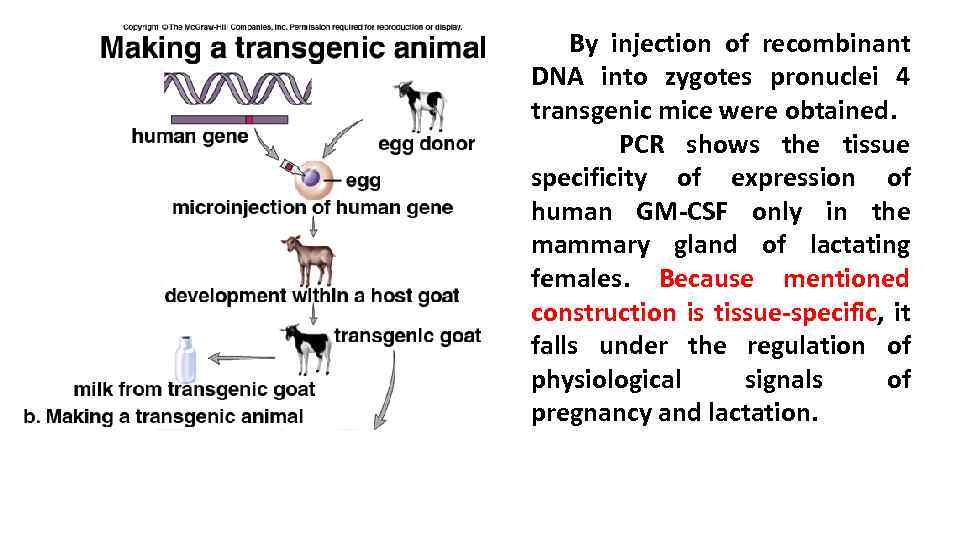
By injection of recombinant DNA into zygotes pronuclei 4 transgenic mice were obtained. PCR shows the tissue specificity of expression of human GM-CSF only in the mammary gland of lactating females. Because mentioned construction is tissue-specific, it falls under the regulation of physiological signals of pregnancy and lactation.
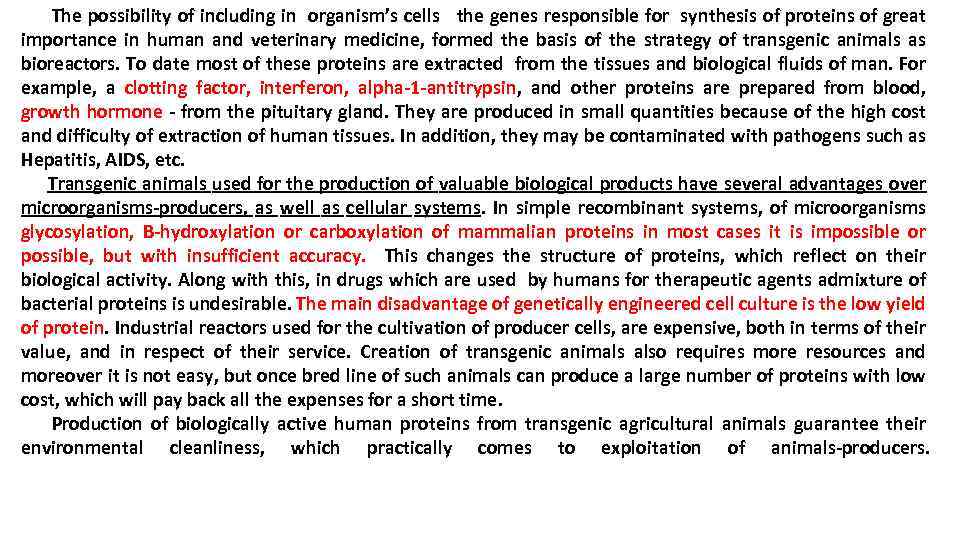
The possibility of including in organism’s cells the genes responsible for synthesis of proteins of great importance in human and veterinary medicine, formed the basis of the strategy of transgenic animals as bioreactors. To date most of these proteins are extracted from the tissues and biological fluids of man. For example, a clotting factor, interferon, alpha-1 -antitrypsin, and other proteins are prepared from blood, growth hormone - from the pituitary gland. They are produced in small quantities because of the high cost and difficulty of extraction of human tissues. In addition, they may be contaminated with pathogens such as Hepatitis, AIDS, etc. Transgenic animals used for the production of valuable biological products have several advantages over microorganisms-producers, as well as cellular systems. In simple recombinant systems, of microorganisms glycosylation, B-hydroxylation or carboxylation of mammalian proteins in most cases it is impossible or possible, but with insufficient accuracy. This changes the structure of proteins, which reflect on their biological activity. Along with this, in drugs which are used by humans for therapeutic agents admixture of bacterial proteins is undesirable. The main disadvantage of genetically engineered cell culture is the low yield of protein. Industrial reactors used for the cultivation of producer cells, are expensive, both in terms of their value, and in respect of their service. Creation of transgenic animals also requires more resources and moreover it is not easy, but once bred line of such animals can produce a large number of proteins with low cost, which will pay back all the expenses for a short time. Production of biologically active human proteins from transgenic agricultural animals guarantee their environmental cleanliness, which practically comes to exploitation of animals-producers.
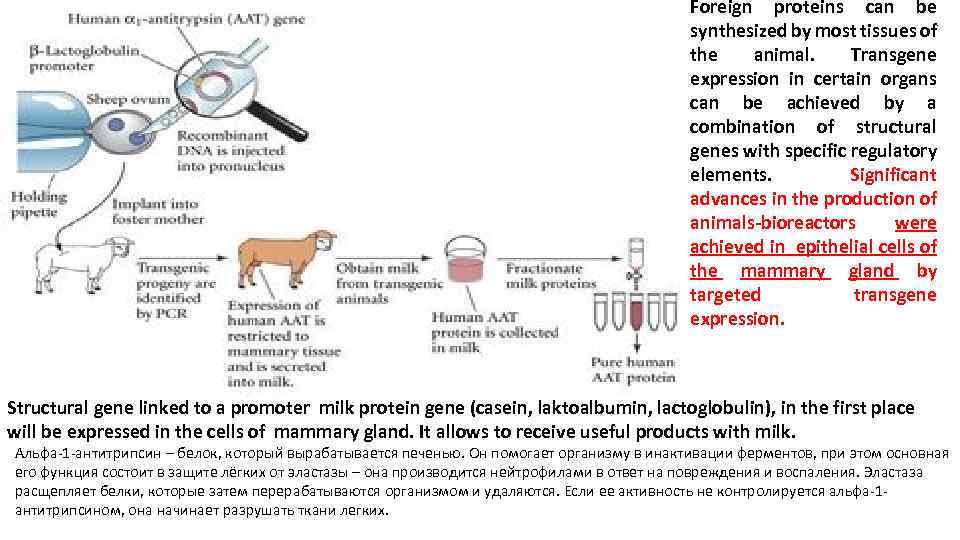
Foreign proteins can be synthesized by most tissues of the animal. Transgene expression in certain organs can be achieved by a combination of structural genes with specific regulatory elements. Significant advances in the production of animals-bioreactors were achieved in epithelial cells of the mammary gland by targeted transgene expression. Structural gene linked to a promoter milk protein gene (casein, laktoalbumin, lactoglobulin), in the first place will be expressed in the cells of mammary gland. It allows to receive useful products with milk. Альфа-1 -антитрипсин – белок, который вырабатывается печенью. Он помогает организму в инактивации ферментов, при этом основная его функция состоит в защите лёгких от эластазы – она производится нейтрофилами в ответ на повреждения и воспаления. Эластаза расщепляет белки, которые затем перерабатываются организмом и удаляются. Если ее активность не контролируется альфа-1 антитрипсином, она начинает разрушать ткани легких.
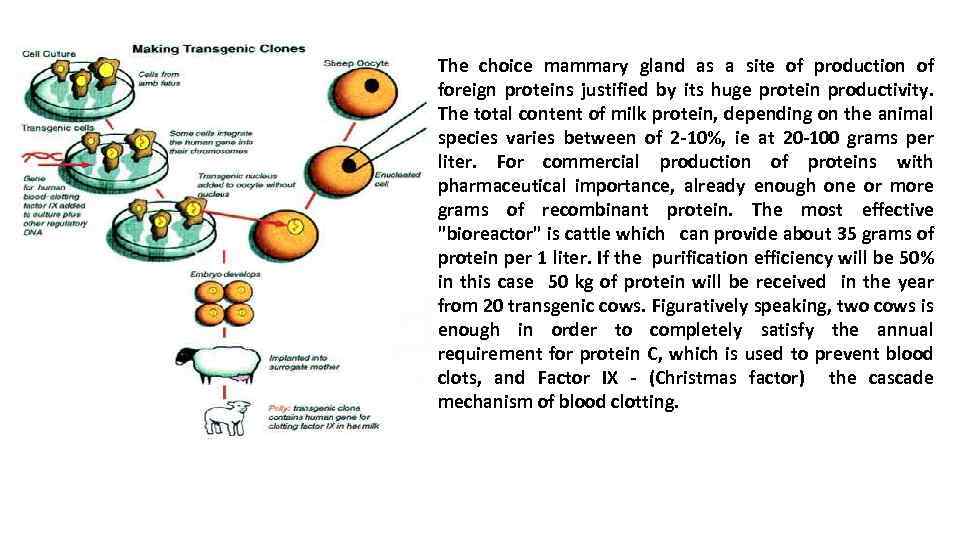
The choice mammary gland as a site of production of foreign proteins justified by its huge protein productivity. The total content of milk protein, depending on the animal species varies between of 2 -10%, ie at 20 -100 grams per liter. For commercial production of proteins with pharmaceutical importance, already enough one or more grams of recombinant protein. The most effective "bioreactor" is cattle which can provide about 35 grams of protein per 1 liter. If the purification efficiency will be 50% in this case 50 kg of protein will be received in the year from 20 transgenic cows. Figuratively speaking, two cows is enough in order to completely satisfy the annual requirement for protein C, which is used to prevent blood clots, and Factor IX - (Christmas factor) the cascade mechanism of blood clotting.
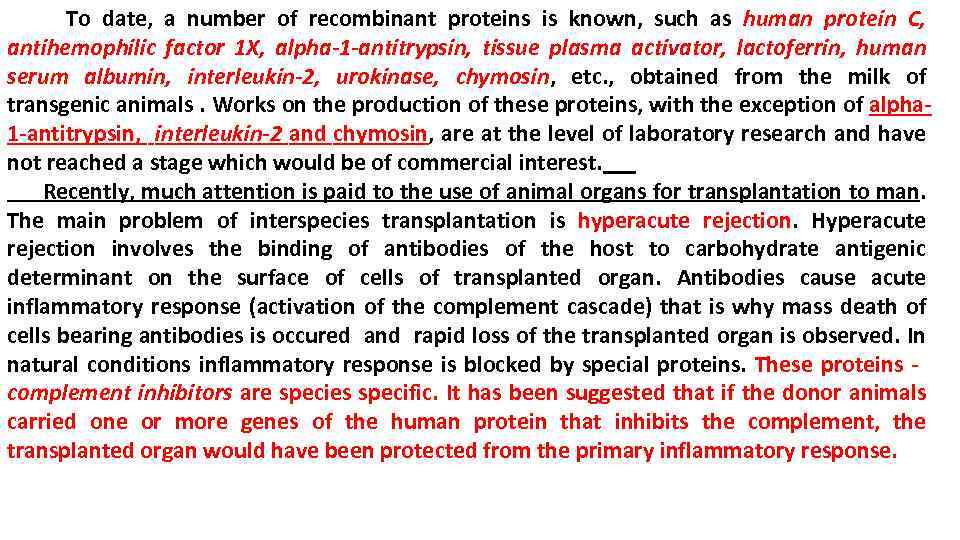
To date, a number of recombinant proteins is known, such as human protein C, antihemophilic factor 1 X, alpha-1 -antitrypsin, tissue plasma activator, lactoferrin, human serum albumin, interleukin-2, urokinase, chymosin, etc. , obtained from the milk of transgenic animals. Works on the production of these proteins, with the exception of alpha 1 -antitrypsin, interleukin-2 and chymosin, are at the level of laboratory research and have not reached a stage which would be of commercial interest. Recently, much attention is paid to the use of animal organs for transplantation to man. The main problem of interspecies transplantation is hyperacute rejection. Hyperacute rejection involves the binding of antibodies of the host to carbohydrate antigenic determinant on the surface of cells of transplanted organ. Antibodies cause acute inflammatory response (activation of the complement cascade) that is why mass death of cells bearing antibodies is occured and rapid loss of the transplanted organ is observed. In natural conditions inflammatory response is blocked by special proteins. These proteins - complement inhibitors are species specific. It has been suggested that if the donor animals carried one or more genes of the human protein that inhibits the complement, the transplanted organ would have been protected from the primary inflammatory response.
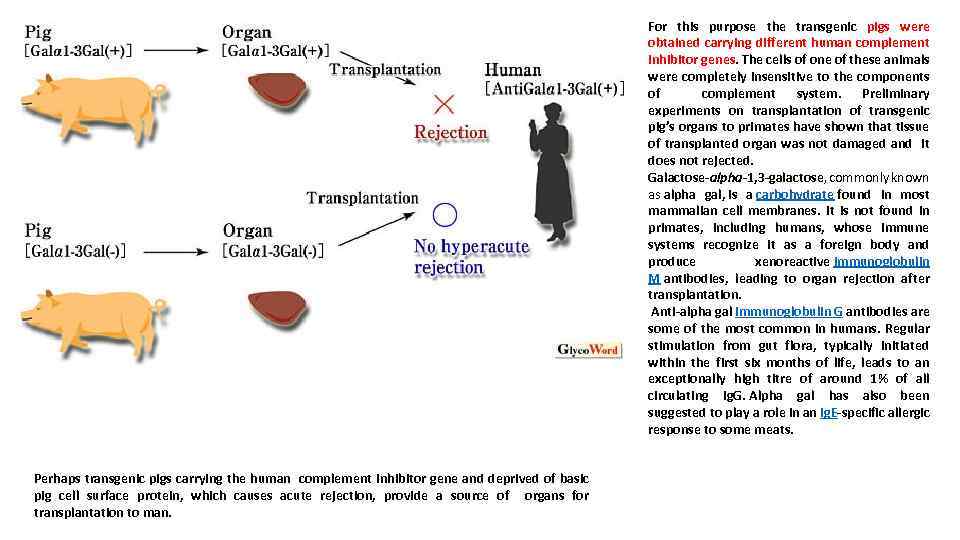
For this purpose the transgenic pigs were obtained carrying different human complement inhibitor genes. The cells of one of these animals were completely insensitive to the components of complement system. Preliminary experiments on transplantation of transgenic pig’s organs to primates have shown that tissue of transplanted organ was not damaged and it does not rejected. Galactose-alpha-1, 3 -galactose, commonly known as alpha gal, is a carbohydrate found in most mammalian cell membranes. It is not found in primates, including humans, whose immune systems recognize it as a foreign body and produce xenoreactive immunoglobulin M antibodies, leading to organ rejection after transplantation. Anti-alpha gal immunoglobulin G antibodies are some of the most common in humans. Regular stimulation from gut flora, typically initiated within the first six months of life, leads to an exceptionally high titre of around 1% of all circulating Ig. G. Alpha gal has also been suggested to play a role in an Ig. E-specific allergic response to some meats. Perhaps transgenic pigs carrying the human complement inhibitor gene and deprived of basic pig cell surface protein, which causes acute rejection, provide a source of organs for transplantation to man.
The first work on getting transgenic animals - producers of interleukin-2 turned out encouraging. Interleukin-2 being a soluble factor of T-helper lymphocytes involved in cell proliferation and differentiation of T-cell killer, plays an important role in ensuring the required level of immunity. Using a gene construct consisting of rabbit beta-casein DNA and structural human interleukin-2 gene, rabbits were obtained secreting with milk active form of the protein. Thus, integration of one or more genes in mammalian embryos is achieved and their expression as well as the transmission to the offspring is proved. However, the difficulties and uncertainties should be emphasized with which still related technique for producing transgenic animals. Mechanism of integration of the gene in mammalian cells is still poorly understood. A)This integration occurs randomly and not connected with a specific region of a chromosome. B) Another difficulty is due to the instability of the cells in which gene (s) is introduced: it may be lost or modified as a result becomes inactive. C) Finally, the activity of genes is determined not only by sequences of nucleotides that provide gene transcription with the formation of m. RNA, but as well as other sequences of nucleotides, which are often far from their own gene. These sequences are administered with a gene to achieve full expression of the it. The results achieved in the field of genetic engineering on getting transgenic mammals allow to deepen our knowledge about gene expression that in the future facilitate gene transfer and identification of factors that contribute to a more complete expression of the genetic information stored in transgene.

Featured Article on lectures J. Livestock Science. -2013. - Vol. 153. -P. 1– 9 Review article Genetically modified farm animals and fish in agriculture: F. Forabosco a, n, M. L. ohmus b, L. Rydhmer a, L. F. Sundstrom. Department of Animal Breeding and Genetics, Swedish University of Agricultural Sciences, Uppsala, Sweden • Department of Chemistry, Environment and Feed Hygiene, Section of Environment and Biosecurity, National Veterinary Institute, Swedish • University of Agricultural. Sciences, Uppsala, Sweden • Department of Ecology and Genetics/Animal Ecology, Evolutionary Biology Centre, Uppsala University, Uppsala, Sweden • • •
RDBVMAH Transgenic animals.pptx