Презентация post lecture4 ats762 nitrogen

























- Размер: 2.9 Mегабайта
- Количество слайдов: 30
Описание презентации Презентация post lecture4 ats762 nitrogen по слайдам
 THENITROGENCYCL
THENITROGENCYCL
 TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed. Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed. Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
 OXIDATIONSTATESOFNITROGEN Nhas 5 electronsinvalenceshell 9 oxidationstatesfrom– 3 to+5 3 0 +1 +2 +3 +4 +5 NH 3 Ammonia NH 4 + Ammonium R 1 N(R 2 )R 3 Organic N N 2 O Nitrous oxide NO Nitric oxide HONO Nitrousacid NO 2 Nitrite NO 2 Nitrogen dioxide HNO 3 Nitricacid NO 3 Nitrate N 2 O 5 Nitrogen pentoxide Decreasingoxidationnumber(reductionreactions) Increasingoxidationnumber(oxidationreactions) Nitrogen: Nitrogenisamajorcomponentoftheatmosphere, butanessentialnutrientinshort supplytolivingorganisms. freeradical
OXIDATIONSTATESOFNITROGEN Nhas 5 electronsinvalenceshell 9 oxidationstatesfrom– 3 to+5 3 0 +1 +2 +3 +4 +5 NH 3 Ammonia NH 4 + Ammonium R 1 N(R 2 )R 3 Organic N N 2 O Nitrous oxide NO Nitric oxide HONO Nitrousacid NO 2 Nitrite NO 2 Nitrogen dioxide HNO 3 Nitricacid NO 3 Nitrate N 2 O 5 Nitrogen pentoxide Decreasingoxidationnumber(reductionreactions) Increasingoxidationnumber(oxidationreactions) Nitrogen: Nitrogenisamajorcomponentoftheatmosphere, butanessentialnutrientinshort supplytolivingorganisms. freeradical
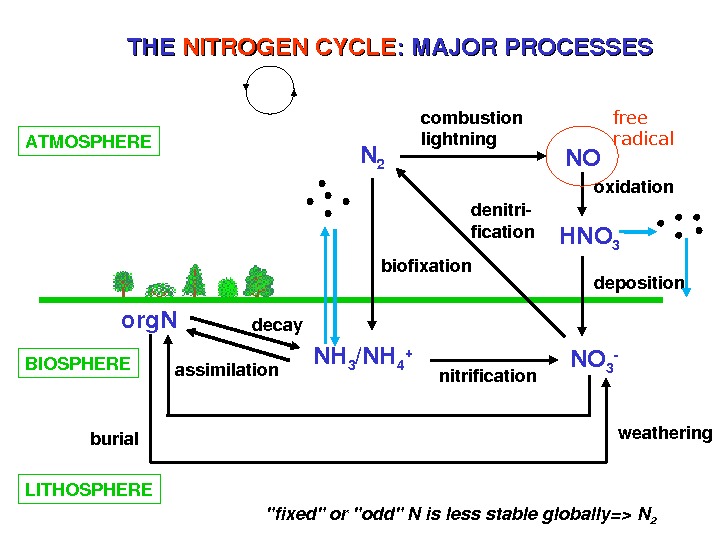
 THETHE NITROGENCYCLE : MAJORPROCESSES ATMOSPHERE N 2 NO HNO 3 NH 3 /NH 4 + NO 3 org. N BIOSPHERE LITHOSPHERE combustion lightning oxidation deposition assimilation decay nitrification denitri fication biofixation burial weatheringfree radical «fixed»or»odd»Nislessstableglobally=>N
THETHE NITROGENCYCLE : MAJORPROCESSES ATMOSPHERE N 2 NO HNO 3 NH 3 /NH 4 + NO 3 org. N BIOSPHERE LITHOSPHERE combustion lightning oxidation deposition assimilation decay nitrification denitri fication biofixation burial weatheringfree radical «fixed»or»odd»Nislessstableglobally=>N
![BOXMODELOFTHENITROGENCYCLE Inventoriesin. Tg. N Flowsin. Tg. Nyr 1 [Jacob, 1999] BOXMODELOFTHENITROGENCYCLE Inventoriesin. Tg. N Flowsin. Tg. Nyr 1 [Jacob, 1999]](/docs//post_lecture4_ats762_nitrogen_images/post_lecture4_ats762_nitrogen_4.jpg) BOXMODELOFTHENITROGENCYCLE Inventoriesin. Tg. N Flowsin. Tg. Nyr 1 [Jacob, 1999]
BOXMODELOFTHENITROGENCYCLE Inventoriesin. Tg. N Flowsin. Tg. Nyr 1 [Jacob, 1999]
 TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
 NOx: KEYTOMAINTAININGTHEOXIDIZINGPOWEROFTHE TROPOSPHERE O 3 O 2 h O 3 OH HO 2 h , H 2 O Deposition NO H 2 O 2 CO, CH 4 NO 2 h STRATOSPHERE TROPOSPHERE 818 km SURFACE • ALSOkeyplayerinstratospheric. O 3 loss
NOx: KEYTOMAINTAININGTHEOXIDIZINGPOWEROFTHE TROPOSPHERE O 3 O 2 h O 3 OH HO 2 h , H 2 O Deposition NO H 2 O 2 CO, CH 4 NO 2 h STRATOSPHERE TROPOSPHERE 818 km SURFACE • ALSOkeyplayerinstratospheric. O 3 loss
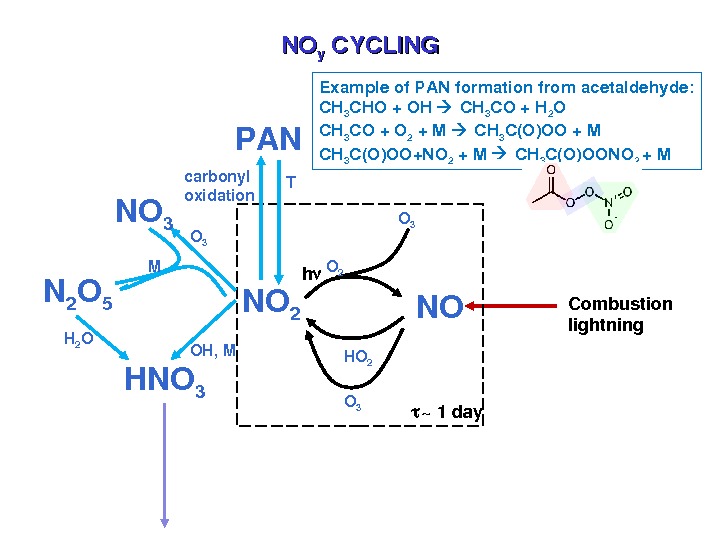
 NONO yy CYCLING HO 2 NONO 2 h O 3 O 2 Combustion lightning HNO 3 OH, MO 3 NO 3 N 2 O 5 M H 2 O PAN carbonyl oxidation T ~1 day. Exampleof. PANformationfromacetaldehyde: CH 3 CHO+OH CH 3 CO+H 2 O CH 3 CO+O 2 +M CH 3 C(O)OO+NO 2 +M CH 3 C(O)OONO 2 +M
NONO yy CYCLING HO 2 NONO 2 h O 3 O 2 Combustion lightning HNO 3 OH, MO 3 NO 3 N 2 O 5 M H 2 O PAN carbonyl oxidation T ~1 day. Exampleof. PANformationfromacetaldehyde: CH 3 CHO+OH CH 3 CO+H 2 O CH 3 CO+O 2 +M CH 3 C(O)OO+NO 2 +M CH 3 C(O)OONO 2 +M
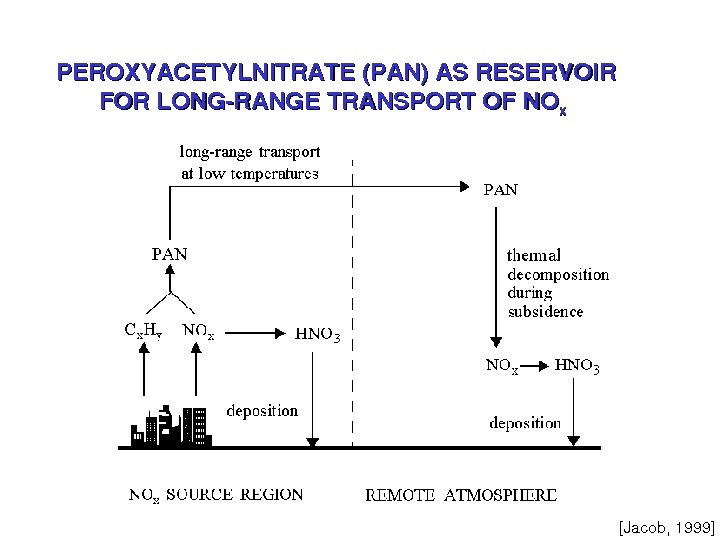
![PEROXYACETYLNITRATE(PAN)ASRESERVOIR FORLONGRANGETRANSPORTOFNO xx [Jacob, 1999] PEROXYACETYLNITRATE(PAN)ASRESERVOIR FORLONGRANGETRANSPORTOFNO xx [Jacob, 1999]](/docs//post_lecture4_ats762_nitrogen_images/post_lecture4_ats762_nitrogen_8.jpg) PEROXYACETYLNITRATE(PAN)ASRESERVOIR FORLONGRANGETRANSPORTOFNO xx [Jacob, 1999]
PEROXYACETYLNITRATE(PAN)ASRESERVOIR FORLONGRANGETRANSPORTOFNO xx [Jacob, 1999]
 TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed. Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed. Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
 NONO xx EMISSIONS(Tg. Nyr 11 )TOTROPOSPHERE Zeldovich. Mechanism : combustionandlightning Athigh. T(~2000 K)oxygenthermolyzes: O 2 O+O O+N 2 NO+N N+O 2 NO+O [IPCC, 2007]
NONO xx EMISSIONS(Tg. Nyr 11 )TOTROPOSPHERE Zeldovich. Mechanism : combustionandlightning Athigh. T(~2000 K)oxygenthermolyzes: O 2 O+O O+N 2 NO+N N+O 2 NO+O [IPCC, 2007]
 USINGSATELLITEOBSERVATIONSOFNO 22 TOMONITORNO xx EMISSIONS SCIAMACHYdata. May. Oct 2004 (R. V. Martin, Dalhousie. U. ) detection limit
USINGSATELLITEOBSERVATIONSOFNO 22 TOMONITORNO xx EMISSIONS SCIAMACHYdata. May. Oct 2004 (R. V. Martin, Dalhousie. U. ) detection limit
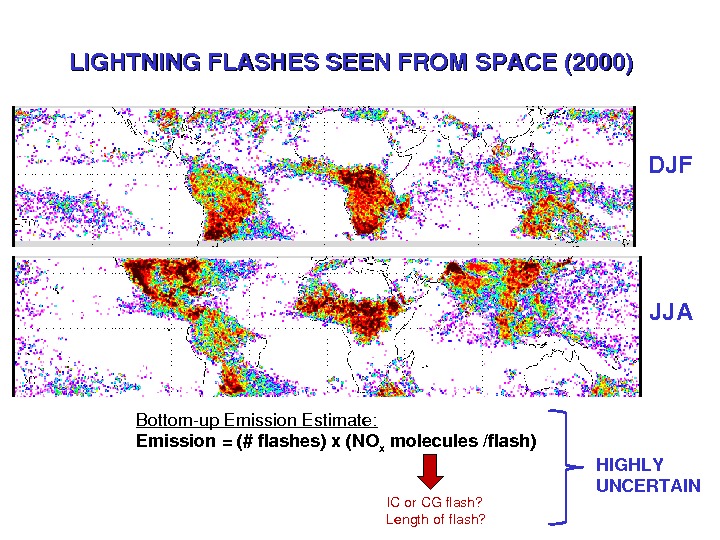
 LIGHTNINGFLASHESSEENFROMSPACE(2000) DJF JJA Bottomup. Emission. Estimate: Emission=(#flashes)x(NO x molecules/flash) ICor. CGflash? Lengthofflash? HIGHLY UNCERTAIN
LIGHTNINGFLASHESSEENFROMSPACE(2000) DJF JJA Bottomup. Emission. Estimate: Emission=(#flashes)x(NO x molecules/flash) ICor. CGflash? Lengthofflash? HIGHLY UNCERTAIN
![TOPDOWNESTIMATESOFGLOBALLIGHTNING NOx. EMISSIONS [Martinetal. , 2007]Using. SCIAMACHY(NO 2 ), OMI(O 3 ), ACEFTS(HNO 3 ): Targetlocations/timeswhere. TOPDOWNESTIMATESOFGLOBALLIGHTNING NOx. EMISSIONS [Martinetal. , 2007]Using. SCIAMACHY(NO 2 ), OMI(O 3 ), ACEFTS(HNO 3 ): Targetlocations/timeswhere.](/docs//post_lecture4_ats762_nitrogen_images/post_lecture4_ats762_nitrogen_13.jpg) TOPDOWNESTIMATESOFGLOBALLIGHTNING NOx. EMISSIONS [Martinetal. , 2007]Using. SCIAMACHY(NO 2 ), OMI(O 3 ), ACEFTS(HNO 3 ): Targetlocations/timeswhere. NO 2 columnisdominatedby lightningsource Globalsourceof 6± 2 Tg. N/yrfromlightning. Obs(satellite) Model(6 Tg. N/yr) Model(48 Tg. N/yr) Model(nolightning)
TOPDOWNESTIMATESOFGLOBALLIGHTNING NOx. EMISSIONS [Martinetal. , 2007]Using. SCIAMACHY(NO 2 ), OMI(O 3 ), ACEFTS(HNO 3 ): Targetlocations/timeswhere. NO 2 columnisdominatedby lightningsource Globalsourceof 6± 2 Tg. N/yrfromlightning. Obs(satellite) Model(6 Tg. N/yr) Model(48 Tg. N/yr) Model(nolightning)
 USINGSATELLITEOBSERVATIONSTOESTIMATE SOILNOx. EMISSIONS Use. GOMEobservationsover. Africa: Soils: 3. 3 Tg. N/year Biomass. Burning: 3. 8 Tg. N/year 40%ofsurface. NOxemissions! Extrapolatingtoallthetropics: 7. 3 Tg. N/yearbiogenicsoil (twicethe. IPCCvalue) Biomass. Burning Soils Fossil+biofuels [Jaegl é etal. , 2004]
USINGSATELLITEOBSERVATIONSTOESTIMATE SOILNOx. EMISSIONS Use. GOMEobservationsover. Africa: Soils: 3. 3 Tg. N/year Biomass. Burning: 3. 8 Tg. N/year 40%ofsurface. NOxemissions! Extrapolatingtoallthetropics: 7. 3 Tg. N/yearbiogenicsoil (twicethe. IPCCvalue) Biomass. Burning Soils Fossil+biofuels [Jaegl é etal. , 2004]
![GROWINGCONTRIBUTIONOFAGRICULTURETO NCYCLE [IPCC, 2007] GROWINGCONTRIBUTIONOFAGRICULTURETO NCYCLE [IPCC, 2007]](/docs//post_lecture4_ats762_nitrogen_images/post_lecture4_ats762_nitrogen_15.jpg) GROWINGCONTRIBUTIONOFAGRICULTURETO NCYCLE [IPCC, 2007]
GROWINGCONTRIBUTIONOFAGRICULTURETO NCYCLE [IPCC, 2007]
 TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed. Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed. Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
 NN 22 O: LOWYIELDPRODUCTOFBACTERIAL NITRIFICATIONANDDENITRIFICATION Importantas • sourceof. NO x radicalsinstratosphere • greenhousegas [IPCC, 2007]
NN 22 O: LOWYIELDPRODUCTOFBACTERIAL NITRIFICATIONANDDENITRIFICATION Importantas • sourceof. NO x radicalsinstratosphere • greenhousegas [IPCC, 2007]
![NN 22 OEMISSIONS(Tg. Nyr 11 )TOTROPOSPHERE [IPCC, 2007]Sourceis. MOSTLY(~75)natural NN 22 OEMISSIONS(Tg. Nyr 11 )TOTROPOSPHERE [IPCC, 2007]Sourceis. MOSTLY(~75)natural](/docs//post_lecture4_ats762_nitrogen_images/post_lecture4_ats762_nitrogen_18.jpg) NN 22 OEMISSIONS(Tg. Nyr 11 )TOTROPOSPHERE [IPCC, 2007]Sourceis. MOSTLY(~75%)natural
NN 22 OEMISSIONS(Tg. Nyr 11 )TOTROPOSPHERE [IPCC, 2007]Sourceis. MOSTLY(~75%)natural
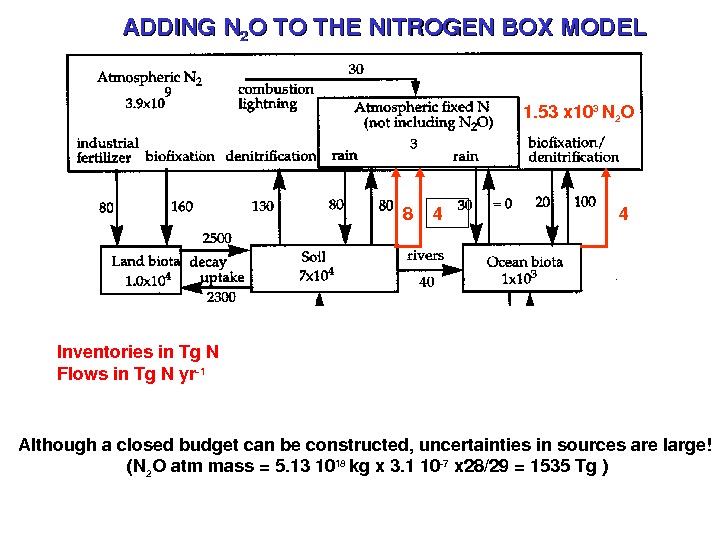
 Inventoriesin. Tg. N Flowsin. Tg. Nyr 1 ADDINGN 22 OTOTHENITROGENBOXMODEL 48 4 1. 53 x 10 3 N 2 O Althoughaclosedbudgetcanbeconstructed, uncertaintiesinsourcesarelarge! (N 2 Oatmmass=5. 1310 18 kgx 3. 110 7 x 28/29=1535 Tg)
Inventoriesin. Tg. N Flowsin. Tg. Nyr 1 ADDINGN 22 OTOTHENITROGENBOXMODEL 48 4 1. 53 x 10 3 N 2 O Althoughaclosedbudgetcanbeconstructed, uncertaintiesinsourcesarelarge! (N 2 Oatmmass=5. 1310 18 kgx 3. 110 7 x 28/29=1535 Tg)
 TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed. Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed. Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
 ANNUALMEANPM 2. 5 CONCENTRATIONSATU. S. SITES Airquality standard Drymassconcentrations
ANNUALMEANPM 2. 5 CONCENTRATIONSATU. S. SITES Airquality standard Drymassconcentrations
 FORMATIONOFSULFATENITRATEAMMONIUMAEROSOLS 2 2 2 4 4 3 3 3 3 4 3( ) 2 ( ) ( ) ( ) €H O H O H SO g SO H NH g NH OH HNO g NO H NH g HNO g NH NO aerosol Sulfatealwaysformsanaqueousaerosol Ammoniadissolvesinthesulfateaerosoltotally oruntiltitrationofacidity, whicheverhappens first Nitrateistakenupbyaerosolif(andonlyif) excess. NH 3 isavailableaftersulfatetitration HNO 3 andexcess. NH 3 canalso formasolidaerosolif. RHislow. Thermodynamicrules: [Parketal. , 2006]HNO 3 NH 4 + H + OH SO
FORMATIONOFSULFATENITRATEAMMONIUMAEROSOLS 2 2 2 4 4 3 3 3 3 4 3( ) 2 ( ) ( ) ( ) €H O H O H SO g SO H NH g NH OH HNO g NO H NH g HNO g NH NO aerosol Sulfatealwaysformsanaqueousaerosol Ammoniadissolvesinthesulfateaerosoltotally oruntiltitrationofacidity, whicheverhappens first Nitrateistakenupbyaerosolif(andonlyif) excess. NH 3 isavailableaftersulfatetitration HNO 3 andexcess. NH 3 canalso formasolidaerosolif. RHislow. Thermodynamicrules: [Parketal. , 2006]HNO 3 NH 4 + H + OH SO
![GLOBALSOURCESOFAMMONIA [Parketal. , 2004]VERYUNCERTAIN! Measurementsaretough, sohardtoverifyregionalestimates. GLOBALSOURCESOFAMMONIA [Parketal. , 2004]VERYUNCERTAIN! Measurementsaretough, sohardtoverifyregionalestimates.](/docs//post_lecture4_ats762_nitrogen_images/post_lecture4_ats762_nitrogen_23.jpg) GLOBALSOURCESOFAMMONIA [Parketal. , 2004]VERYUNCERTAIN! Measurementsaretough, sohardtoverifyregionalestimates.
GLOBALSOURCESOFAMMONIA [Parketal. , 2004]VERYUNCERTAIN! Measurementsaretough, sohardtoverifyregionalestimates.
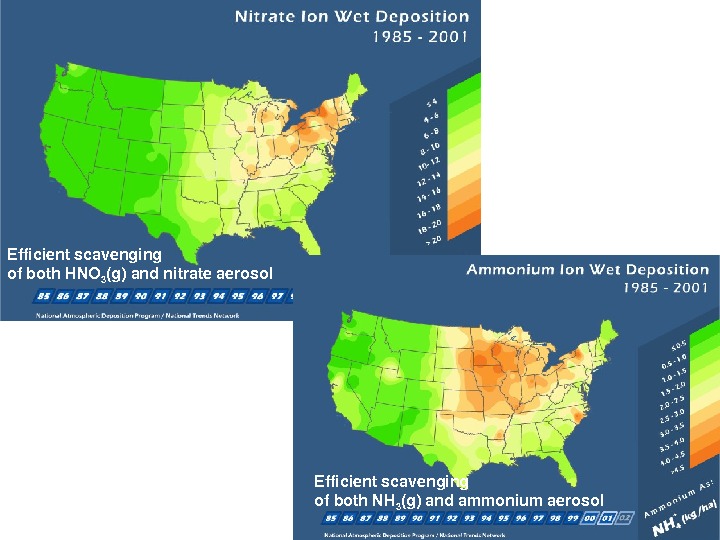
 Efficientscavenging ofboth. HNO 3 (g)andnitrateaerosol Efficientscavenging ofboth. NH 3 (g)andammoniumaerosol
Efficientscavenging ofboth. HNO 3 (g)andnitrateaerosol Efficientscavenging ofboth. NH 3 (g)andammoniumaerosol
 TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed. Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
TOPICSFORTODAY 1. The. Nitrogen. Cycle 2. Fixed. Nitrogeninthe. Atmosphere 3. Sourcesof. NOx 4. Whatabout. N 2 O? 5. Nitrogen. Cycle: ontheparticleside 6. Howmightthenitrogencyclebeaffectedbyclimate change?
![PREDICTEDCHANGESINANTHROPOGENICNO XX EMISSIONS [IPCC 2007(WG 3)]Emissionsdecliningin. NA, EU, growingin. AS(transportation), butpredictedto leveloff(maypeakasearlyas 2015). Whataboutnaturalsources? Note: thisincludeaviation. PREDICTEDCHANGESINANTHROPOGENICNO XX EMISSIONS [IPCC 2007(WG 3)]Emissionsdecliningin. NA, EU, growingin. AS(transportation), butpredictedto leveloff(maypeakasearlyas 2015). Whataboutnaturalsources? Note: thisincludeaviation.](/docs//post_lecture4_ats762_nitrogen_images/post_lecture4_ats762_nitrogen_26.jpg) PREDICTEDCHANGESINANTHROPOGENICNO XX EMISSIONS [IPCC 2007(WG 3)]Emissionsdecliningin. NA, EU, growingin. AS(transportation), butpredictedto leveloff(maypeakasearlyas 2015). Whataboutnaturalsources? Note: thisincludeaviation. NOxsourceswhicharesmallbutin. UTandhavegrown from 0. 55 to 0. 7 Tg/yrfrom 19922002(maydoubleinnext 20 years)
PREDICTEDCHANGESINANTHROPOGENICNO XX EMISSIONS [IPCC 2007(WG 3)]Emissionsdecliningin. NA, EU, growingin. AS(transportation), butpredictedto leveloff(maypeakasearlyas 2015). Whataboutnaturalsources? Note: thisincludeaviation. NOxsourceswhicharesmallbutin. UTandhavegrown from 0. 55 to 0. 7 Tg/yrfrom 19922002(maydoubleinnext 20 years)
 CHANGINGLNO XX ? ? Warmerclimate=morethunderclouds=morelightning Impact: (1) increasing. UTozoneformation(positiveforcing) (2) Increasing. OHleadstosmallreductionsin. CH 4 (negative forcing) Modelspredict +460%LNO x per °K
CHANGINGLNO XX ? ? Warmerclimate=morethunderclouds=morelightning Impact: (1) increasing. UTozoneformation(positiveforcing) (2) Increasing. OHleadstosmallreductionsin. CH 4 (negative forcing) Modelspredict +460%LNO x per °K
 THETHE NITROGENCYCLE : MAJORPROCESSES ATMOSPHERE N 2 NO HNO 3 NH 3 /NH 4 + NO 3 org. N BIOSPHERE LITHOSPHERE combustion lightning oxidation deposition assimilation decay nitrification denitri fication biofixation burial weathering «fixed»or»odd»Nislessstableglobally=>N
THETHE NITROGENCYCLE : MAJORPROCESSES ATMOSPHERE N 2 NO HNO 3 NH 3 /NH 4 + NO 3 org. N BIOSPHERE LITHOSPHERE combustion lightning oxidation deposition assimilation decay nitrification denitri fication biofixation burial weathering «fixed»or»odd»Nislessstableglobally=>N
 NONO yy CYCLING HO 2 NONO 2 h O 3 O 2 Combustion lightning HNO 3 OH, MO 3 NO 3 N 2 O 5 M H 2 O PAN carbonyl oxidation T ~1 day. Exampleof. PANformationfromacetaldehyde: CH 3 CHO+OH CH 3 CO+H 2 O CH 3 CO+O 2 +M CH 3 C(O)OO+NO 2 +M CH 3 C(O)OONO 2 +M
NONO yy CYCLING HO 2 NONO 2 h O 3 O 2 Combustion lightning HNO 3 OH, MO 3 NO 3 N 2 O 5 M H 2 O PAN carbonyl oxidation T ~1 day. Exampleof. PANformationfromacetaldehyde: CH 3 CHO+OH CH 3 CO+H 2 O CH 3 CO+O 2 +M CH 3 C(O)OO+NO 2 +M CH 3 C(O)OONO 2 +M

