Применение рентгеновского излучения_Лекция_4.pptx
- Количество слайдов: 36
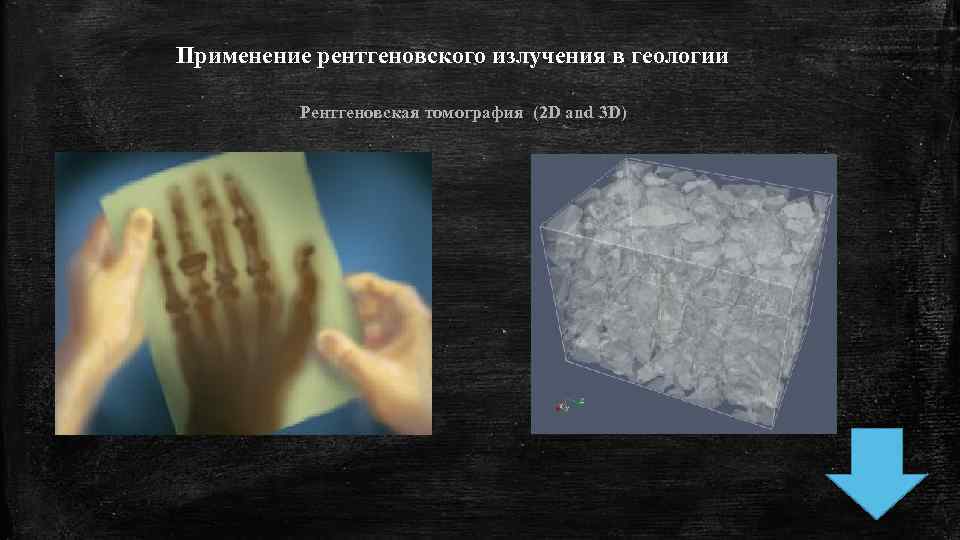 Применение рентгеновского излучения в геологии Рентгеновская томография (2 D and 3 D)
Применение рентгеновского излучения в геологии Рентгеновская томография (2 D and 3 D)
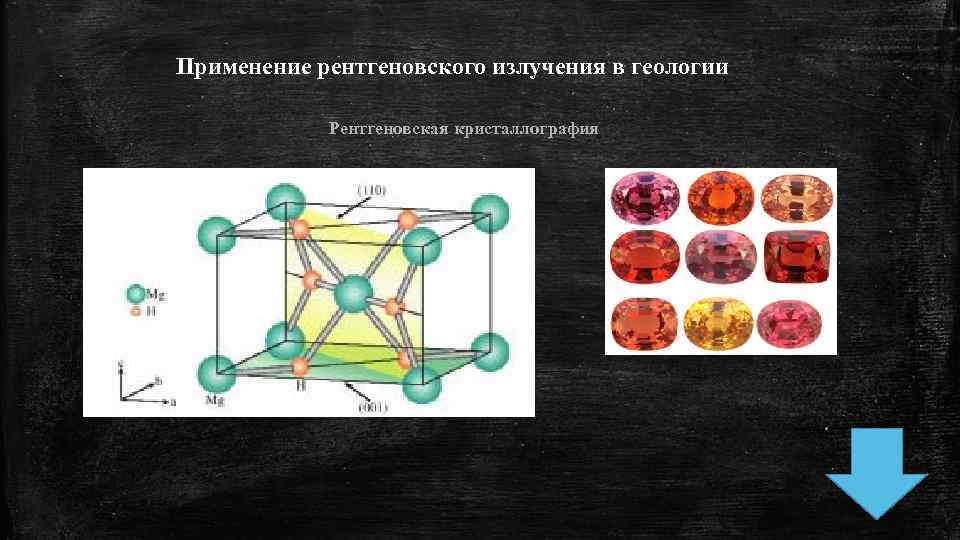 Применение рентгеновского излучения в геологии Рентгеновская кристаллография
Применение рентгеновского излучения в геологии Рентгеновская кристаллография
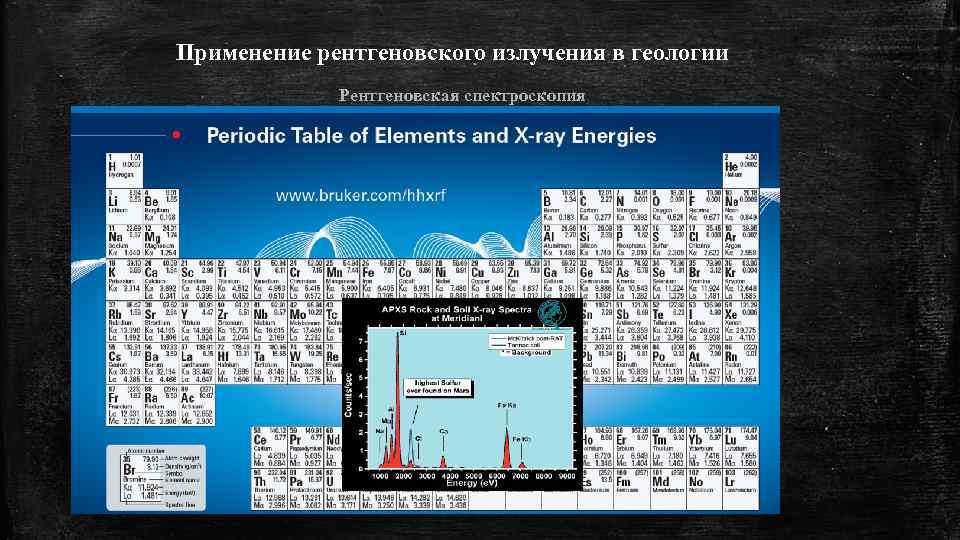 Применение рентгеновского излучения в геологии Рентгеновская спектроскопия
Применение рентгеновского излучения в геологии Рентгеновская спектроскопия
 Рентгеновское излучение было открыто в 1895 году. Автор открытия – Вильгельм Конрад Рентген стал первым физиком, удостоенным Нобелевской премии в 1901 году. На первом рентгеновском снимке было теневое изображение руки с кольцом на безымянном пальце супруги Вильгельма Рентгена
Рентгеновское излучение было открыто в 1895 году. Автор открытия – Вильгельм Конрад Рентген стал первым физиком, удостоенным Нобелевской премии в 1901 году. На первом рентгеновском снимке было теневое изображение руки с кольцом на безымянном пальце супруги Вильгельма Рентгена
 Нобелевские премии, тем или иным образом связанные с рентгеновским излучением. From: http: //www. nobelprize. org/educational/physics/x-rays/
Нобелевские премии, тем или иным образом связанные с рентгеновским излучением. From: http: //www. nobelprize. org/educational/physics/x-rays/
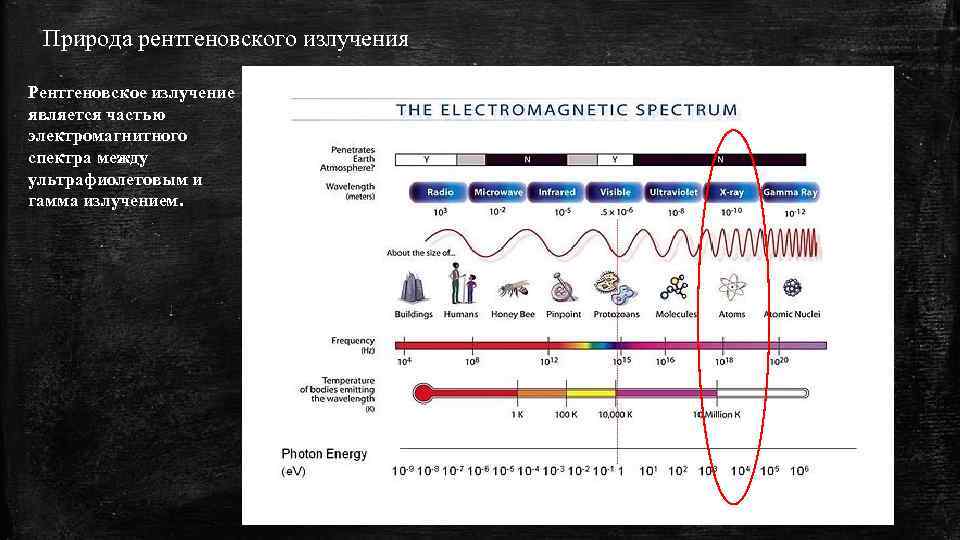 Природа рентгеновского излучения Рентгеновское излучение является частью электромагнитного спектра между ультрафиолетовым и гамма излучением.
Природа рентгеновского излучения Рентгеновское излучение является частью электромагнитного спектра между ультрафиолетовым и гамма излучением.
 Природа рентгеновского излучения Волны = ФОТОНЫ Частицы • В явлениях дифракции рентгеновское излучение рассматривается как электромагнитная волна с длиной волны λ • В явлениях поглощения и рассеяния рентгеновское излучение рассматривается, удобнее всего, как фотоны с определенной энергией E.
Природа рентгеновского излучения Волны = ФОТОНЫ Частицы • В явлениях дифракции рентгеновское излучение рассматривается как электромагнитная волна с длиной волны λ • В явлениях поглощения и рассеяния рентгеновское излучение рассматривается, удобнее всего, как фотоны с определенной энергией E.
 Природа рентгеновского излучения Соотношение, связывающее энергетические и волновые свойства электромагнитного излучения : где: h – постоянная Планка (6. 6254 x 10 -34 Дж с) c - скорость света (3. 00 x 108 м/с) λ - длина волны в метрах E - энергия в Джоулях. Если длина волны измеряется в ангстремах (1Å = 0, 1 нм = 10 -10 м), а энергия измеряется в кило-электронвольтах (1 кэ. В = 1. 6 x 10 -16 джоуля), тогда соотношение между энергией и длиной волны примет вид:
Природа рентгеновского излучения Соотношение, связывающее энергетические и волновые свойства электромагнитного излучения : где: h – постоянная Планка (6. 6254 x 10 -34 Дж с) c - скорость света (3. 00 x 108 м/с) λ - длина волны в метрах E - энергия в Джоулях. Если длина волны измеряется в ангстремах (1Å = 0, 1 нм = 10 -10 м), а энергия измеряется в кило-электронвольтах (1 кэ. В = 1. 6 x 10 -16 джоуля), тогда соотношение между энергией и длиной волны примет вид:
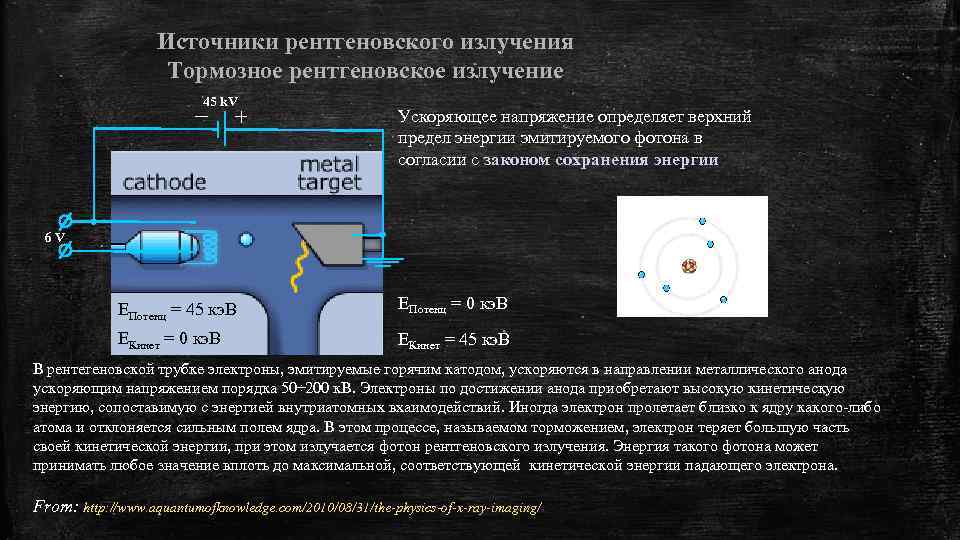 Источники рентгеновского излучения Тормозное рентгеновское излучение 45 k. V Ускоряющее напряжение определяет верхний предел энергии эмитируемого фотона в согласии с законом сохранения энергии 6 V EПотенц = 45 кэ. В EПотенц = 0 кэ. В EКинет = 45 кэ. В В рентегеновской трубке электроны, эмитируемые горячим катодом, ускоряются в направлении металлического анода ускоряющим напряжением порядка 50÷ 200 к. В. Электроны по достижении анода приобретают высокую кинетическую энергию, сопоставимую с энергией внутриатомных вхаимодействий. Иногда электрон пролетает близко к ядру какого-либо атома и отклоняется сильным полем ядра. В этом процессе, называемом торможением, электрон теряет большую часть своей кинетической энергии, при этом излучается фотон рентгеновского излучения. Энергия такого фотона может принимать любое значение вплоть до максимальной, соответствующей кинетической энергии падающего электрона. From: http: //www. aquantumofknowledge. com/2010/08/31/the-physics-of-x-ray-imaging/
Источники рентгеновского излучения Тормозное рентгеновское излучение 45 k. V Ускоряющее напряжение определяет верхний предел энергии эмитируемого фотона в согласии с законом сохранения энергии 6 V EПотенц = 45 кэ. В EПотенц = 0 кэ. В EКинет = 45 кэ. В В рентегеновской трубке электроны, эмитируемые горячим катодом, ускоряются в направлении металлического анода ускоряющим напряжением порядка 50÷ 200 к. В. Электроны по достижении анода приобретают высокую кинетическую энергию, сопоставимую с энергией внутриатомных вхаимодействий. Иногда электрон пролетает близко к ядру какого-либо атома и отклоняется сильным полем ядра. В этом процессе, называемом торможением, электрон теряет большую часть своей кинетической энергии, при этом излучается фотон рентгеновского излучения. Энергия такого фотона может принимать любое значение вплоть до максимальной, соответствующей кинетической энергии падающего электрона. From: http: //www. aquantumofknowledge. com/2010/08/31/the-physics-of-x-ray-imaging/
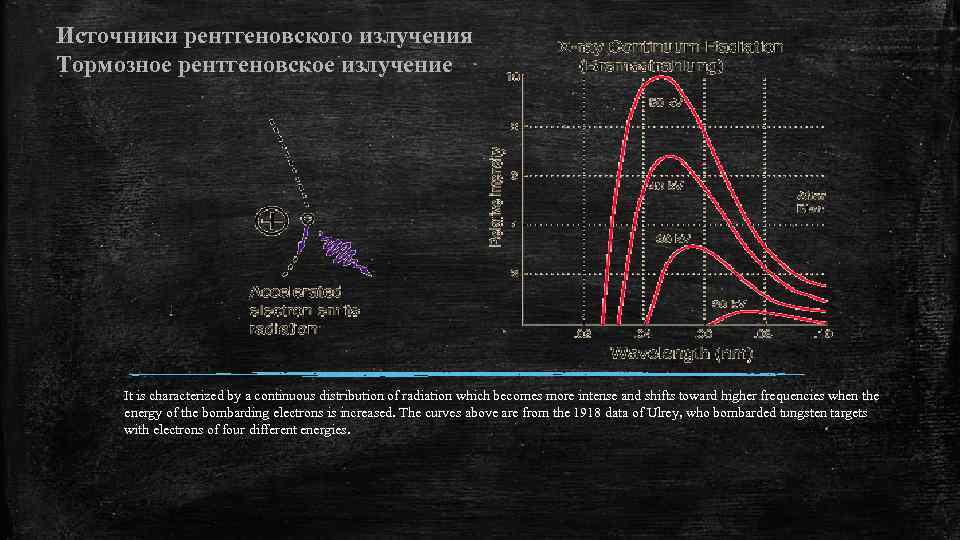 Источники рентгеновского излучения Тормозное рентгеновское излучение It is characterized by a continuous distribution of radiation which becomes more intense and shifts toward higher frequencies when the energy of the bombarding electrons is increased. The curves above are from the 1918 data of Ulrey, who bombarded tungsten targets with electrons of four different energies.
Источники рентгеновского излучения Тормозное рентгеновское излучение It is characterized by a continuous distribution of radiation which becomes more intense and shifts toward higher frequencies when the energy of the bombarding electrons is increased. The curves above are from the 1918 data of Ulrey, who bombarded tungsten targets with electrons of four different energies.
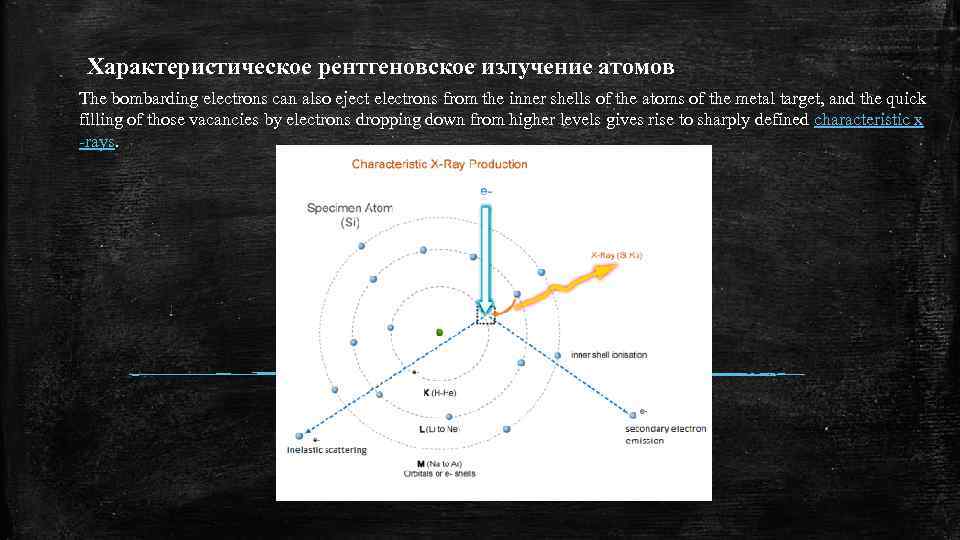 Характеристическое рентгеновское излучение атомов The bombarding electrons can also eject electrons from the inner shells of the atoms of the metal target, and the quick filling of those vacancies by electrons dropping down from higher levels gives rise to sharply defined characteristic x -rays.
Характеристическое рентгеновское излучение атомов The bombarding electrons can also eject electrons from the inner shells of the atoms of the metal target, and the quick filling of those vacancies by electrons dropping down from higher levels gives rise to sharply defined characteristic x -rays.
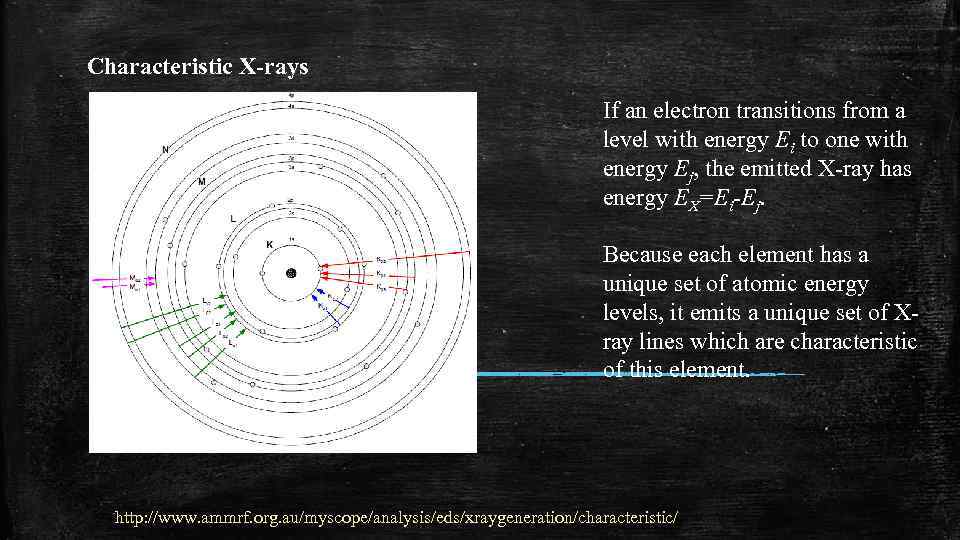 Characteristic X-rays If an electron transitions from a level with energy Ei to one with energy Ej, the emitted X-ray has energy EX=Ei-Ej. Because each element has a unique set of atomic energy levels, it emits a unique set of Xray lines which are characteristic of this element. http: //www. ammrf. org. au/myscope/analysis/eds/xraygeneration/characteristic/
Characteristic X-rays If an electron transitions from a level with energy Ei to one with energy Ej, the emitted X-ray has energy EX=Ei-Ej. Because each element has a unique set of atomic energy levels, it emits a unique set of Xray lines which are characteristic of this element. http: //www. ammrf. org. au/myscope/analysis/eds/xraygeneration/characteristic/
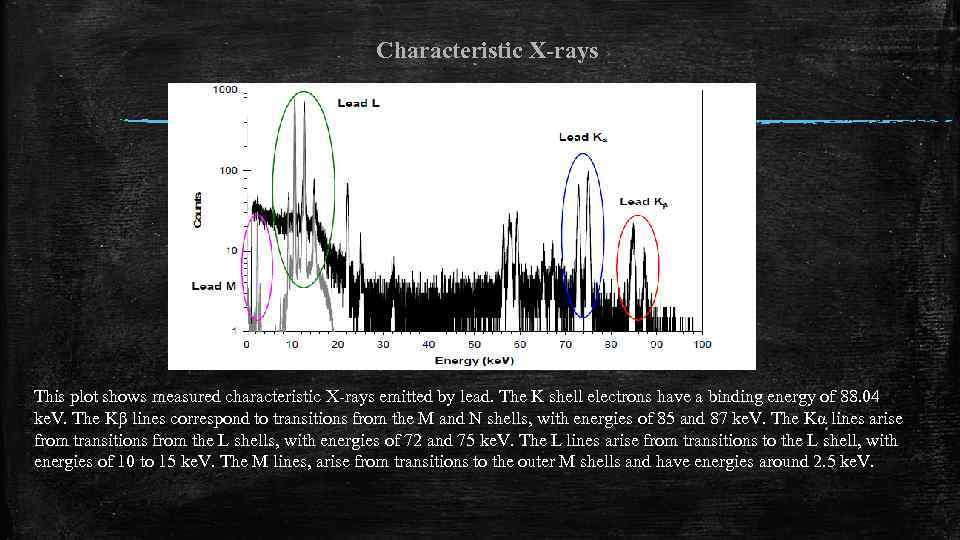 Characteristic X-rays This plot shows measured characteristic X-rays emitted by lead. The K shell electrons have a binding energy of 88. 04 ke. V. The Kβ lines correspond to transitions from the M and N shells, with energies of 85 and 87 ke. V. The Kα lines arise from transitions from the L shells, with energies of 72 and 75 ke. V. The L lines arise from transitions to the L shell, with energies of 10 to 15 ke. V. The M lines, arise from transitions to the outer M shells and have energies around 2. 5 ke. V.
Characteristic X-rays This plot shows measured characteristic X-rays emitted by lead. The K shell electrons have a binding energy of 88. 04 ke. V. The Kβ lines correspond to transitions from the M and N shells, with energies of 85 and 87 ke. V. The Kα lines arise from transitions from the L shells, with energies of 72 and 75 ke. V. The L lines arise from transitions to the L shell, with energies of 10 to 15 ke. V. The M lines, arise from transitions to the outer M shells and have energies around 2. 5 ke. V.
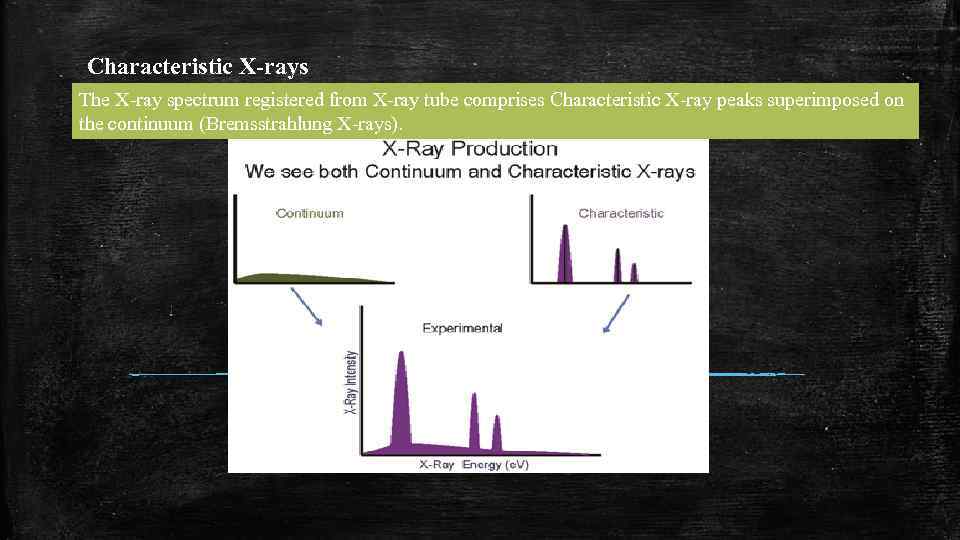 Characteristic X-rays The X-ray spectrum registered from X-ray tube comprises Characteristic X-ray peaks superimposed on the continuum (Bremsstrahlung X-rays).
Characteristic X-rays The X-ray spectrum registered from X-ray tube comprises Characteristic X-ray peaks superimposed on the continuum (Bremsstrahlung X-rays).
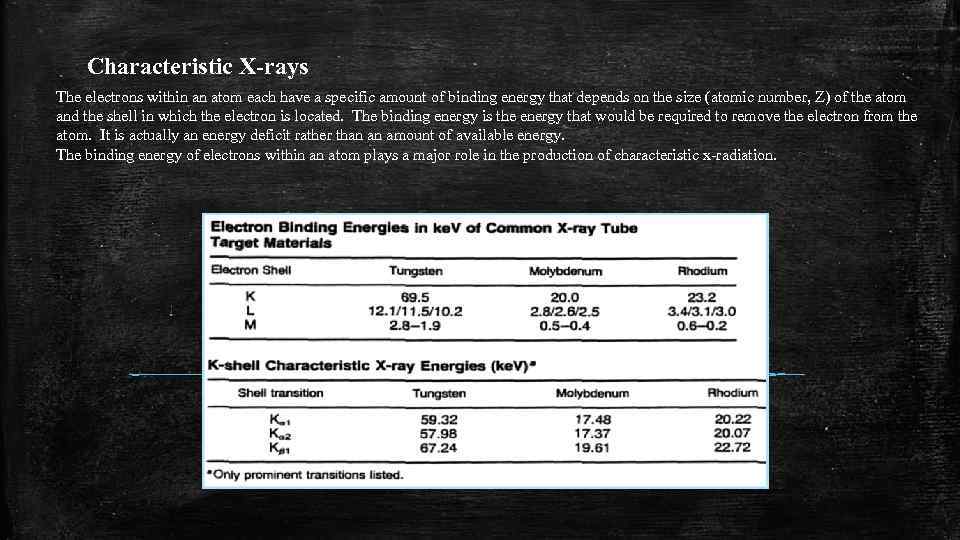 Characteristic X-rays The electrons within an atom each have a specific amount of binding energy that depends on the size (atomic number, Z) of the atom and the shell in which the electron is located. The binding energy is the energy that would be required to remove the electron from the atom. It is actually an energy deficit rather than an amount of available energy. The binding energy of electrons within an atom plays a major role in the production of characteristic x-radiation.
Characteristic X-rays The electrons within an atom each have a specific amount of binding energy that depends on the size (atomic number, Z) of the atom and the shell in which the electron is located. The binding energy is the energy that would be required to remove the electron from the atom. It is actually an energy deficit rather than an amount of available energy. The binding energy of electrons within an atom plays a major role in the production of characteristic x-radiation.
 Short resume The production of Characteristic X-rays is a two-stage process: ionization followed by relaxation. Firstly, an electron is removed from one of the inner shells of the atom by an electron from the primary beam so that the atom is ionized and in an unstable state. Secondly, the atom regains stability when an electron from an outer shell fills the inner shell vacancy and an X-ray photon is emitted. The energy of the emitted X-ray is equal to the difference between the ionization energies of the electrons involved in the transition. Note that inner shell ionization and Characteristic X-ray emission can result from irradiation by a primary beam of protons (PIXE - proton induced x-ray emission) or X-rays (XRF - X-ray fluorescence) as well as electrons (EDS/WDS – these abbreviations are related to additional analytical capability on a scanning electron microscope – energy-dispersive or wavelength-dispersive x-ray spectroscopy).
Short resume The production of Characteristic X-rays is a two-stage process: ionization followed by relaxation. Firstly, an electron is removed from one of the inner shells of the atom by an electron from the primary beam so that the atom is ionized and in an unstable state. Secondly, the atom regains stability when an electron from an outer shell fills the inner shell vacancy and an X-ray photon is emitted. The energy of the emitted X-ray is equal to the difference between the ionization energies of the electrons involved in the transition. Note that inner shell ionization and Characteristic X-ray emission can result from irradiation by a primary beam of protons (PIXE - proton induced x-ray emission) or X-rays (XRF - X-ray fluorescence) as well as electrons (EDS/WDS – these abbreviations are related to additional analytical capability on a scanning electron microscope – energy-dispersive or wavelength-dispersive x-ray spectroscopy).
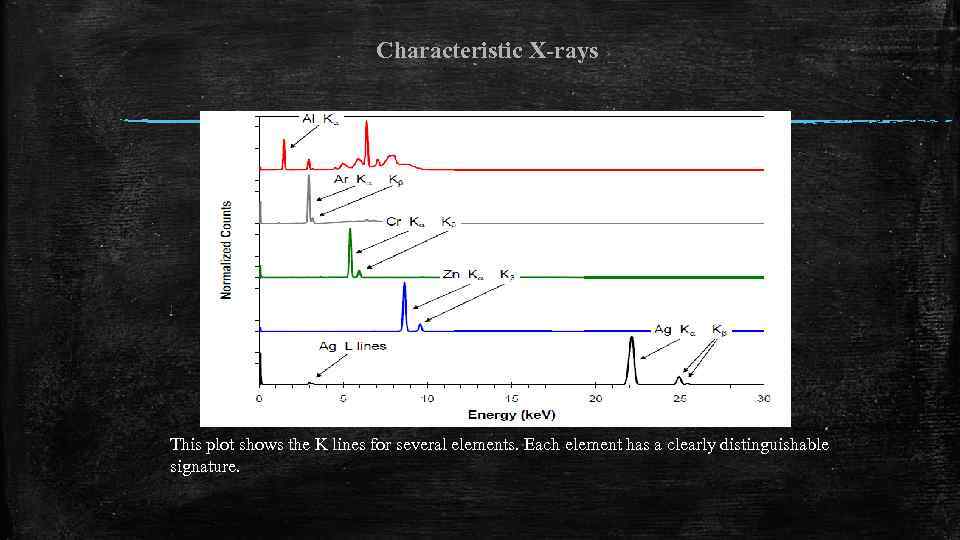 Characteristic X-rays This plot shows the K lines for several elements. Each element has a clearly distinguishable signature.
Characteristic X-rays This plot shows the K lines for several elements. Each element has a clearly distinguishable signature.
 NOMENCLATURE FOR CHARACTERISTIC X-RAYS NOTE. The emission is governed by quantum mechanical selection rules: Δn > 0, Δ l = ± 1, and Δ j = 0 or ± 1. The energies of the characteristic X-rays can be found in many different references. An excellent reference is available online, at http: //xdb. lbl. gov/Section 1/Periodic_Table/X-ray_Elements. html. This is the online version of the X-ray data booklet, from the Lawrence Berkely National Laboratory.
NOMENCLATURE FOR CHARACTERISTIC X-RAYS NOTE. The emission is governed by quantum mechanical selection rules: Δn > 0, Δ l = ± 1, and Δ j = 0 or ± 1. The energies of the characteristic X-rays can be found in many different references. An excellent reference is available online, at http: //xdb. lbl. gov/Section 1/Periodic_Table/X-ray_Elements. html. This is the online version of the X-ray data booklet, from the Lawrence Berkely National Laboratory.
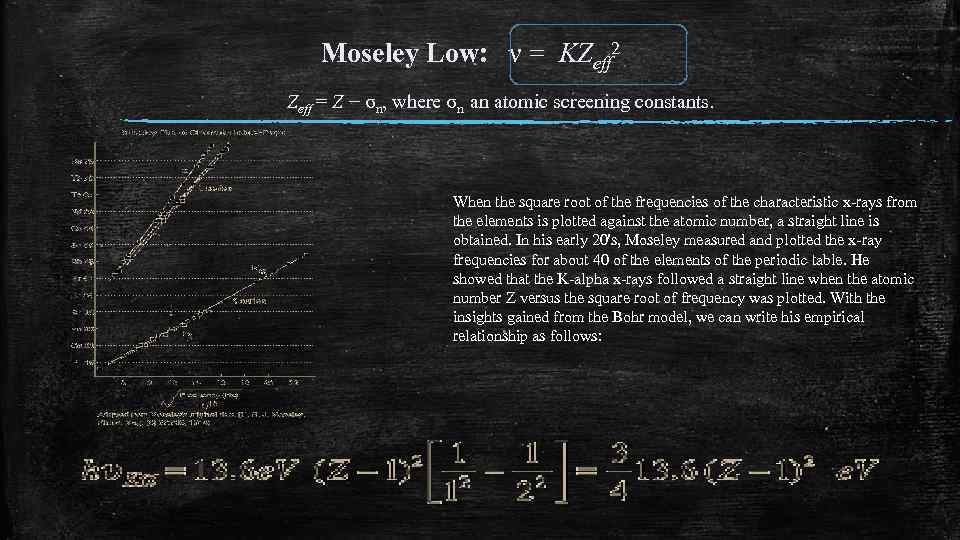 Moseley Low: ν = KZeff 2 Zeff = Z − σn, where σn an atomic screening constants. When the square root of the frequencies of the characteristic x-rays from the elements is plotted against the atomic number, a straight line is obtained. In his early 20's, Moseley measured and plotted the x-ray frequencies for about 40 of the elements of the periodic table. He showed that the K-alpha x-rays followed a straight line when the atomic number Z versus the square root of frequency was plotted. With the insights gained from the Bohr model, we can write his empirical relationship as follows:
Moseley Low: ν = KZeff 2 Zeff = Z − σn, where σn an atomic screening constants. When the square root of the frequencies of the characteristic x-rays from the elements is plotted against the atomic number, a straight line is obtained. In his early 20's, Moseley measured and plotted the x-ray frequencies for about 40 of the elements of the periodic table. He showed that the K-alpha x-rays followed a straight line when the atomic number Z versus the square root of frequency was plotted. With the insights gained from the Bohr model, we can write his empirical relationship as follows:
 Henry Moseley In 1914, Henry Mosely planned to continue his physics reasearch at Oxford so he resigned from his position at Manchester. His plans were never materialised because when the first World War broke out he decided to enlist in the British Army. On August 10, 1915 he was shot dead during the Battle of Gallipoli, in Turkey. This great physicist died very young at the age of twenty-seven but his contribution to the scientific world will never be forgotten.
Henry Moseley In 1914, Henry Mosely planned to continue his physics reasearch at Oxford so he resigned from his position at Manchester. His plans were never materialised because when the first World War broke out he decided to enlist in the British Army. On August 10, 1915 he was shot dead during the Battle of Gallipoli, in Turkey. This great physicist died very young at the age of twenty-seven but his contribution to the scientific world will never be forgotten.
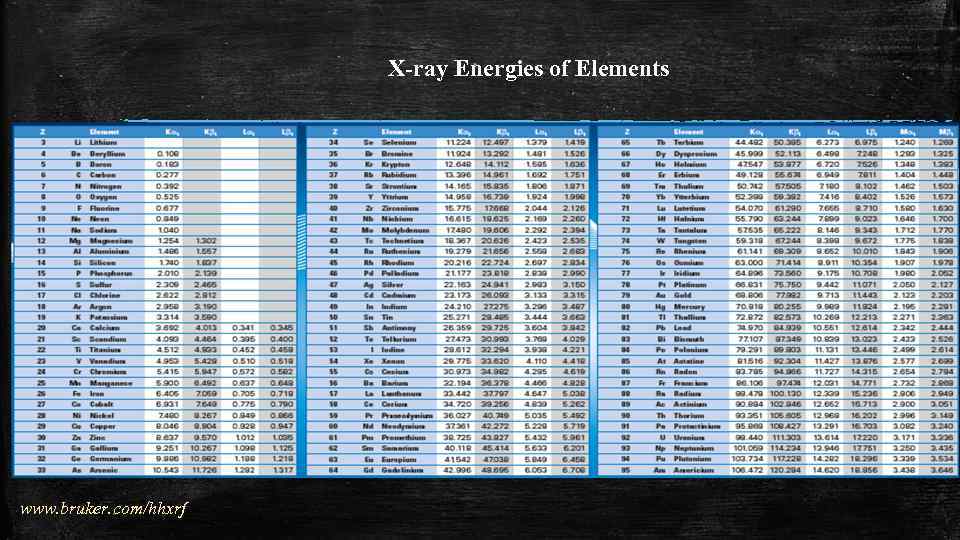 X-ray Energies of Elements www. bruker. com/hhxrf
X-ray Energies of Elements www. bruker. com/hhxrf
 Summary in short 1. x-rays are electromagnetic radiation with an energy in the same range as the binding energy or inner (K, L-shell) electrons 2. the main interaction is the photo-electric effect 3. the decay of the vacancy created the PE-effect causes the emission of characteristic x-rays 4. there are K, L and M x-ray lines
Summary in short 1. x-rays are electromagnetic radiation with an energy in the same range as the binding energy or inner (K, L-shell) electrons 2. the main interaction is the photo-electric effect 3. the decay of the vacancy created the PE-effect causes the emission of characteristic x-rays 4. there are K, L and M x-ray lines
 Chemical analyses by X-ray techniques - Electron probe microanalysis (EPMA) - X-ray fluorescence spectroscopy (XRF)
Chemical analyses by X-ray techniques - Electron probe microanalysis (EPMA) - X-ray fluorescence spectroscopy (XRF)
 Electron probe microanalysis (EPMA) EPMA is one of several particle-beam techniques. A beam of accelerated electrons is focused on the surface of a specimen using a series of electromagnetic lenses, and these energetic electrons produce characteristic X-rays within a small volume (typically 1 to 9 cubic microns) of the specimen. The characteristic X-rays are detected at particular wavelengths, and their intensities are measured to determine concentrations. All elements (except H, He, and Li) can be detected because each element has a specific set of X-rays that it emits. This analytical technique has a high spatial resolution and sensitivity, and individual analyses are reasonably short, requiring only a minute or two in most cases. Additionally, the electron microprobe can function like a scanning electron microscope (SEM) and obtain highly magnified secondary- and backscattered-electron images of a sample.
Electron probe microanalysis (EPMA) EPMA is one of several particle-beam techniques. A beam of accelerated electrons is focused on the surface of a specimen using a series of electromagnetic lenses, and these energetic electrons produce characteristic X-rays within a small volume (typically 1 to 9 cubic microns) of the specimen. The characteristic X-rays are detected at particular wavelengths, and their intensities are measured to determine concentrations. All elements (except H, He, and Li) can be detected because each element has a specific set of X-rays that it emits. This analytical technique has a high spatial resolution and sensitivity, and individual analyses are reasonably short, requiring only a minute or two in most cases. Additionally, the electron microprobe can function like a scanning electron microscope (SEM) and obtain highly magnified secondary- and backscattered-electron images of a sample.
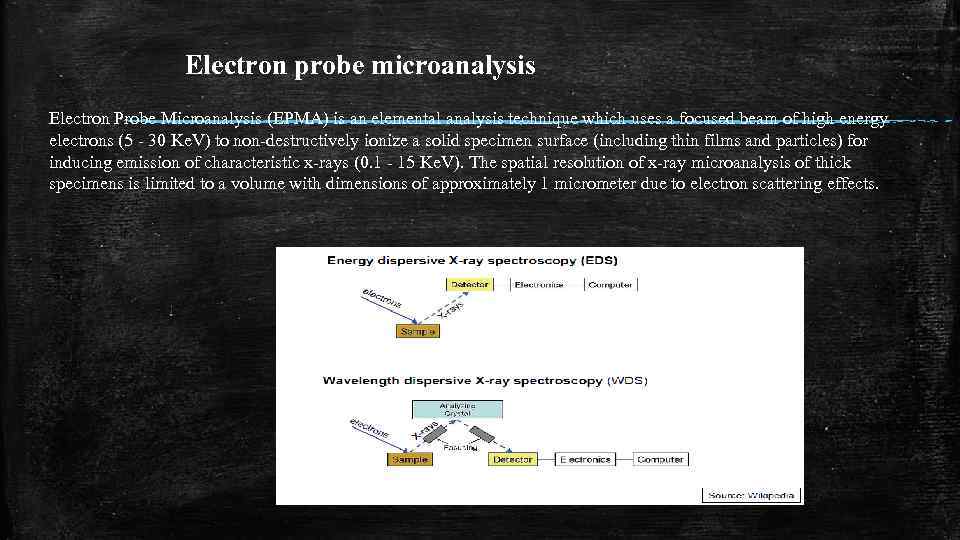 Electron probe microanalysis Electron Probe Microanalysis (EPMA) is an elemental analysis technique which uses a focused beam of high energy electrons (5 - 30 Ke. V) to non-destructively ionize a solid specimen surface (including thin films and particles) for inducing emission of characteristic x-rays (0. 1 - 15 Ke. V). The spatial resolution of x-ray microanalysis of thick specimens is limited to a volume with dimensions of approximately 1 micrometer due to electron scattering effects.
Electron probe microanalysis Electron Probe Microanalysis (EPMA) is an elemental analysis technique which uses a focused beam of high energy electrons (5 - 30 Ke. V) to non-destructively ionize a solid specimen surface (including thin films and particles) for inducing emission of characteristic x-rays (0. 1 - 15 Ke. V). The spatial resolution of x-ray microanalysis of thick specimens is limited to a volume with dimensions of approximately 1 micrometer due to electron scattering effects.
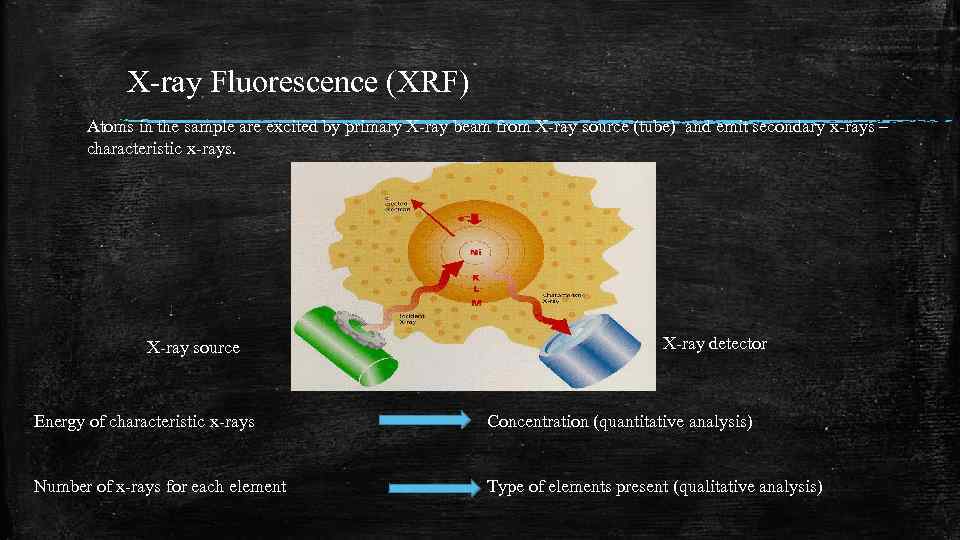 X-ray Fluorescence (XRF) Atoms in the sample are excited by primary X-ray beam from X-ray source (tube) and emit secondary x-rays – characteristic x-rays. X-ray source X-ray detector Energy of characteristic x-rays Concentration (quantitative analysis) Number of x-rays for each element Type of elements present (qualitative analysis)
X-ray Fluorescence (XRF) Atoms in the sample are excited by primary X-ray beam from X-ray source (tube) and emit secondary x-rays – characteristic x-rays. X-ray source X-ray detector Energy of characteristic x-rays Concentration (quantitative analysis) Number of x-rays for each element Type of elements present (qualitative analysis)
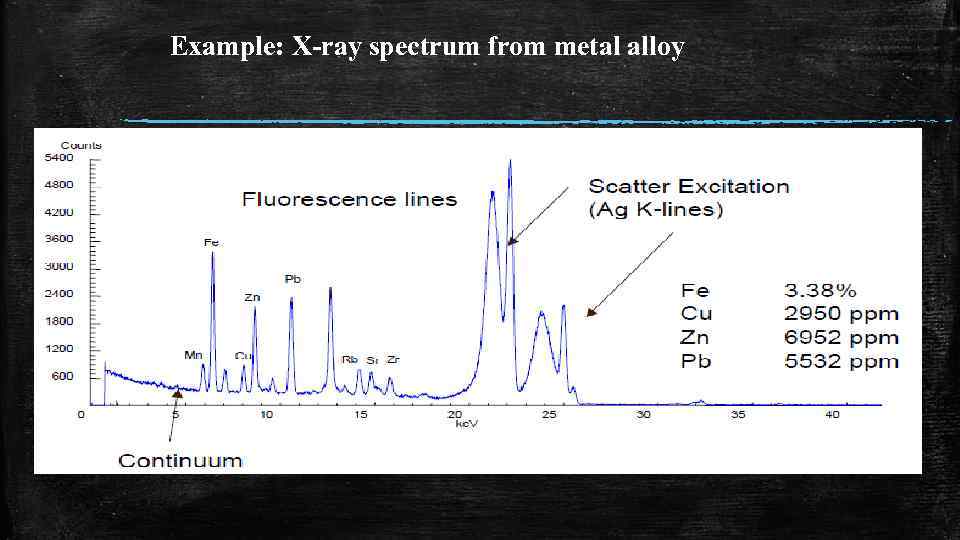 Example: X-ray spectrum from metal alloy
Example: X-ray spectrum from metal alloy
 Analytical characteristics of XRF • • • instrumental analytical technique with multi-element capability elemental analysis of solids and liquids minimal sample treatment can be operated without vacuum concentration range: ppm to % (trace, minor and major elem. ) element range: from boron to uranium (in theory)
Analytical characteristics of XRF • • • instrumental analytical technique with multi-element capability elemental analysis of solids and liquids minimal sample treatment can be operated without vacuum concentration range: ppm to % (trace, minor and major elem. ) element range: from boron to uranium (in theory)
 Types of XRF instruments How can we classify XRF methods? Based on the excitation • Tube excited XRF • Radio-isotope excited XRF • Secondary target, • Synchrotron, • Total reflection. . . Based on the detection • Wavelength dispersive (WD-XRF) • Energy dispersive (ED-XRF) • Filter instruments (proportional counter)
Types of XRF instruments How can we classify XRF methods? Based on the excitation • Tube excited XRF • Radio-isotope excited XRF • Secondary target, • Synchrotron, • Total reflection. . . Based on the detection • Wavelength dispersive (WD-XRF) • Energy dispersive (ED-XRF) • Filter instruments (proportional counter)
 Analytical characteristics of XRF • • • instrumental analytical technique with multi-element capability elemental analysis of solids and liquids minimal sample treatment can be operated without vacuum concentration range: ppm to % (trace, minor and major elem. ) element range: from boron to uranium (in theory)
Analytical characteristics of XRF • • • instrumental analytical technique with multi-element capability elemental analysis of solids and liquids minimal sample treatment can be operated without vacuum concentration range: ppm to % (trace, minor and major elem. ) element range: from boron to uranium (in theory)
 Summary in short: • • • 1. in XRF x-rays are used to excite the sample 2. characteristic x-rays are measured in the form of a spectrum 3. the spectrum tells which elements are present and how much 4. element range: B – U; concentration range ppm - % 5. used in various fields 6. there are wavelength and energy-dispersive instruments
Summary in short: • • • 1. in XRF x-rays are used to excite the sample 2. characteristic x-rays are measured in the form of a spectrum 3. the spectrum tells which elements are present and how much 4. element range: B – U; concentration range ppm - % 5. used in various fields 6. there are wavelength and energy-dispersive instruments
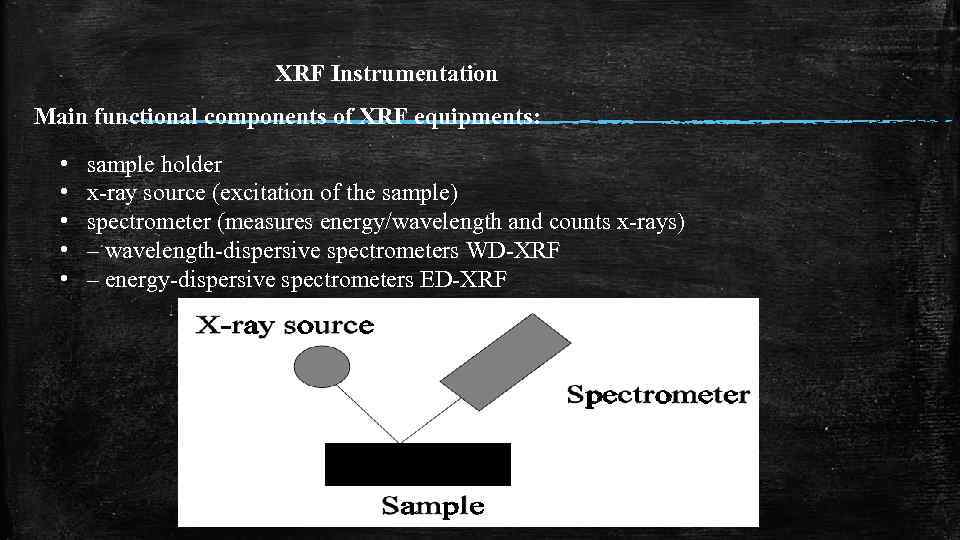 XRF Instrumentation Main functional components of XRF equipments: • • • sample holder x-ray source (excitation of the sample) spectrometer (measures energy/wavelength and counts x-rays) – wavelength-dispersive spectrometers WD-XRF – energy-dispersive spectrometers ED-XRF
XRF Instrumentation Main functional components of XRF equipments: • • • sample holder x-ray source (excitation of the sample) spectrometer (measures energy/wavelength and counts x-rays) – wavelength-dispersive spectrometers WD-XRF – energy-dispersive spectrometers ED-XRF
 XRF Instrumentation Configurations:
XRF Instrumentation Configurations:
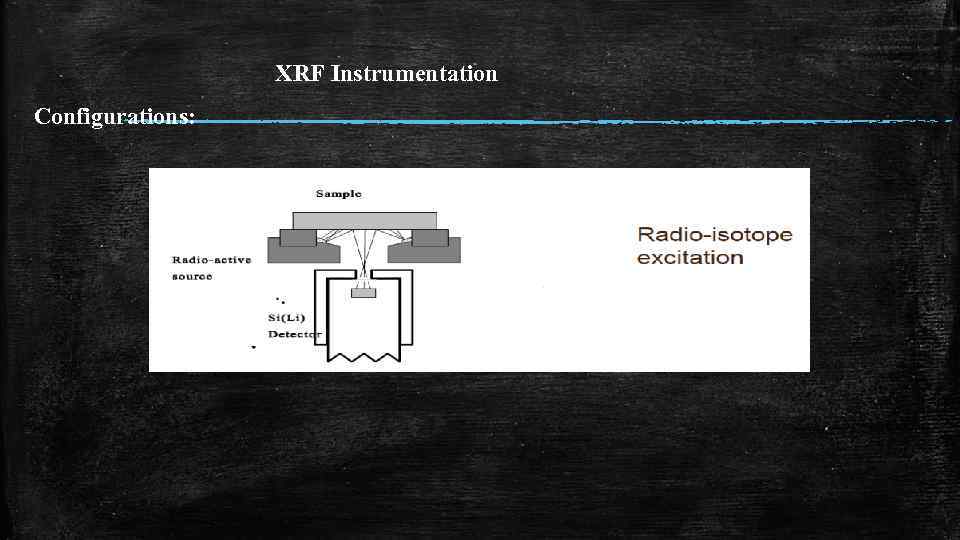 XRF Instrumentation Configurations:
XRF Instrumentation Configurations: