8749887bfc9b0a3d2093761c26ff8819.ppt
- Количество слайдов: 21
 Primer Design Dave Palmer dpalmer@zdap. com
Primer Design Dave Palmer dpalmer@zdap. com
 Why Are Primers Important? Primers are what gives PCR its SPECIFICITY!!! Good primer design: PCR works great. Bad primer design: PCR works terrible.
Why Are Primers Important? Primers are what gives PCR its SPECIFICITY!!! Good primer design: PCR works great. Bad primer design: PCR works terrible.
 Very-Brief PCR Reminder PCR is a method to amplify large quantities of a DNA covering a specific sequence.
Very-Brief PCR Reminder PCR is a method to amplify large quantities of a DNA covering a specific sequence.
 Factors That Affect Priming Melting / Annealing Temperature n Of primers to target Secondary Structure n Within target Complementarity n n Primers to target Primers to each other
Factors That Affect Priming Melting / Annealing Temperature n Of primers to target Secondary Structure n Within target Complementarity n n Primers to target Primers to each other
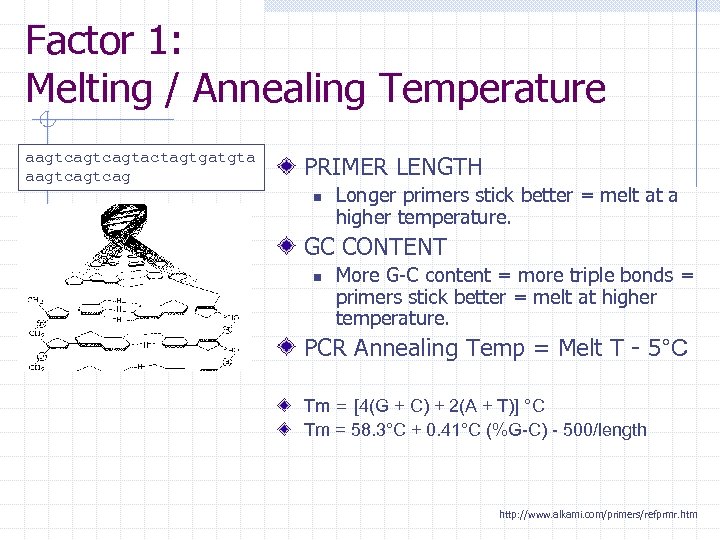 Factor 1: Melting / Annealing Temperature aagtcagtactagtgatgta aagtcag PRIMER LENGTH n Longer primers stick better = melt at a higher temperature. GC CONTENT n More G-C content = more triple bonds = primers stick better = melt at higher temperature. PCR Annealing Temp = Melt T - 5°C Tm = [4(G + C) + 2(A + T)] °C Tm = 58. 3°C + 0. 41°C (%G-C) - 500/length http: //www. alkami. com/primers/refprmr. htm
Factor 1: Melting / Annealing Temperature aagtcagtactagtgatgta aagtcag PRIMER LENGTH n Longer primers stick better = melt at a higher temperature. GC CONTENT n More G-C content = more triple bonds = primers stick better = melt at higher temperature. PCR Annealing Temp = Melt T - 5°C Tm = [4(G + C) + 2(A + T)] °C Tm = 58. 3°C + 0. 41°C (%G-C) - 500/length http: //www. alkami. com/primers/refprmr. htm
 Factor 2: Secondary Structure Primers will have difficulty annealing: if they anneal to regions of secondary structure within the target that have a higher melting point than the primer. http: //www. alkami. com/primers/refprmr. htm
Factor 2: Secondary Structure Primers will have difficulty annealing: if they anneal to regions of secondary structure within the target that have a higher melting point than the primer. http: //www. alkami. com/primers/refprmr. htm
 Factor 3: Complementarity PRIMER-PRIMER (BAD) atcggactatcga n gctatacttatggcca Excessive similarity between primers, especially at the 3’ ends, leads to the formation of “primer dimers” PRIMER-TARGET (GOOD) atcggactatcga n tagcctgatagctatacttatggcca n Ideally should be 100% similar for maximal specificity. Primers don’t HAVE to be perfectly similar to target to work. http: //www. alkami. com/primers/refprmr. htm
Factor 3: Complementarity PRIMER-PRIMER (BAD) atcggactatcga n gctatacttatggcca Excessive similarity between primers, especially at the 3’ ends, leads to the formation of “primer dimers” PRIMER-TARGET (GOOD) atcggactatcga n tagcctgatagctatacttatggcca n Ideally should be 100% similar for maximal specificity. Primers don’t HAVE to be perfectly similar to target to work. http: //www. alkami. com/primers/refprmr. htm
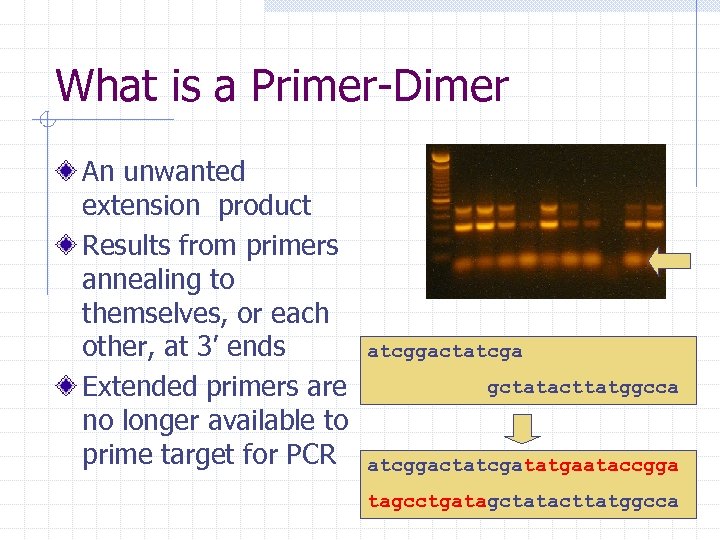 What is a Primer-Dimer An unwanted extension product Results from primers annealing to themselves, or each other, at 3’ ends Extended primers are no longer available to prime target for PCR atcggactatcga gctatacttatggcca atcggactatcgatatgaataccgga tagcctgatagctatacttatggcca
What is a Primer-Dimer An unwanted extension product Results from primers annealing to themselves, or each other, at 3’ ends Extended primers are no longer available to prime target for PCR atcggactatcga gctatacttatggcca atcggactatcgatatgaataccgga tagcctgatagctatacttatggcca
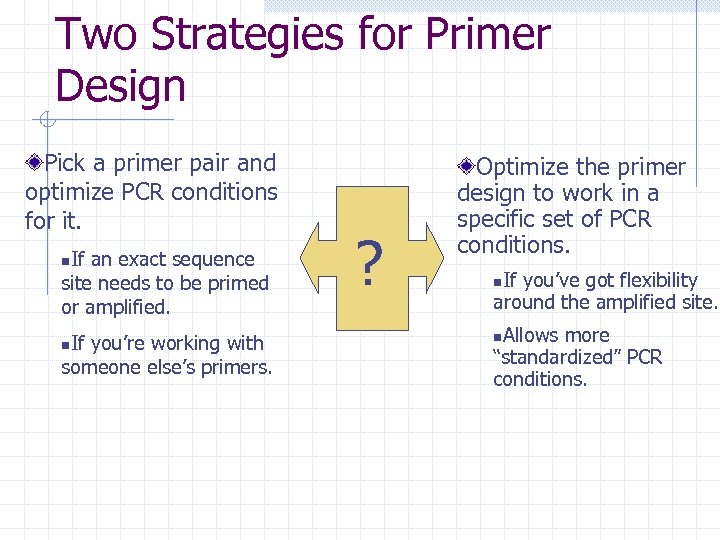 Two Strategies for Primer Design Pick a primer pair and optimize PCR conditions for it. If an exact sequence site needs to be primed or amplified. n If you’re working with someone else’s primers. n ? Optimize the primer design to work in a specific set of PCR conditions. If you’ve got flexibility around the amplified site. n Allows more “standardized” PCR conditions. n
Two Strategies for Primer Design Pick a primer pair and optimize PCR conditions for it. If an exact sequence site needs to be primed or amplified. n If you’re working with someone else’s primers. n ? Optimize the primer design to work in a specific set of PCR conditions. If you’ve got flexibility around the amplified site. n Allows more “standardized” PCR conditions. n
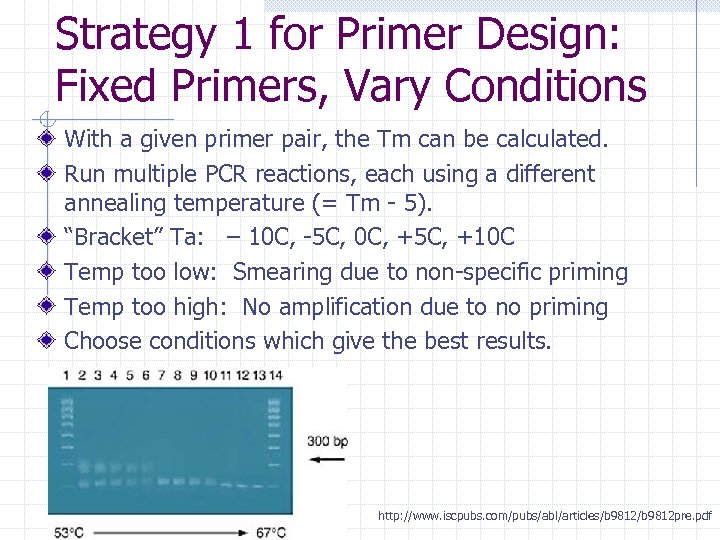 Strategy 1 for Primer Design: Fixed Primers, Vary Conditions With a given primer pair, the Tm can be calculated. Run multiple PCR reactions, each using a different annealing temperature (= Tm - 5). “Bracket” Ta: – 10 C, -5 C, 0 C, +5 C, +10 C Temp too low: Smearing due to non-specific priming Temp too high: No amplification due to no priming Choose conditions which give the best results. http: //www. iscpubs. com/pubs/abl/articles/b 9812 pre. pdf
Strategy 1 for Primer Design: Fixed Primers, Vary Conditions With a given primer pair, the Tm can be calculated. Run multiple PCR reactions, each using a different annealing temperature (= Tm - 5). “Bracket” Ta: – 10 C, -5 C, 0 C, +5 C, +10 C Temp too low: Smearing due to non-specific priming Temp too high: No amplification due to no priming Choose conditions which give the best results. http: //www. iscpubs. com/pubs/abl/articles/b 9812 pre. pdf
 Strategy 2 for Primer Design: Optimizing Primers for Set Conditions PCR conditions (esp. annealing temp) are kept constant. Select primers for a theoretical Tm. Best to select multiple primers, then experiment to see which combination works best. 95 C – 65 C – 72 C F 1: atcgatcgatcagtcatcg F 2: gtactgagctgcagctc R 1: atgactgagctgctagcttg R 2: atgctcgtgactgtg R 1 R 2 F 1/R 1 F 1/R 2 F 2/R 1 F 2/R 2
Strategy 2 for Primer Design: Optimizing Primers for Set Conditions PCR conditions (esp. annealing temp) are kept constant. Select primers for a theoretical Tm. Best to select multiple primers, then experiment to see which combination works best. 95 C – 65 C – 72 C F 1: atcgatcgatcagtcatcg F 2: gtactgagctgcagctc R 1: atgactgagctgctagcttg R 2: atgctcgtgactgtg R 1 R 2 F 1/R 1 F 1/R 2 F 2/R 1 F 2/R 2
 Designing Primers Primer Design on the Web n Example: “Primer 3” http: //frodo. wi. mit. edu/ Example gene: GFP 5 Green Fluorescent Protein n GFP 5, Genebank 1848286 1 ggatccaagg agatataaca atgagtaaag gagaagaact tttcactgga gttgtcccaa 61 ttcttgttga attagatggt gatgttaatg ggcacaaatt ttctgtcagt ggagagggtg 121 aaggtgatgc aacatacgga aaacttaccc ttaaatttat ttgcactact ggaaaactac 181 ctgttccatg gccaacactt gtcactactt tctcttatgg tgttcaatgc ttttcaagat 241 acccagatca tatgaagcgg cacgacttct tcaagagcgc catgcctgag ggatacgtgc 301 aggagaggac catcttcttc aaggacgacg ggaactacaa gacacgtgct gaagtcaagt 361 ttgagggaga caccctcgtc aacaggatcg agcttaaggg aatcgatttc aaggaggacg 421 gaaacatcct cggccacaag ttggaataca actacaactc ccacaacgta tacatcatgg 481 ccgacaagca aaagaacggc atcaaagcca acttcaagac ccgccacaac atcgaagacg 541 gcggcgtgca actcgctgat cattatcaac aaaatactcc aattggcgat ggccctgtcc 601 ttttaccaga caaccattac ctgtccacac aatctgccct ttcgaaagat cccaacgaaa 661 agagagacca catggtcctt cttgagtttg taacagctgc tgggattaca catgg 721 atgaactata caaataagag ctc
Designing Primers Primer Design on the Web n Example: “Primer 3” http: //frodo. wi. mit. edu/ Example gene: GFP 5 Green Fluorescent Protein n GFP 5, Genebank 1848286 1 ggatccaagg agatataaca atgagtaaag gagaagaact tttcactgga gttgtcccaa 61 ttcttgttga attagatggt gatgttaatg ggcacaaatt ttctgtcagt ggagagggtg 121 aaggtgatgc aacatacgga aaacttaccc ttaaatttat ttgcactact ggaaaactac 181 ctgttccatg gccaacactt gtcactactt tctcttatgg tgttcaatgc ttttcaagat 241 acccagatca tatgaagcgg cacgacttct tcaagagcgc catgcctgag ggatacgtgc 301 aggagaggac catcttcttc aaggacgacg ggaactacaa gacacgtgct gaagtcaagt 361 ttgagggaga caccctcgtc aacaggatcg agcttaaggg aatcgatttc aaggaggacg 421 gaaacatcct cggccacaag ttggaataca actacaactc ccacaacgta tacatcatgg 481 ccgacaagca aaagaacggc atcaaagcca acttcaagac ccgccacaac atcgaagacg 541 gcggcgtgca actcgctgat cattatcaac aaaatactcc aattggcgat ggccctgtcc 601 ttttaccaga caaccattac ctgtccacac aatctgccct ttcgaaagat cccaacgaaa 661 agagagacca catggtcctt cttgagtttg taacagctgc tgggattaca catgg 721 atgaactata caaataagag ctc
 Designing Primers Primer Design on the Web Using Primer 3 Enter sequence Pick Primers
Designing Primers Primer Design on the Web Using Primer 3 Enter sequence Pick Primers
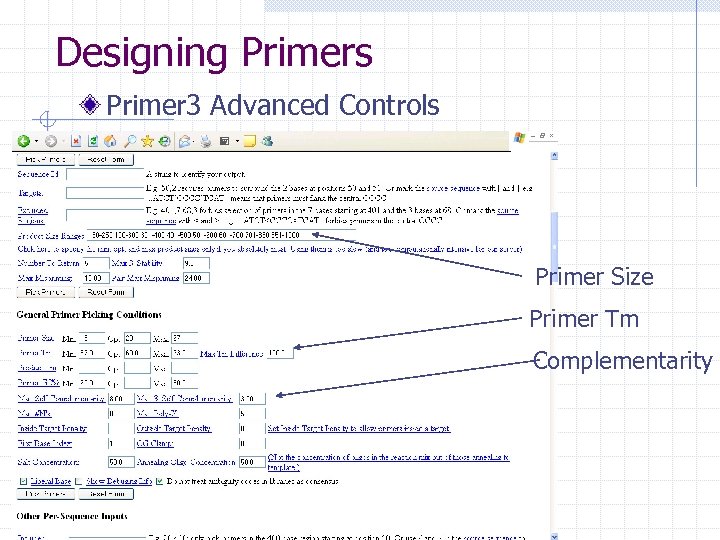 Designing Primers Primer 3 Advanced Controls Primer Size Primer Tm Complementarity
Designing Primers Primer 3 Advanced Controls Primer Size Primer Tm Complementarity
 Designing Primers Primer 3 Output Details: -Start -Length -Tm -GC -Sequence Where they bind:
Designing Primers Primer 3 Output Details: -Start -Length -Tm -GC -Sequence Where they bind:
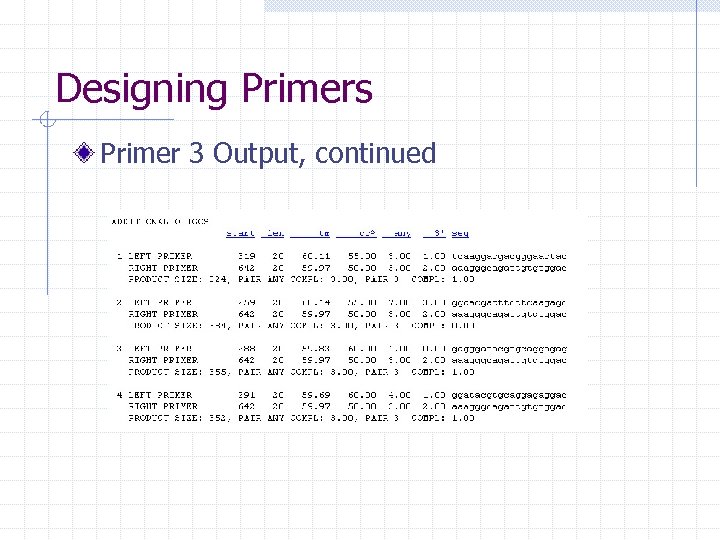 Designing Primers Primer 3 Output, continued
Designing Primers Primer 3 Output, continued
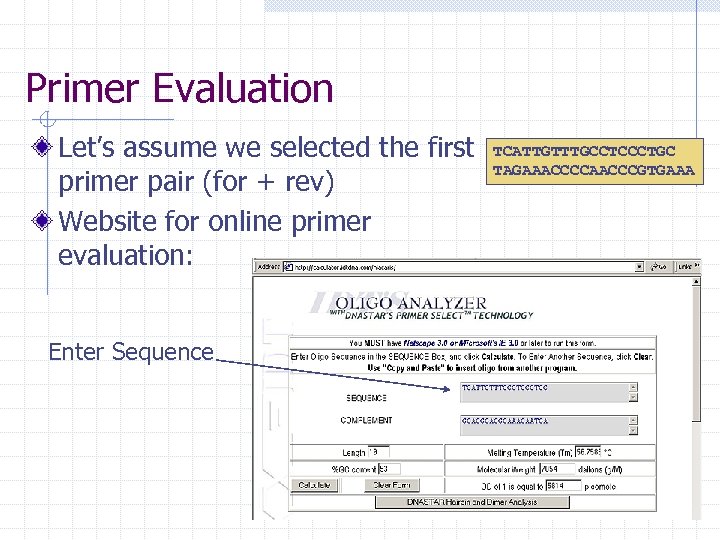 Primer Evaluation Let’s assume we selected the first primer pair (for + rev) Website for online primer evaluation: Enter Sequence TCATTGTTTGCCTCCCTGC TAGAAACCCCAACCCGTGAAA
Primer Evaluation Let’s assume we selected the first primer pair (for + rev) Website for online primer evaluation: Enter Sequence TCATTGTTTGCCTCCCTGC TAGAAACCCCAACCCGTGAAA
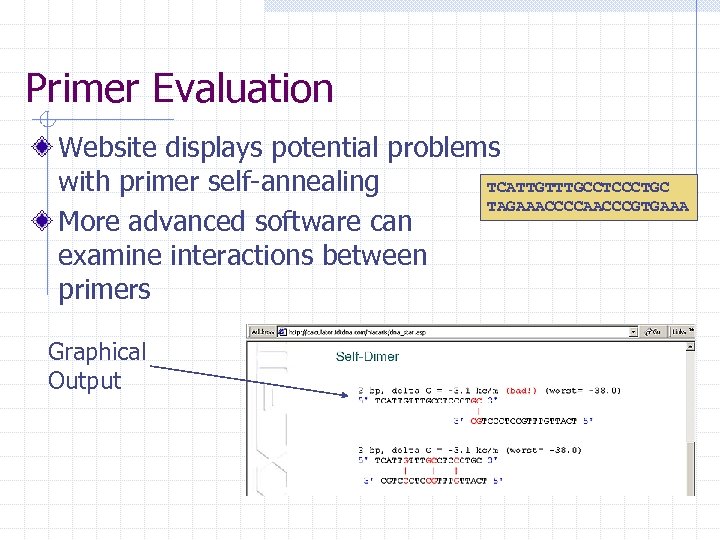 Primer Evaluation Website displays potential problems with primer self-annealing TCATTGTTTGCCTCCCTGC TAGAAACCCCAACCCGTGAAA More advanced software can examine interactions between primers Graphical Output
Primer Evaluation Website displays potential problems with primer self-annealing TCATTGTTTGCCTCCCTGC TAGAAACCCCAACCCGTGAAA More advanced software can examine interactions between primers Graphical Output
 Primer Evaluation Just for fun, let’s assume we selected a really BAD primer. . . GGGCCCCTCACCAACCCGTGCCCGGG
Primer Evaluation Just for fun, let’s assume we selected a really BAD primer. . . GGGCCCCTCACCAACCCGTGCCCGGG
 Live Example Primer Design Workflow: 1. Pick a gene. ie. BRCA 1 2. Pull up sequence for the gene. a. http: //www. ncbi. nlm. nih. gov/ b. search Nucleotide Database for brca 1 c. scroll through accessions for desired one 3. Copy sequence to text editor. 4. Pull up a primer design website. a. http: //frodo. wi. mit. edu/ b. copy sequence c. select options and choose Pick Primers 4 b. Verify primers find target (optional) a. http: //www. ncbi. nlm. nih. gov/BLAST/ b. select nucleotide blast c. enter primer sequence, choose blast 4 c. Analyse and double-check the primers a. http: //www. idtdna. com/analyzer/Applications/Oligo. Analyzer/Default. aspx b. enter sequence, view 5. Order oligos. a. http: //www. operon. com
Live Example Primer Design Workflow: 1. Pick a gene. ie. BRCA 1 2. Pull up sequence for the gene. a. http: //www. ncbi. nlm. nih. gov/ b. search Nucleotide Database for brca 1 c. scroll through accessions for desired one 3. Copy sequence to text editor. 4. Pull up a primer design website. a. http: //frodo. wi. mit. edu/ b. copy sequence c. select options and choose Pick Primers 4 b. Verify primers find target (optional) a. http: //www. ncbi. nlm. nih. gov/BLAST/ b. select nucleotide blast c. enter primer sequence, choose blast 4 c. Analyse and double-check the primers a. http: //www. idtdna. com/analyzer/Applications/Oligo. Analyzer/Default. aspx b. enter sequence, view 5. Order oligos. a. http: //www. operon. com
 End of Primer Design
End of Primer Design


