b448de438fb232289d7e76845d4f929b.ppt
- Количество слайдов: 31

Probe design for microarrays using Oligo. Wiz
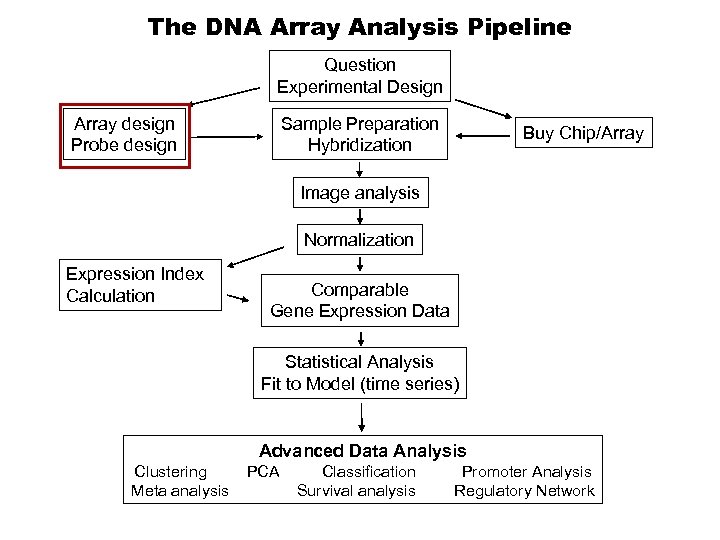
The DNA Array Analysis Pipeline Question Experimental Design Array design Probe design Sample Preparation Hybridization Buy Chip/Array Image analysis Normalization Expression Index Calculation Comparable Gene Expression Data Statistical Analysis Fit to Model (time series) Advanced Data Analysis Clustering Meta analysis PCA Classification Survival analysis Promoter Analysis Regulatory Network
Probe design for microarrays -What is a Probe -Different Probe Types -Oligo. Wiz -Probe Design -Cross Hybridization and Complexity -Affinity -Position

An Ideal Probe must - Discriminate well between its intended target and all other targets in the target pool - Detect concentration differences under the hybridization conditions
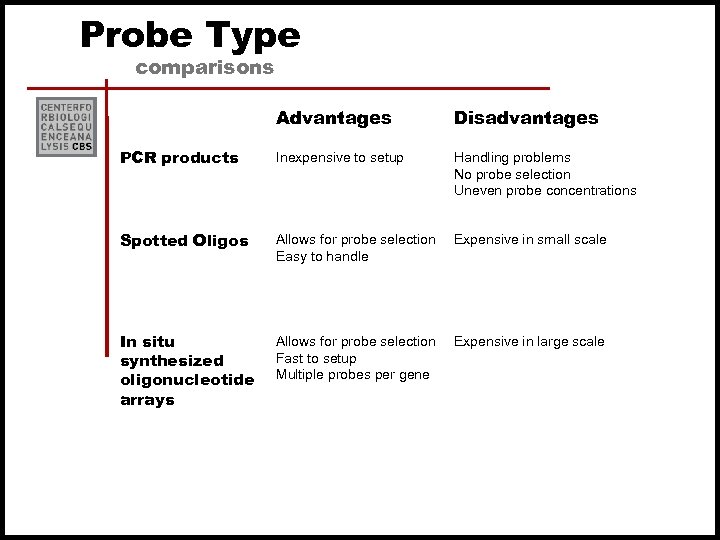
Probe Type comparisons Advantages PCR products Inexpensive to setup Handling problems No probe selection Uneven probe concentrations Spotted Oligos Allows for probe selection Easy to handle Expensive in small scale In situ synthesized oligonucleotide arrays Disadvantages Allows for probe selection Fast to setup Multiple probes per gene Expensive in large scale
Custom Microarrays When on virgin ground Some technologies available for custom arrays Spotted arrays in situ synthesized Nimble. Expressェ Array Program
Oligo. Wiz a Tool for flexible probe design

How does it work? Probe selection 1. Optimal melting temperature (Tm) for the DNA: DNA or RNA: DNA hybridization for probes of the given length is determined. 2. Optimal probe length are determined for all possible probes along the input sequence 3. Five scores are calculated for each of these probes 4. Best probes are selected based on a weighted sum of these scores
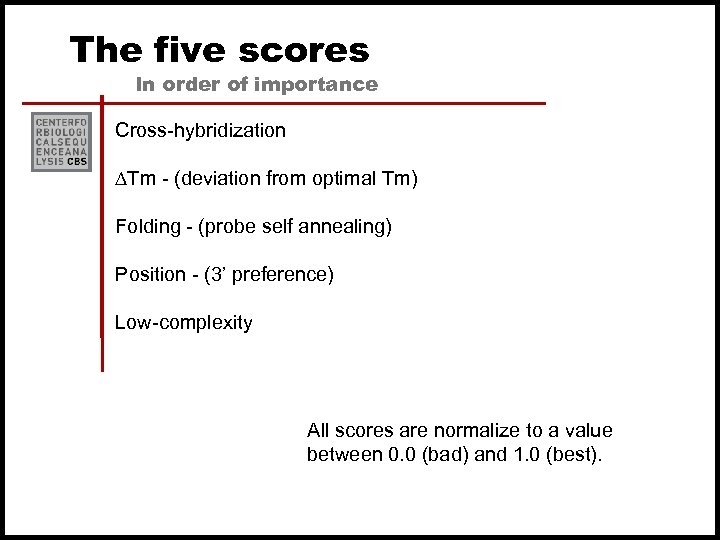
The five scores In order of importance Cross-hybridization ∆Tm - (deviation from optimal Tm) Folding - (probe self annealing) Position - (3’ preference) Low-complexity All scores are normalize to a value between 0. 0 (bad) and 1. 0 (best).

How to Avoid cross-hybridization From Kane et al. (2000) we learn that a 50’mer probe can detect significant false signal from a target that has >75 -80% homology to a 50’mer oligo or a continuous stretch of >15 complementary bases If we have substantial sequence information on the given organism, we can try to avoid this by choosing oligos that are not similar to any other expressed sequences.

Probe Specificity Hughes et al. 2001
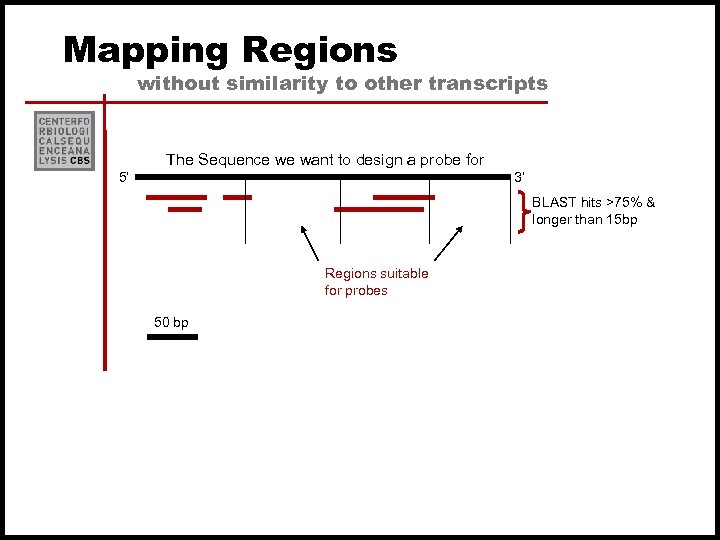
Mapping Regions without similarity to other transcripts The Sequence we want to design a probe for 5’ 3’ BLAST hits >75% & longer than 15 bp Regions suitable for probes 50 bp

Filtering Self Detecting BLAST hits out The Sequence we want to design a oligo for 3’ 5’ BLAST hits >75% & longer than 15 bp Sequence identical or very similar to the query sequence Therefore no BLAST hits with homology > 97% and with a ‘hit length vs. query length’ ratio > 0. 8, are considered. 50 bp
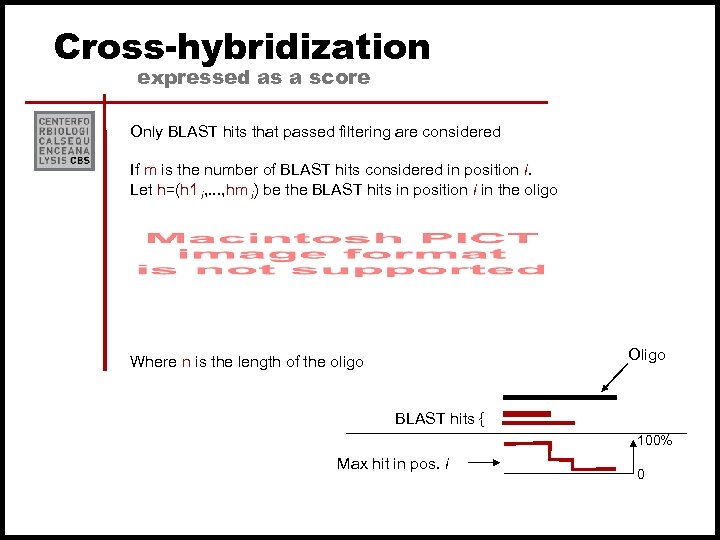
Cross-hybridization expressed as a score Only BLAST hits that passed filtering are considered If m is the number of BLAST hits considered in position i. Let h=(h 1 i, . . . , hm i) be the BLAST hits in position i in the oligo Oligo Where n is the length of the oligo BLAST hits { 100% Max hit in pos. i 0

Similar Affinity for all oligos Another way of ensuring a optimal discrimination between target and non-target under hybridization is to design all the oligos on an array with similar affinity for their targets. This will allow the experimentalist to optimize the hybridization conditions for all oligos by choosing the right hybridization temperature and salt concentration. Commonly Melting Temperature (Tm) is used as a measure for DNA: DNA or RNA: DNA hybrid affinity.

Melting Temperature difference Where DH (Kcal/mol) is the sum of the nearest neighbor enthalpy, A is a constant for helix initiation corrections, DS is the sum of the nearest neighbor entropy changes, R is the Gas Constant (1. 987 cal deg-1 mol-1) and Ct is the total molar concentration of strands. Where N is all oligos in all sequences.

Tm distributions for 30’mers and 50’mers
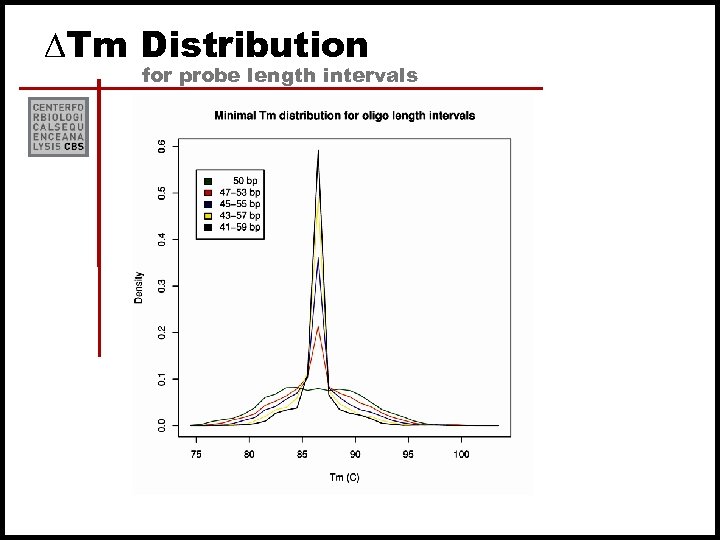
DTm Distribution for probe length intervals
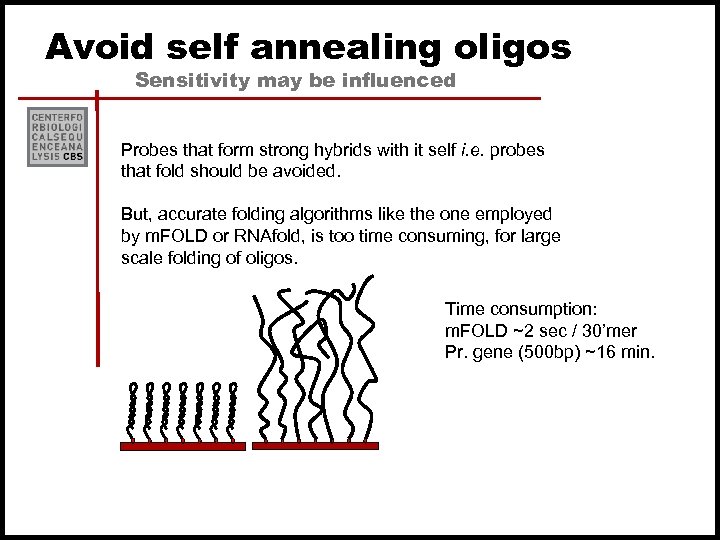
Avoid self annealing oligos Sensitivity may be influenced Probes that form strong hybrids with it self i. e. probes that fold should be avoided. But, accurate folding algorithms like the one employed by m. FOLD or RNAfold, is too time consuming, for large scale folding of oligos. Time consumption: m. FOLD ~2 sec / 30’mer Pr. gene (500 bp) ~16 min.
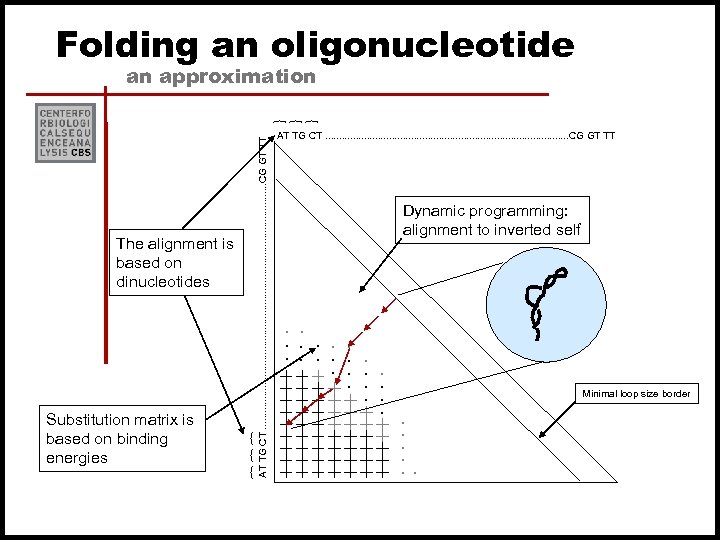
Folding an oligonucleotide an approximation Minimal loop size border . . . { { { Dynamic programming: alignment to inverted self . . . Substitution matrix is based on binding energies AT TG CT. . . . . . CG GT TT { { { The alignment is based on dinucleotides AT TG CT. . . . . . CG GT TT
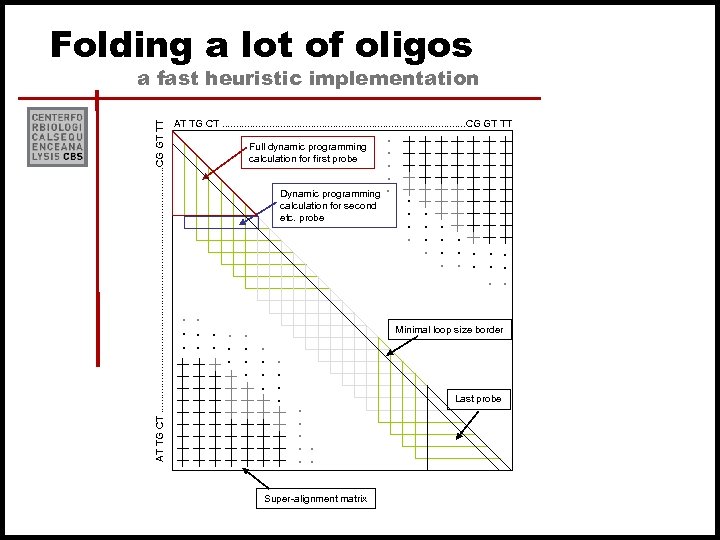
Folding a lot of oligos AT TG CT. . . . . . CG GT TT Full dynamic programming calculation for first probe Dynamic programming calculation for second etc. probe . . . . Minimal loop size border Last probe . . . . AT TG CT. . . . . . CG GT TT a fast heuristic implementation Super-alignment matrix

Reasonably folding prediction compared to m. FOLD

Probes With Very Common sub sequences may result in unspecific signal If the sub-fractions of an oligo are very common we define it as ‘low-complex’ Oligo with low-complexity: AAAAAAAGGAGTTTTCAAAAAACTTTTTAAAAAAGCTTTAGGTTTTTA (Human) Oligo without low-complexity: CGTGACAGCTGACTGCTAGCCATGCAACGTCATAGTACGATGACT (Human)

Low-complexity expressed as a score For a given transcriptome a list of information content from all ‘words’ with length wl (8 bp) is calculated: Where f(w) is the number of occurrences of a pattern and tf(w) is the total number of patterns of length wl. A low-complexity score for a given oligo is defined as: Low-complexity = 1 -norm Where norm is a function that normalizes to between 1 and 0, L is the length of the oligo and Wi is the pattern in position i.
Location of Oligo within transcript Labeling include reverse transcription of the m. RNA and is sensitive to: - RNA degradation - Premature termination of c. DNA synthesis - Premature termination of c. RNA transcription (IVT) Eukaryote Position Score: 3’ preference Prokaryote Position score Preference toward 3’, but avoid ~50 most 3’ bases Typically eukaryote sample labeling is done by poly-T and Bacterial samples by random labeling
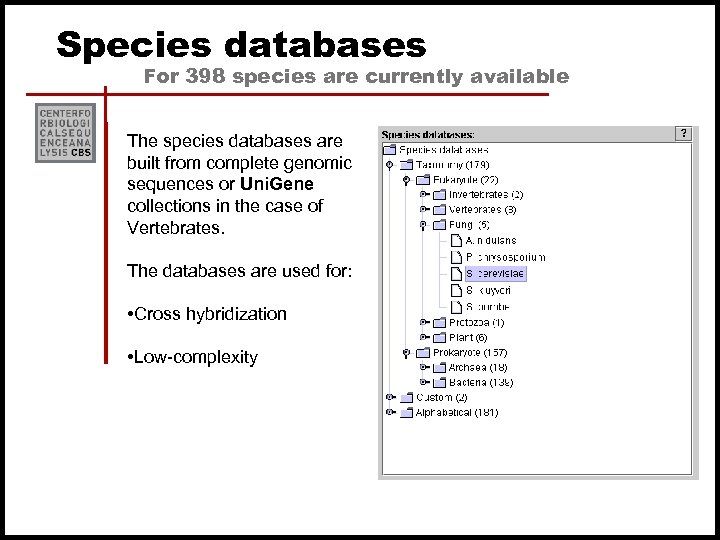
Species databases For 398 species are currently available The species databases are built from complete genomic sequences or Uni. Gene collections in the case of Vertebrates. The databases are used for: • Cross hybridization • Low-complexity
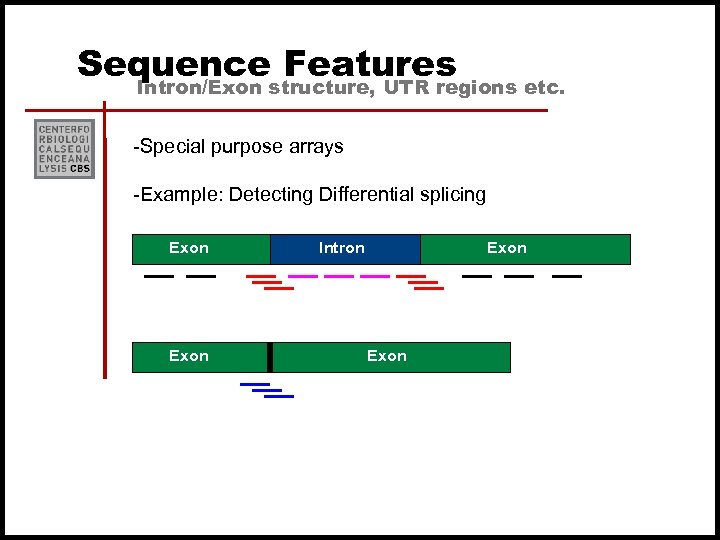
Sequence Features Intron/Exon structure, UTR regions etc. -Special purpose arrays -Example: Detecting Differential splicing Exon Intron Exon
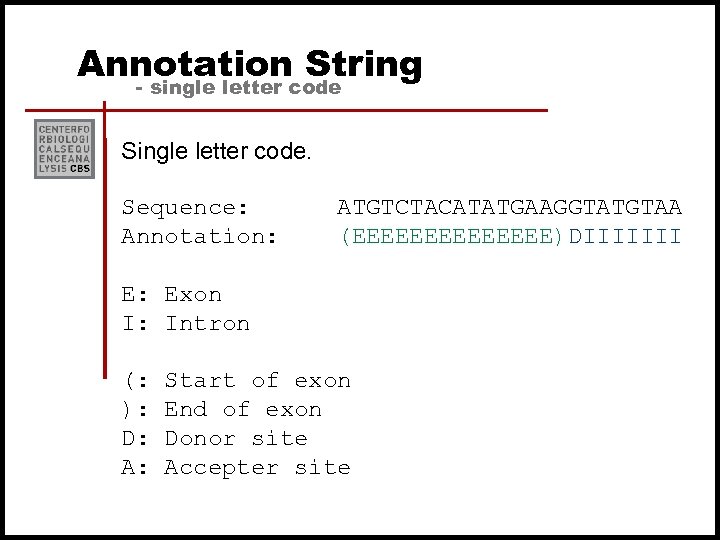
Annotation String - single letter code Single letter code. Sequence: Annotation: ATGTCTACATATGAAGGTATGTAA (EEEEEEE)DIIIIIII E: Exon I: Intron (: ): D: A: Start of exon End of exon Donor site Accepter site
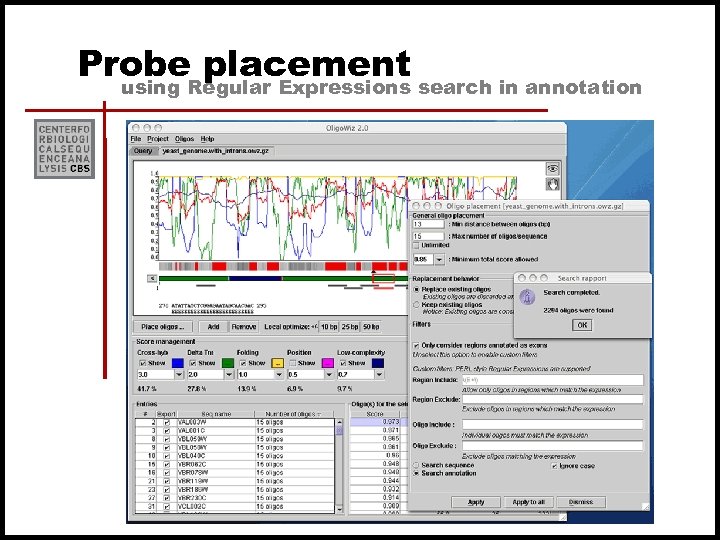
Probe placement using Regular Expressions search in annotation

Extracting annotation from Gen. Bank files -Feature. Extract server -www. cbs. dtu. dk/services/Feature. Extract

Exercise • Running Oligo. Wiz 2. 0 • Java 1. 4. 1 or better is required • Input data • Sequence only (FASTA) • Sequence and annotation • Rule-based placement of multiple probes • Distance criteria • Annotation criteria • Please go to the exercise web-page linked from the course program
b448de438fb232289d7e76845d4f929b.ppt