e35408218d6b2d69cfb33a79c41ad4c3.ppt
- Количество слайдов: 63
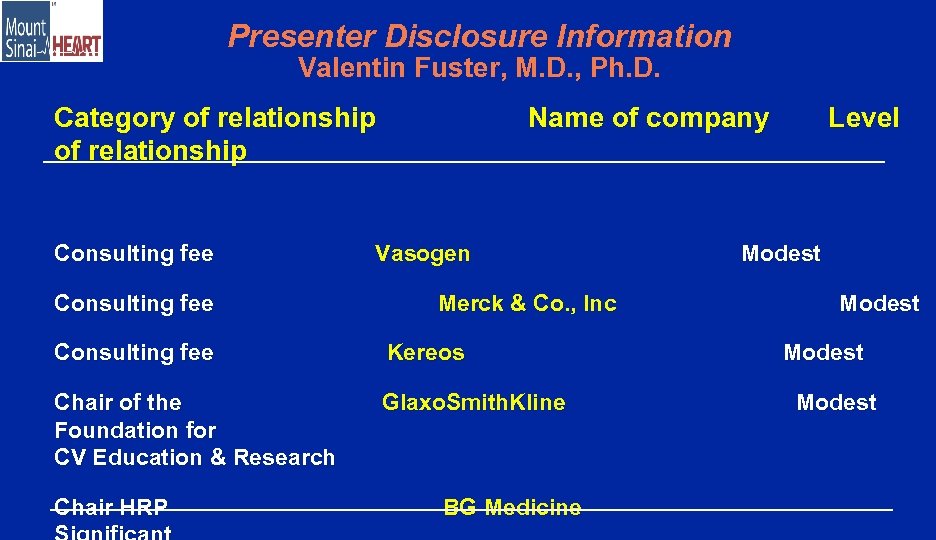 Presenter Disclosure Information Valentin Fuster, M. D. , Ph. D. Category of relationship Consulting fee Name of company Vasogen Merck & Co. , Inc Consulting fee Kereos Chair of the Foundation for CV Education & Research Glaxo. Smith. Kline Chair HRP BG Medicine Level Modest
Presenter Disclosure Information Valentin Fuster, M. D. , Ph. D. Category of relationship Consulting fee Name of company Vasogen Merck & Co. , Inc Consulting fee Kereos Chair of the Foundation for CV Education & Research Glaxo. Smith. Kline Chair HRP BG Medicine Level Modest
 CARDIOVASCULAR GENE AND CELL THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
CARDIOVASCULAR GENE AND CELL THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
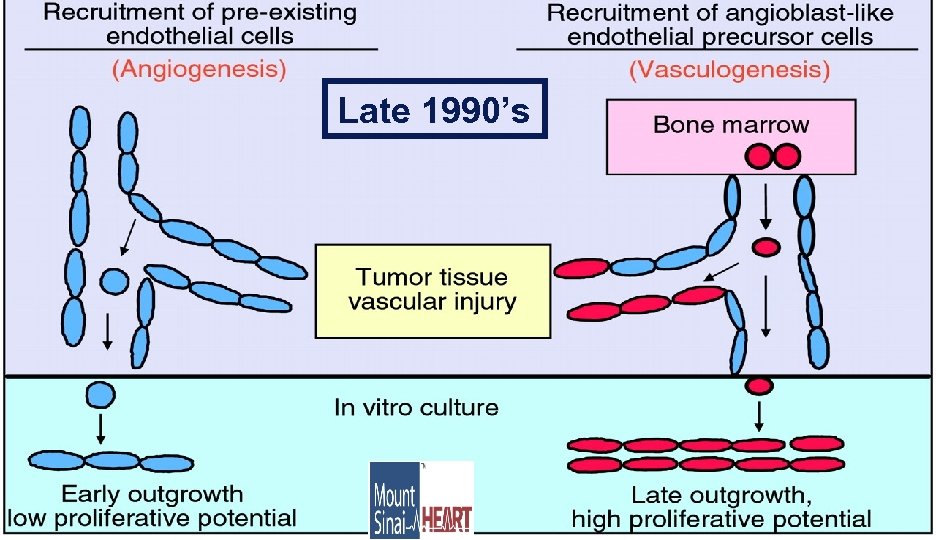 Late 1990’s
Late 1990’s
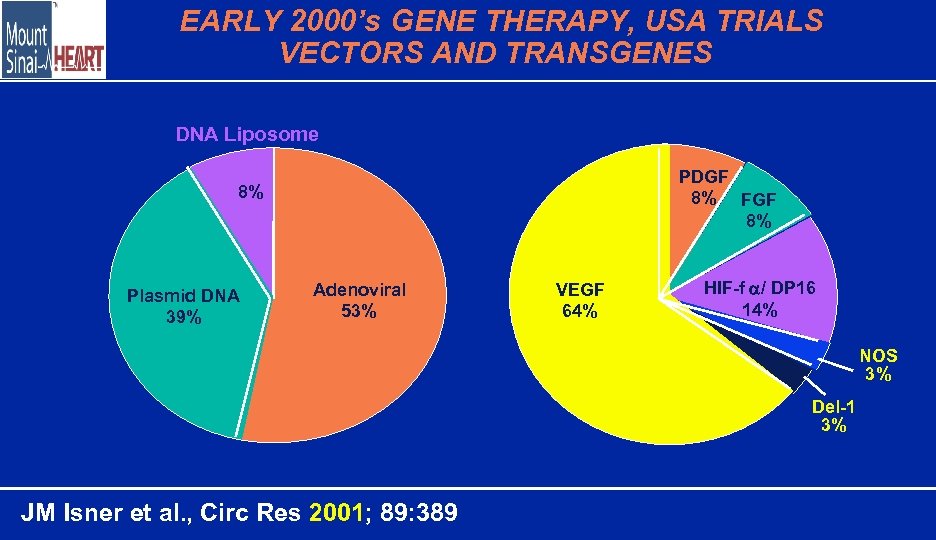 EARLY 2000’s GENE THERAPY, USA TRIALS VECTORS AND TRANSGENES DNA Liposome PDGF 8% FGF 8% 8% Plasmid DNA 39% Adenoviral 53% VEGF 64% HIF-f / DP 16 14% NOS 3% Del-1 3% JM Isner et al. , Circ Res 2001; 89: 389
EARLY 2000’s GENE THERAPY, USA TRIALS VECTORS AND TRANSGENES DNA Liposome PDGF 8% FGF 8% 8% Plasmid DNA 39% Adenoviral 53% VEGF 64% HIF-f / DP 16 14% NOS 3% Del-1 3% JM Isner et al. , Circ Res 2001; 89: 389
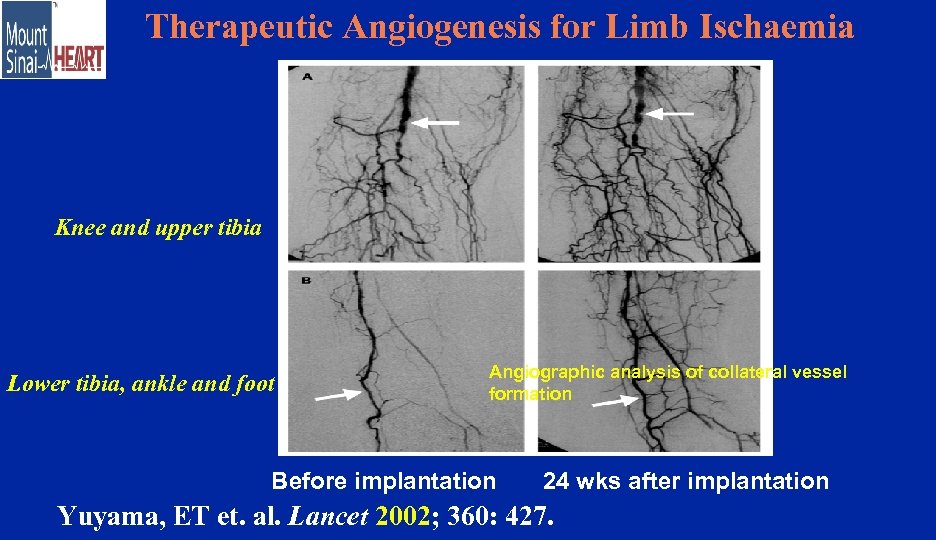 Therapeutic Angiogenesis for Limb Ischaemia Knee and upper tibia Lower tibia, ankle and foot Angiographic analysis of collateral vessel formation Before implantation 24 wks after implantation Yuyama, ET et. al. Lancet 2002; 360: 427.
Therapeutic Angiogenesis for Limb Ischaemia Knee and upper tibia Lower tibia, ankle and foot Angiographic analysis of collateral vessel formation Before implantation 24 wks after implantation Yuyama, ET et. al. Lancet 2002; 360: 427.
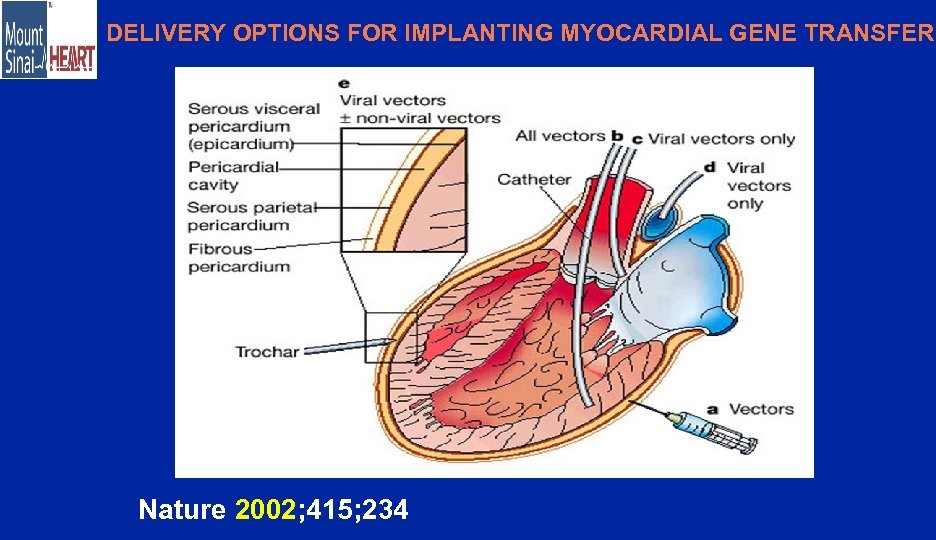 DELIVERY OPTIONS FOR IMPLANTING MYOCARDIAL GENE TRANSFER Nature 2002; 415; 234
DELIVERY OPTIONS FOR IMPLANTING MYOCARDIAL GENE TRANSFER Nature 2002; 415; 234
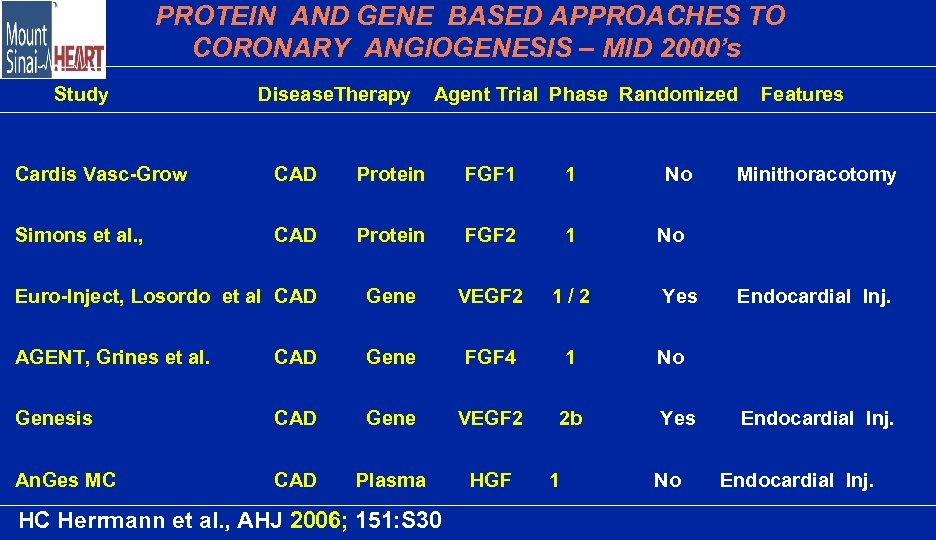 PROTEIN AND GENE BASED APPROACHES TO CORONARY ANGIOGENESIS – MID 2000’s Study Disease. Therapy Agent Trial Phase Randomized Cardis Vasc-Grow CAD Protein FGF 1 1 Simons et al. , CAD Protein FGF 2 1 Euro-Inject, Losordo et al CAD Gene VEGF 2 1/2 AGENT, Grines et al. CAD Gene FGF 4 1 Genesis CAD Gene VEGF 2 2 b An. Ges MC CAD Plasma HGF HC Herrmann et al. , AHJ 2006; 151: S 30 1 No Features Minithoracotomy No Yes Endocardial Inj. No Yes No Endocardial Inj.
PROTEIN AND GENE BASED APPROACHES TO CORONARY ANGIOGENESIS – MID 2000’s Study Disease. Therapy Agent Trial Phase Randomized Cardis Vasc-Grow CAD Protein FGF 1 1 Simons et al. , CAD Protein FGF 2 1 Euro-Inject, Losordo et al CAD Gene VEGF 2 1/2 AGENT, Grines et al. CAD Gene FGF 4 1 Genesis CAD Gene VEGF 2 2 b An. Ges MC CAD Plasma HGF HC Herrmann et al. , AHJ 2006; 151: S 30 1 No Features Minithoracotomy No Yes Endocardial Inj. No Yes No Endocardial Inj.
 Angiogenic Agents – 2005 • PHASE III CLINICAL TRIALS 0
Angiogenic Agents – 2005 • PHASE III CLINICAL TRIALS 0
 Potential Reasons for Early Failures in Angiogenesis Study – Trials in the mid 2000’s DELIVERY / VECTOR ISSUES • Route • Immunity • Pharmacokinetics of vector systems CONCEPTUAL ISSUES • Single Growth Factor Approach Simplistic • Persistent expression (VEGF) needed • Heterogeneity of Responses
Potential Reasons for Early Failures in Angiogenesis Study – Trials in the mid 2000’s DELIVERY / VECTOR ISSUES • Route • Immunity • Pharmacokinetics of vector systems CONCEPTUAL ISSUES • Single Growth Factor Approach Simplistic • Persistent expression (VEGF) needed • Heterogeneity of Responses
 The History of Regeneration – Early 2000’s
The History of Regeneration – Early 2000’s
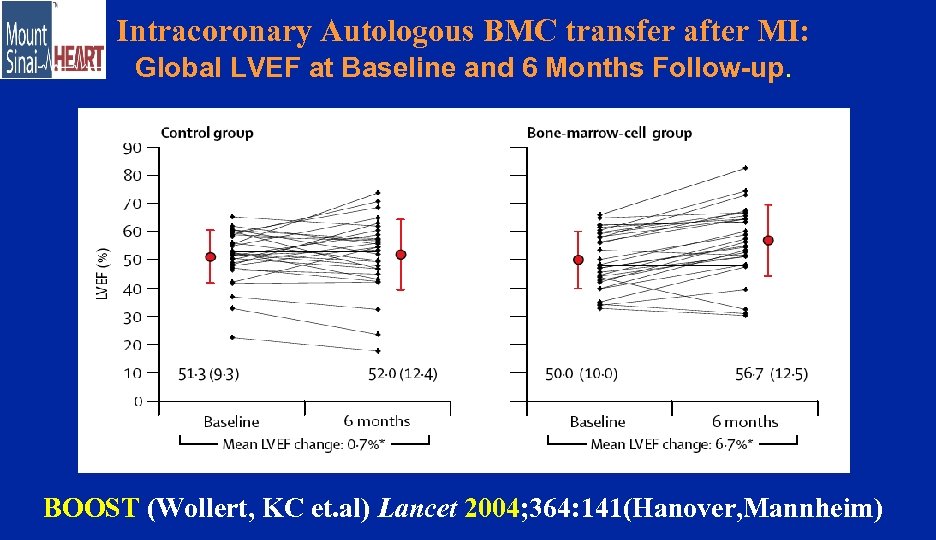 Intracoronary Autologous BMC transfer after MI: Global LVEF at Baseline and 6 Months Follow-up. P< 0. 003 BOOST (Wollert, KC et. al) Lancet 2004; 364: 141(Hanover, Mannheim)
Intracoronary Autologous BMC transfer after MI: Global LVEF at Baseline and 6 Months Follow-up. P< 0. 003 BOOST (Wollert, KC et. al) Lancet 2004; 364: 141(Hanover, Mannheim)
 Intracoronary Bone Marrow Cell Transfer After Myocardial Infarction Eighteen Months’ Follow-Up Data From the Randomized, Controlled BOOST (BOne marr. Ow transfer to enhance ST-elevation infarct regeneration) In this study, a single dose of intracoronary BMCs did not provide long-term benefit on LV systolic function after AMI compared with a randomized control group, however, the study suggests an acceleration of LV ejection fraction recovery after AMI by BMC therapy. Circulation 2006; 113: 1287
Intracoronary Bone Marrow Cell Transfer After Myocardial Infarction Eighteen Months’ Follow-Up Data From the Randomized, Controlled BOOST (BOne marr. Ow transfer to enhance ST-elevation infarct regeneration) In this study, a single dose of intracoronary BMCs did not provide long-term benefit on LV systolic function after AMI compared with a randomized control group, however, the study suggests an acceleration of LV ejection fraction recovery after AMI by BMC therapy. Circulation 2006; 113: 1287
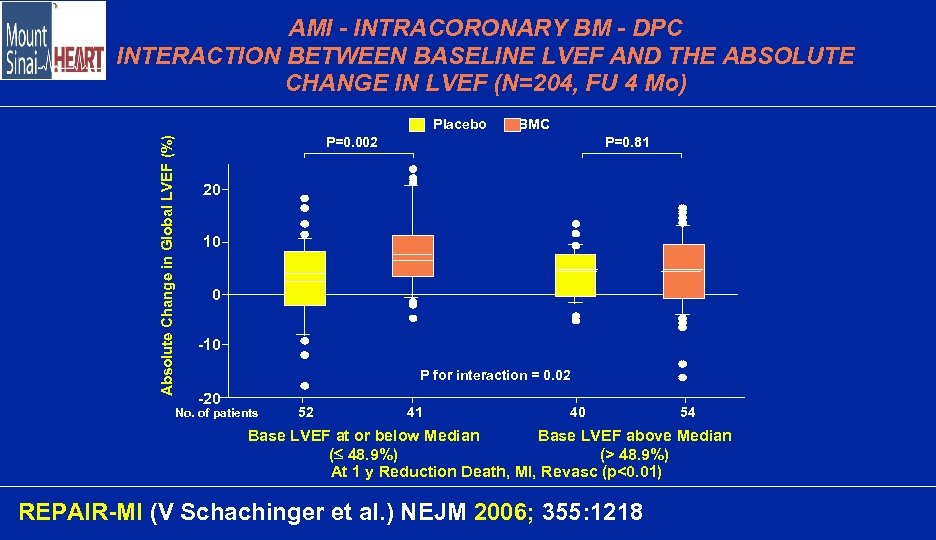 AMI - INTRACORONARY BM - DPC INTERACTION BETWEEN BASELINE LVEF AND THE ABSOLUTE CHANGE IN LVEF (N=204, FU 4 Mo) Absolute Change in Global LVEF (%) Placebo BMC P=0. 002 P=0. 81 20 10 0 -10 P for interaction = 0. 02 -20 No. of patients 52 41 40 54 Base LVEF at or below Median Base LVEF above Median ( 48. 9%) (> 48. 9%) At 1 y Reduction Death, MI, Revasc (p<0. 01) REPAIR-MI (V Schachinger et al. ) NEJM 2006; 355: 1218
AMI - INTRACORONARY BM - DPC INTERACTION BETWEEN BASELINE LVEF AND THE ABSOLUTE CHANGE IN LVEF (N=204, FU 4 Mo) Absolute Change in Global LVEF (%) Placebo BMC P=0. 002 P=0. 81 20 10 0 -10 P for interaction = 0. 02 -20 No. of patients 52 41 40 54 Base LVEF at or below Median Base LVEF above Median ( 48. 9%) (> 48. 9%) At 1 y Reduction Death, MI, Revasc (p<0. 01) REPAIR-MI (V Schachinger et al. ) NEJM 2006; 355: 1218
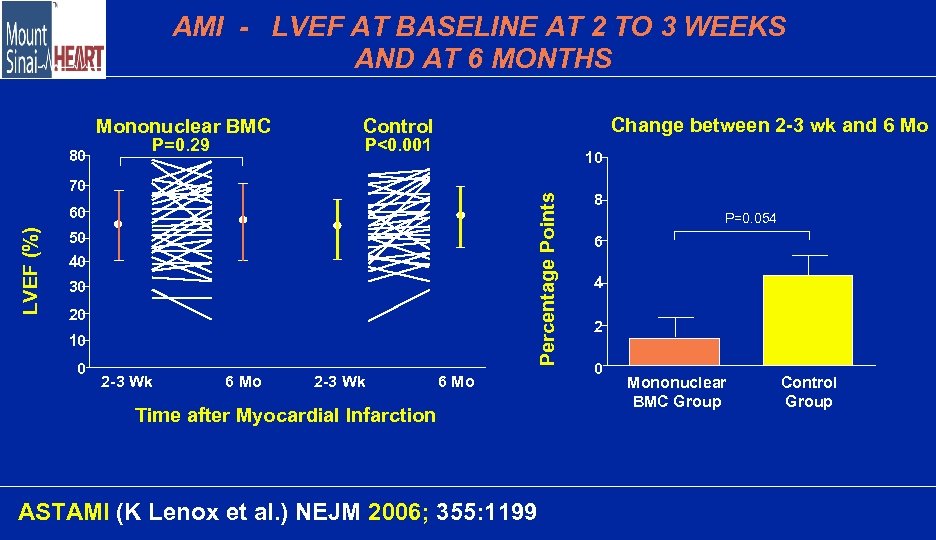 AMI - LVEF AT BASELINE AT 2 TO 3 WEEKS AND AT 6 MONTHS Mononuclear BMC 80 P=0. 29 Change between 2 -3 wk and 6 Mo Control P<0. 001 10 Percentage Points 70 LVEF (%) 60 50 40 30 20 10 0 2 -3 Wk 6 Mo Time after Myocardial Infarction ASTAMI (K Lenox et al. ) NEJM 2006; 355: 1199 8 P=0. 054 6 4 2 0 Mononuclear BMC Group Control Group
AMI - LVEF AT BASELINE AT 2 TO 3 WEEKS AND AT 6 MONTHS Mononuclear BMC 80 P=0. 29 Change between 2 -3 wk and 6 Mo Control P<0. 001 10 Percentage Points 70 LVEF (%) 60 50 40 30 20 10 0 2 -3 Wk 6 Mo Time after Myocardial Infarction ASTAMI (K Lenox et al. ) NEJM 2006; 355: 1199 8 P=0. 054 6 4 2 0 Mononuclear BMC Group Control Group
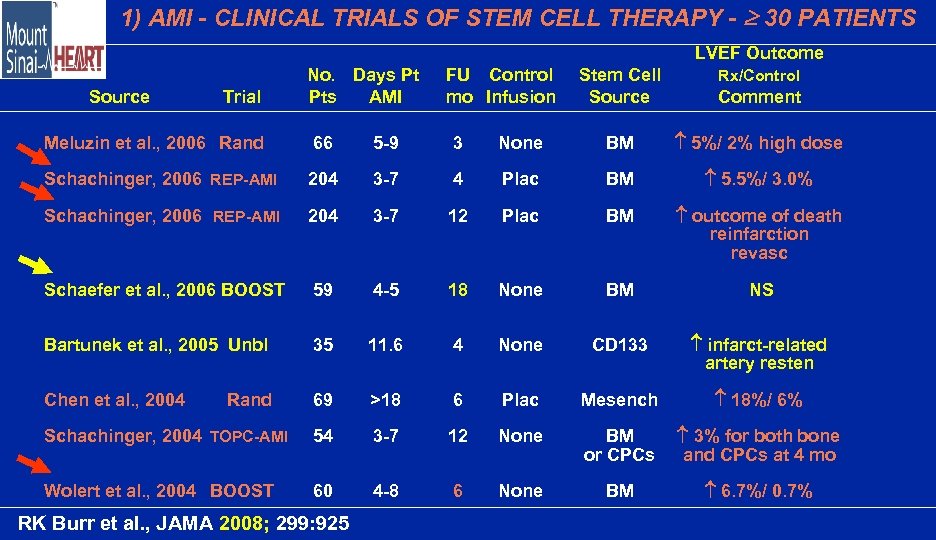 1) AMI - CLINICAL TRIALS OF STEM CELL THERAPY - 30 PATIENTS LVEF Outcome Source Trial No. Days Pt Pts AMI FU Control mo Infusion Stem Cell Source Comment Rx/Control Meluzin et al. , 2006 Rand 66 5 -9 3 None BM 5%/ 2% high dose Schachinger, 2006 REP-AMI 204 3 -7 4 Plac BM 5. 5%/ 3. 0% Schachinger, 2006 REP-AMI 204 3 -7 12 Plac BM outcome of death reinfarction revasc Schaefer et al. , 2006 BOOST 59 4 -5 18 None BM NS Bartunek et al. , 2005 Unbl 35 11. 6 4 None CD 133 infarct-related artery resten Chen et al. , 2004 69 >18 6 Plac Mesench 18%/ 6% Schachinger, 2004 TOPC-AMI 54 3 -7 12 None BM or CPCs 3% for both bone and CPCs at 4 mo Wolert et al. , 2004 BOOST 60 4 -8 6 None BM 6. 7%/ 0. 7% Rand RK Burr et al. , JAMA 2008; 299: 925
1) AMI - CLINICAL TRIALS OF STEM CELL THERAPY - 30 PATIENTS LVEF Outcome Source Trial No. Days Pt Pts AMI FU Control mo Infusion Stem Cell Source Comment Rx/Control Meluzin et al. , 2006 Rand 66 5 -9 3 None BM 5%/ 2% high dose Schachinger, 2006 REP-AMI 204 3 -7 4 Plac BM 5. 5%/ 3. 0% Schachinger, 2006 REP-AMI 204 3 -7 12 Plac BM outcome of death reinfarction revasc Schaefer et al. , 2006 BOOST 59 4 -5 18 None BM NS Bartunek et al. , 2005 Unbl 35 11. 6 4 None CD 133 infarct-related artery resten Chen et al. , 2004 69 >18 6 Plac Mesench 18%/ 6% Schachinger, 2004 TOPC-AMI 54 3 -7 12 None BM or CPCs 3% for both bone and CPCs at 4 mo Wolert et al. , 2004 BOOST 60 4 -8 6 None BM 6. 7%/ 0. 7% Rand RK Burr et al. , JAMA 2008; 299: 925
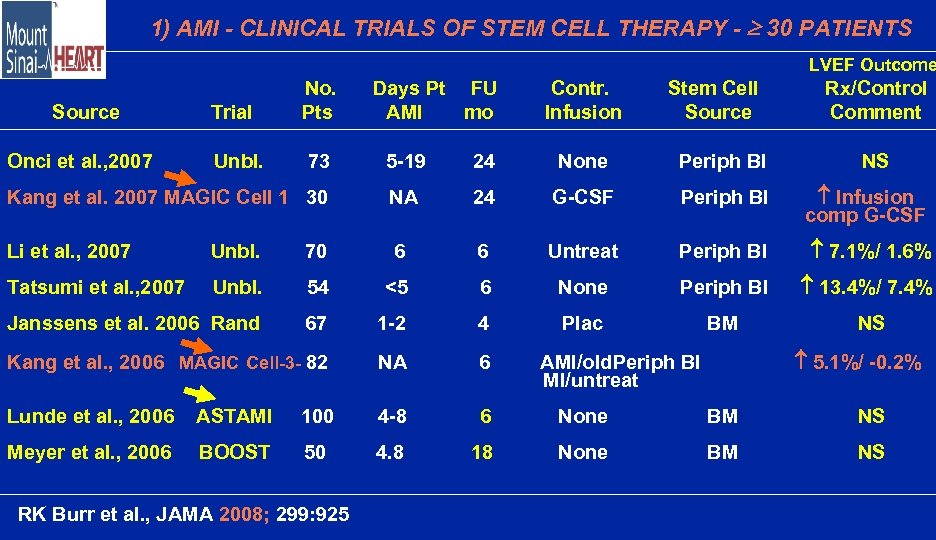 1) AMI - CLINICAL TRIALS OF STEM CELL THERAPY - 30 PATIENTS LVEF Outcome Source Onci et al. , 2007 Trial Unbl. No. Pts 73 Days Pt FU AMI mo Contr. Infusion Stem Cell Source Rx/Control Comment 5 -19 24 None Periph Bl Kang et al. 2007 MAGIC Cell 1 30 NA 24 G-CSF Periph Bl Infusion comp G-CSF Li et al. , 2007 Unbl. 70 6 6 Untreat Periph Bl 7. 1%/ 1. 6% Tatsumi et al. , 2007 Unbl. 54 <5 6 None Periph Bl 13. 4%/ 7. 4% Janssens et al. 2006 Rand 67 1 -2 4 Plac BM NS Kang et al. , 2006 MAGIC Cell-3 - 82 NA 6 Lunde et al. , 2006 ASTAMI 100 4 -8 6 None BM NS Meyer et al. , 2006 BOOST 50 4. 8 18 None BM NS RK Burr et al. , JAMA 2008; 299: 925 NS 5. 1%/ -0. 2% AMI/old. Periph Bl MI/untreat
1) AMI - CLINICAL TRIALS OF STEM CELL THERAPY - 30 PATIENTS LVEF Outcome Source Onci et al. , 2007 Trial Unbl. No. Pts 73 Days Pt FU AMI mo Contr. Infusion Stem Cell Source Rx/Control Comment 5 -19 24 None Periph Bl Kang et al. 2007 MAGIC Cell 1 30 NA 24 G-CSF Periph Bl Infusion comp G-CSF Li et al. , 2007 Unbl. 70 6 6 Untreat Periph Bl 7. 1%/ 1. 6% Tatsumi et al. , 2007 Unbl. 54 <5 6 None Periph Bl 13. 4%/ 7. 4% Janssens et al. 2006 Rand 67 1 -2 4 Plac BM NS Kang et al. , 2006 MAGIC Cell-3 - 82 NA 6 Lunde et al. , 2006 ASTAMI 100 4 -8 6 None BM NS Meyer et al. , 2006 BOOST 50 4. 8 18 None BM NS RK Burr et al. , JAMA 2008; 299: 925 NS 5. 1%/ -0. 2% AMI/old. Periph Bl MI/untreat
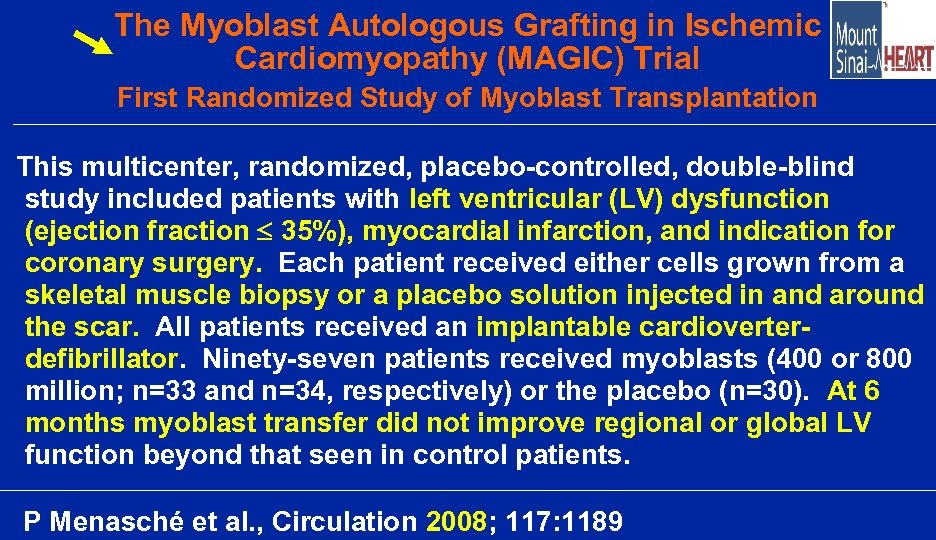 The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) Trial First Randomized Study of Myoblast Transplantation This multicenter, randomized, placebo-controlled, double-blind study included patients with left ventricular (LV) dysfunction (ejection fraction 35%), myocardial infarction, and indication for coronary surgery. Each patient received either cells grown from a skeletal muscle biopsy or a placebo solution injected in and around the scar. All patients received an implantable cardioverterdefibrillator. Ninety-seven patients received myoblasts (400 or 800 million; n=33 and n=34, respectively) or the placebo (n=30). At 6 months myoblast transfer did not improve regional or global LV function beyond that seen in control patients. P Menasché et al. , Circulation 2008; 117: 1189
The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) Trial First Randomized Study of Myoblast Transplantation This multicenter, randomized, placebo-controlled, double-blind study included patients with left ventricular (LV) dysfunction (ejection fraction 35%), myocardial infarction, and indication for coronary surgery. Each patient received either cells grown from a skeletal muscle biopsy or a placebo solution injected in and around the scar. All patients received an implantable cardioverterdefibrillator. Ninety-seven patients received myoblasts (400 or 800 million; n=33 and n=34, respectively) or the placebo (n=30). At 6 months myoblast transfer did not improve regional or global LV function beyond that seen in control patients. P Menasché et al. , Circulation 2008; 117: 1189
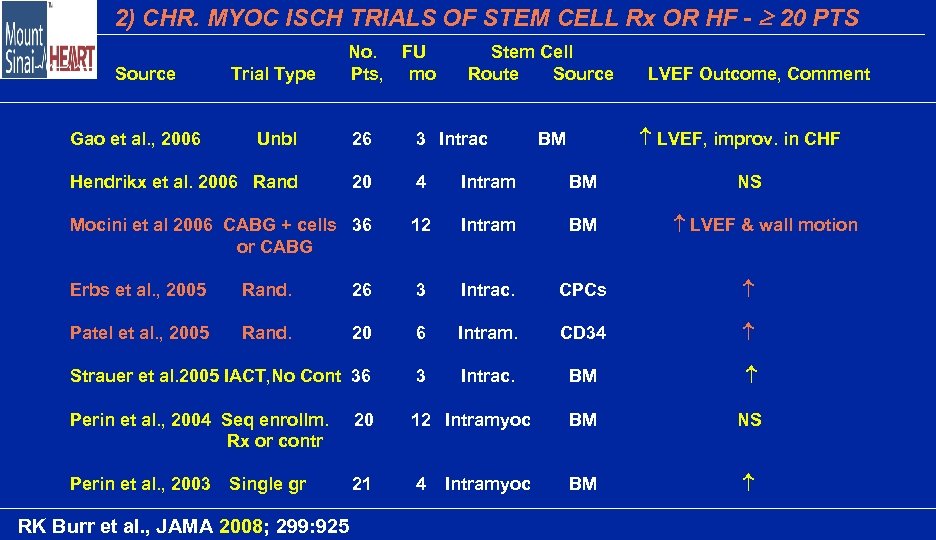 2) CHR. MYOC ISCH TRIALS OF STEM CELL Rx OR HF - 20 PTS Trial Type No. Pts, Unbl 26 3 Intrac Hendrikx et al. 2006 Rand 20 4 Intram BM Mocini et al 2006 CABG + cells 36 or CABG 12 Intram BM Erbs et al. , 2005 Rand. 26 3 Intrac. CPCs Patel et al. , 2005 Rand. 20 6 Intram. CD 34 Strauer et al. 2005 IACT, No Cont 36 3 Intrac. BM Perin et al. , 2004 Seq enrollm. Rx or contr 20 12 Intramyoc BM NS Perin et al. , 2003 Single gr 21 4 BM Source Gao et al. , 2006 RK Burr et al. , JAMA 2008; 299: 925 FU mo Stem Cell Route Source Intramyoc LVEF Outcome, Comment LVEF, improv. in CHF BM NS LVEF & wall motion
2) CHR. MYOC ISCH TRIALS OF STEM CELL Rx OR HF - 20 PTS Trial Type No. Pts, Unbl 26 3 Intrac Hendrikx et al. 2006 Rand 20 4 Intram BM Mocini et al 2006 CABG + cells 36 or CABG 12 Intram BM Erbs et al. , 2005 Rand. 26 3 Intrac. CPCs Patel et al. , 2005 Rand. 20 6 Intram. CD 34 Strauer et al. 2005 IACT, No Cont 36 3 Intrac. BM Perin et al. , 2004 Seq enrollm. Rx or contr 20 12 Intramyoc BM NS Perin et al. , 2003 Single gr 21 4 BM Source Gao et al. , 2006 RK Burr et al. , JAMA 2008; 299: 925 FU mo Stem Cell Route Source Intramyoc LVEF Outcome, Comment LVEF, improv. in CHF BM NS LVEF & wall motion
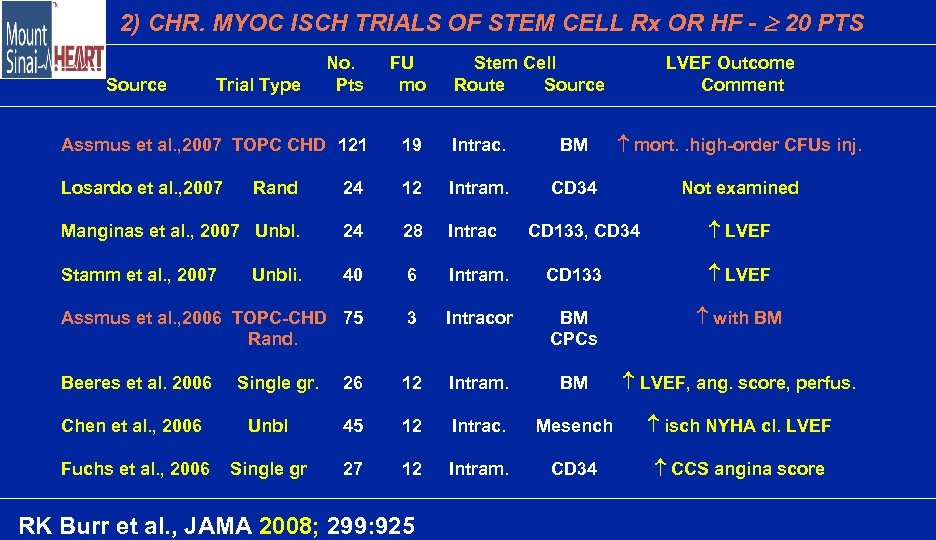 2) CHR. MYOC ISCH TRIALS OF STEM CELL Rx OR HF - 20 PTS No. Pts FU mo Assmus et al. , 2007 TOPC CHD 121 19 Intrac. BM mort. . high-order CFUs inj. Losardo et al. , 2007 CD 34 Not examined Source Trial Type Stem Cell Route Source LVEF Outcome Comment Rand 24 12 Intram. Manginas et al. , 2007 Unbl. 24 28 Intrac Stamm et al. , 2007 40 6 Intram. CD 133 LVEF Assmus et al. , 2006 TOPC-CHD 75 Rand. 3 Intracor BM CPCs with BM Beeres et al. 2006 26 12 Intram. BM LVEF, ang. score, perfus. Unbli. Single gr. CD 133, CD 34 LVEF Chen et al. , 2006 Unbl 45 12 Intrac. Mesench isch NYHA cl. LVEF Fuchs et al. , 2006 Single gr 27 12 Intram. CD 34 CCS angina score RK Burr et al. , JAMA 2008; 299: 925
2) CHR. MYOC ISCH TRIALS OF STEM CELL Rx OR HF - 20 PTS No. Pts FU mo Assmus et al. , 2007 TOPC CHD 121 19 Intrac. BM mort. . high-order CFUs inj. Losardo et al. , 2007 CD 34 Not examined Source Trial Type Stem Cell Route Source LVEF Outcome Comment Rand 24 12 Intram. Manginas et al. , 2007 Unbl. 24 28 Intrac Stamm et al. , 2007 40 6 Intram. CD 133 LVEF Assmus et al. , 2006 TOPC-CHD 75 Rand. 3 Intracor BM CPCs with BM Beeres et al. 2006 26 12 Intram. BM LVEF, ang. score, perfus. Unbli. Single gr. CD 133, CD 34 LVEF Chen et al. , 2006 Unbl 45 12 Intrac. Mesench isch NYHA cl. LVEF Fuchs et al. , 2006 Single gr 27 12 Intram. CD 34 CCS angina score RK Burr et al. , JAMA 2008; 299: 925
 CARDIOVASCULAR GENE AND CELL THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
CARDIOVASCULAR GENE AND CELL THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
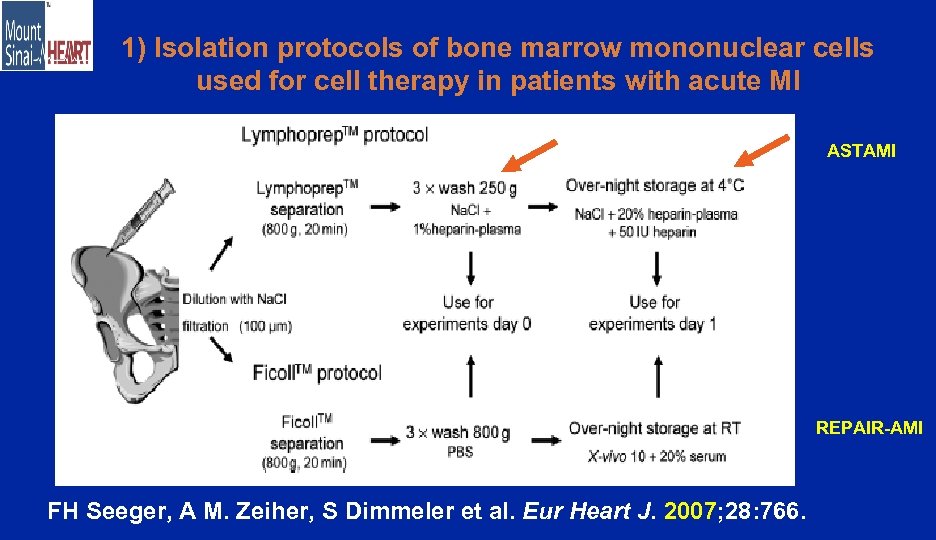 1) Isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute MI ASTAMI REPAIR-AMI FH Seeger, A M. Zeiher, S Dimmeler et al. Eur Heart J. 2007; 28: 766.
1) Isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute MI ASTAMI REPAIR-AMI FH Seeger, A M. Zeiher, S Dimmeler et al. Eur Heart J. 2007; 28: 766.
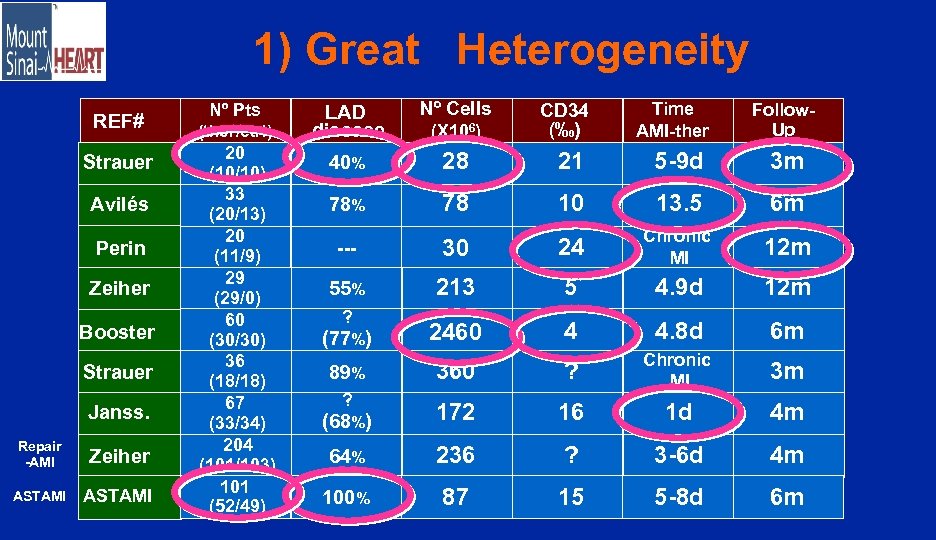 1) Great Heterogeneity REF# Strauer Avilés Perin Zeiher Booster Strauer Janss. Repair -AMI Zeiher ASTAMI Nº Pts (ther/ctrl) 20 (10/10) 33 (20/13) 20 (11/9) 29 (29/0) 60 (30/30) 36 (18/18) 67 (33/34) 204 (101/103) 101 (52/49) LAD disease Nº Cells 40% 28 21 5 -9 d 3 m 78% 78 10 13. 5 6 m --- 30 24 Chronic MI 12 m 55% 213 5 4. 9 d 12 m (77%) 2460 4 4. 8 d 6 m 89% 360 ? 3 m ? Chronic MI (68%) 172 16 1 d 4 m 64% 236 ? 3 -6 d 4 m 100% 87 15 5 -8 d 6 m ? (X 106) CD 34 (‰) Time AMI-ther Follow. Up
1) Great Heterogeneity REF# Strauer Avilés Perin Zeiher Booster Strauer Janss. Repair -AMI Zeiher ASTAMI Nº Pts (ther/ctrl) 20 (10/10) 33 (20/13) 20 (11/9) 29 (29/0) 60 (30/30) 36 (18/18) 67 (33/34) 204 (101/103) 101 (52/49) LAD disease Nº Cells 40% 28 21 5 -9 d 3 m 78% 78 10 13. 5 6 m --- 30 24 Chronic MI 12 m 55% 213 5 4. 9 d 12 m (77%) 2460 4 4. 8 d 6 m 89% 360 ? 3 m ? Chronic MI (68%) 172 16 1 d 4 m 64% 236 ? 3 -6 d 4 m 100% 87 15 5 -8 d 6 m ? (X 106) CD 34 (‰) Time AMI-ther Follow. Up
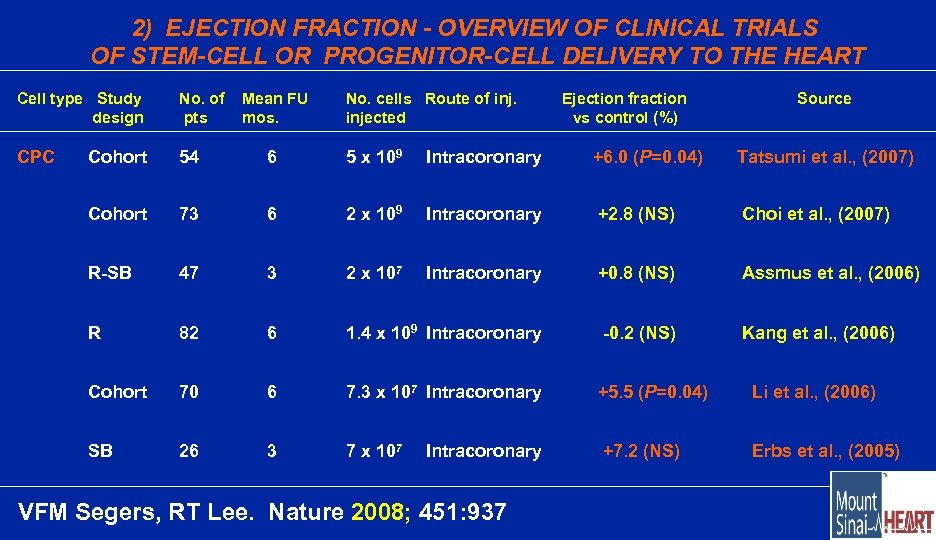 2) EJECTION FRACTION - OVERVIEW OF CLINICAL TRIALS OF STEM-CELL OR PROGENITOR-CELL DELIVERY TO THE HEART Cell type Study design No. of pts Mean FU mos. No. cells Route of injected CPC Cohort 54 6 5 x 109 Intracoronary +6. 0 (P=0. 04) Tatsumi et al. , (2007) Cohort 73 6 2 x 109 Intracoronary +2. 8 (NS) Choi et al. , (2007) R-SB 47 3 2 x 107 Intracoronary +0. 8 (NS) Assmus et al. , (2006) R 82 6 1. 4 x 109 Intracoronary -0. 2 (NS) Kang et al. , (2006) Cohort 70 6 7. 3 x 107 Intracoronary +5. 5 (P=0. 04) Li et al. , (2006) SB 26 3 7 x 107 +7. 2 (NS) Erbs et al. , (2005) Intracoronary VFM Segers, RT Lee. Nature 2008; 451: 937 Ejection fraction vs control (%) Source
2) EJECTION FRACTION - OVERVIEW OF CLINICAL TRIALS OF STEM-CELL OR PROGENITOR-CELL DELIVERY TO THE HEART Cell type Study design No. of pts Mean FU mos. No. cells Route of injected CPC Cohort 54 6 5 x 109 Intracoronary +6. 0 (P=0. 04) Tatsumi et al. , (2007) Cohort 73 6 2 x 109 Intracoronary +2. 8 (NS) Choi et al. , (2007) R-SB 47 3 2 x 107 Intracoronary +0. 8 (NS) Assmus et al. , (2006) R 82 6 1. 4 x 109 Intracoronary -0. 2 (NS) Kang et al. , (2006) Cohort 70 6 7. 3 x 107 Intracoronary +5. 5 (P=0. 04) Li et al. , (2006) SB 26 3 7 x 107 +7. 2 (NS) Erbs et al. , (2005) Intracoronary VFM Segers, RT Lee. Nature 2008; 451: 937 Ejection fraction vs control (%) Source
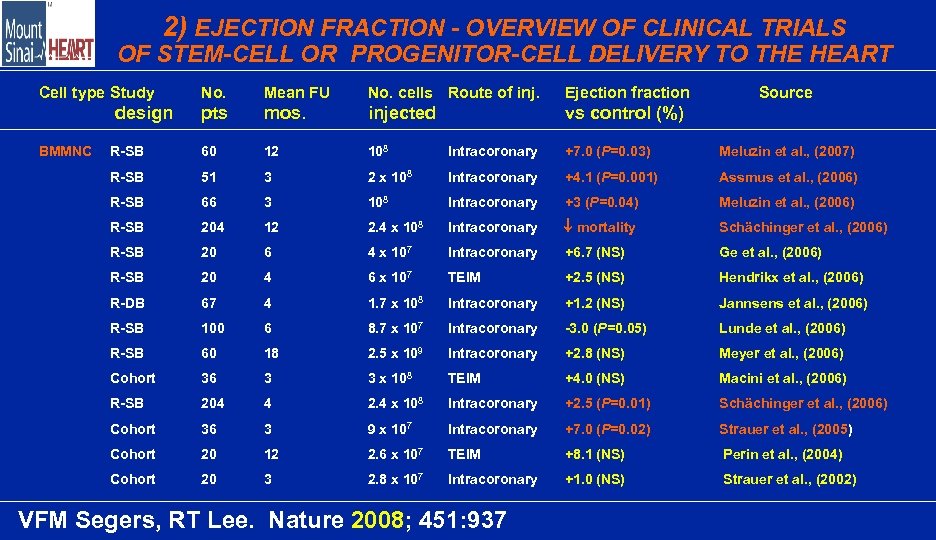 2) EJECTION FRACTION - OVERVIEW OF CLINICAL TRIALS OF STEM-CELL OR PROGENITOR-CELL DELIVERY TO THE HEART Cell type Study No. Mean FU No. cells Route of inj. Ejection fraction pts mos. injected vs control (%) R-SB 60 12 108 Intracoronary +7. 0 (P=0. 03) Meluzin et al. , (2007) R-SB 51 3 2 x 108 Intracoronary +4. 1 (P=0. 001) Assmus et al. , (2006) R-SB 66 3 108 Intracoronary +3 (P=0. 04) Meluzin et al. , (2006) R-SB 204 12 2. 4 x 108 Intracoronary mortality Schächinger et al. , (2006) R-SB 20 6 4 x 107 Intracoronary +6. 7 (NS) Ge et al. , (2006) R-SB 20 4 6 x 107 TEIM +2. 5 (NS) Hendrikx et al. , (2006) R-DB 67 4 1. 7 x 108 Intracoronary +1. 2 (NS) Jannsens et al. , (2006) R-SB 100 6 8. 7 x 107 Intracoronary -3. 0 (P=0. 05) Lunde et al. , (2006) R-SB 60 18 2. 5 x 109 Intracoronary +2. 8 (NS) Meyer et al. , (2006) Cohort 36 3 3 x 108 TEIM +4. 0 (NS) Macini et al. , (2006) R-SB 204 4 2. 4 x 108 Intracoronary +2. 5 (P=0. 01) Schächinger et al. , (2006) Cohort 36 3 9 x 107 Intracoronary +7. 0 (P=0. 02) Strauer et al. , (2005) Cohort 20 12 2. 6 x 107 TEIM +8. 1 (NS) Perin et al. , (2004) Cohort 20 3 2. 8 x 107 Intracoronary +1. 0 (NS) Strauer et al. , (2002) design BMMNC VFM Segers, RT Lee. Nature 2008; 451: 937 Source
2) EJECTION FRACTION - OVERVIEW OF CLINICAL TRIALS OF STEM-CELL OR PROGENITOR-CELL DELIVERY TO THE HEART Cell type Study No. Mean FU No. cells Route of inj. Ejection fraction pts mos. injected vs control (%) R-SB 60 12 108 Intracoronary +7. 0 (P=0. 03) Meluzin et al. , (2007) R-SB 51 3 2 x 108 Intracoronary +4. 1 (P=0. 001) Assmus et al. , (2006) R-SB 66 3 108 Intracoronary +3 (P=0. 04) Meluzin et al. , (2006) R-SB 204 12 2. 4 x 108 Intracoronary mortality Schächinger et al. , (2006) R-SB 20 6 4 x 107 Intracoronary +6. 7 (NS) Ge et al. , (2006) R-SB 20 4 6 x 107 TEIM +2. 5 (NS) Hendrikx et al. , (2006) R-DB 67 4 1. 7 x 108 Intracoronary +1. 2 (NS) Jannsens et al. , (2006) R-SB 100 6 8. 7 x 107 Intracoronary -3. 0 (P=0. 05) Lunde et al. , (2006) R-SB 60 18 2. 5 x 109 Intracoronary +2. 8 (NS) Meyer et al. , (2006) Cohort 36 3 3 x 108 TEIM +4. 0 (NS) Macini et al. , (2006) R-SB 204 4 2. 4 x 108 Intracoronary +2. 5 (P=0. 01) Schächinger et al. , (2006) Cohort 36 3 9 x 107 Intracoronary +7. 0 (P=0. 02) Strauer et al. , (2005) Cohort 20 12 2. 6 x 107 TEIM +8. 1 (NS) Perin et al. , (2004) Cohort 20 3 2. 8 x 107 Intracoronary +1. 0 (NS) Strauer et al. , (2002) design BMMNC VFM Segers, RT Lee. Nature 2008; 451: 937 Source
 Myocardial Cell Therapy At The Crossroads B Nadal-Ginard, V Fuster Nature CP Cardiov Med 2007; 4: 1 Cardiac Cell Therapy: Bench or Bedside? Steering Committee NHLBI Cardiovascular Cell Therapy Research Network Nature CP Cardiov Med 2007; 4: 403
Myocardial Cell Therapy At The Crossroads B Nadal-Ginard, V Fuster Nature CP Cardiov Med 2007; 4: 1 Cardiac Cell Therapy: Bench or Bedside? Steering Committee NHLBI Cardiovascular Cell Therapy Research Network Nature CP Cardiov Med 2007; 4: 403
 CARDIOVASCULAR GENE AND CELL THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
CARDIOVASCULAR GENE AND CELL THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
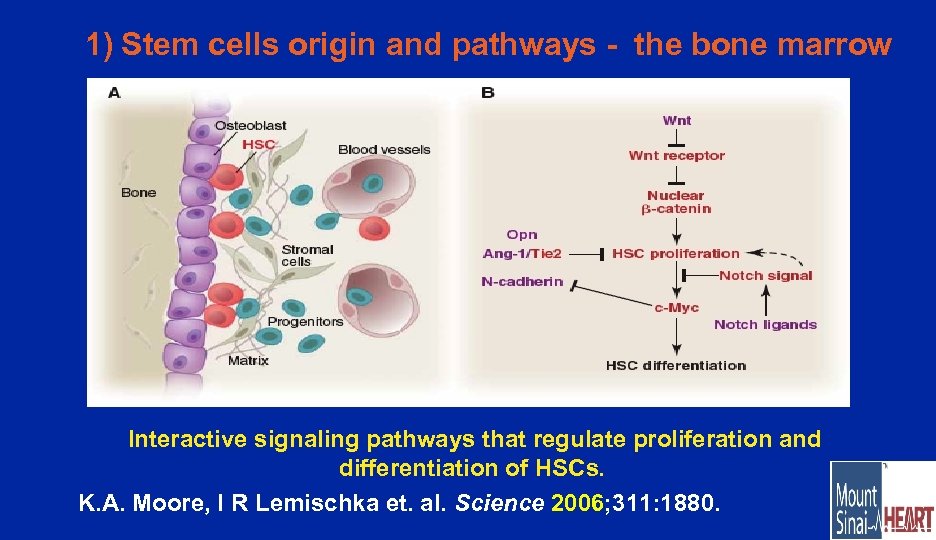 1) Stem cells origin and pathways - the bone marrow Interactive signaling pathways that regulate proliferation and differentiation of HSCs. K. A. Moore, I R Lemischka et. al. Science 2006; 311: 1880.
1) Stem cells origin and pathways - the bone marrow Interactive signaling pathways that regulate proliferation and differentiation of HSCs. K. A. Moore, I R Lemischka et. al. Science 2006; 311: 1880.
 2) Haematopoietic Stem Cell Release Is Regulated By Circadian Oscillations The cyclical release of HSCs and expression of Cxel 12 are regulated by core genes of the molecular clock through circadian noradrenaline secretion by the sympathetic nervous system. These adrenergic signals are locally delivered by nerves in the bone marrow. These data indicate that a circadian, neurally driven release of HSC during the animal’s resting period may promote the regeneration of the stem cell niche and possibly other tissues. S Méndez-Ferrer et al. , Nature 2008 (In Press)
2) Haematopoietic Stem Cell Release Is Regulated By Circadian Oscillations The cyclical release of HSCs and expression of Cxel 12 are regulated by core genes of the molecular clock through circadian noradrenaline secretion by the sympathetic nervous system. These adrenergic signals are locally delivered by nerves in the bone marrow. These data indicate that a circadian, neurally driven release of HSC during the animal’s resting period may promote the regeneration of the stem cell niche and possibly other tissues. S Méndez-Ferrer et al. , Nature 2008 (In Press)
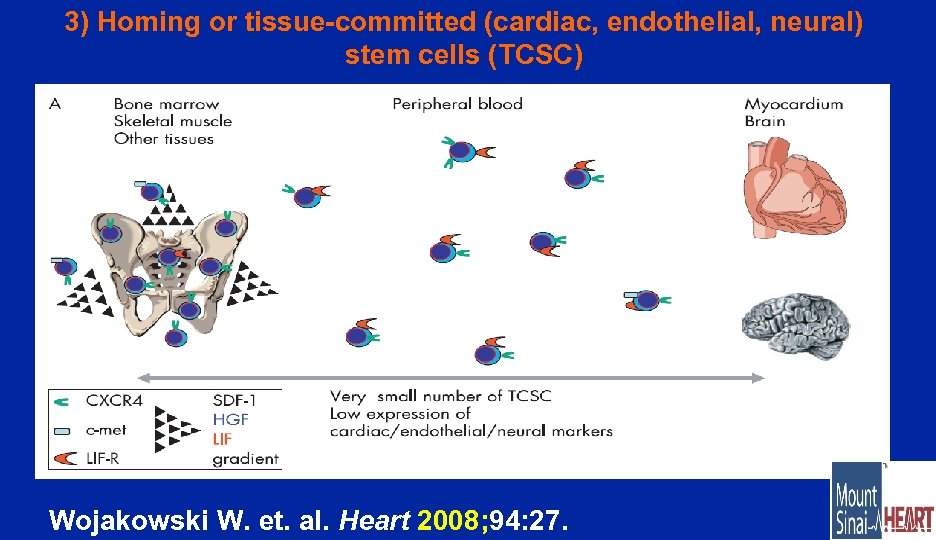 3) Homing or tissue-committed (cardiac, endothelial, neural) stem cells (TCSC) Wojakowski W. et. al. Heart 2008; 94: 27.
3) Homing or tissue-committed (cardiac, endothelial, neural) stem cells (TCSC) Wojakowski W. et. al. Heart 2008; 94: 27.
 3 a) EVEN IF WE FIND THE RIGHT CELL, DOES IT MATTER? • Can Stem Cells Survive in the hostile environment of the ischemic myocardium without a known niche? • Can the end stage heart truly be reverse remodeled by stem cells? Even the liver, which is one of the most regenerative organs in the body, cannot be regenerated once it becomes cirrhotic.
3 a) EVEN IF WE FIND THE RIGHT CELL, DOES IT MATTER? • Can Stem Cells Survive in the hostile environment of the ischemic myocardium without a known niche? • Can the end stage heart truly be reverse remodeled by stem cells? Even the liver, which is one of the most regenerative organs in the body, cannot be regenerated once it becomes cirrhotic.
 3 a) Cardiac Stem Cells in the Real World We prospectively screened 32 endomyocardial biopsies harvested from heart transplant recipients (off rejection episodes) and 18 right appendage biopsies collected during coronary artery bypass surgery, and processed the tissue specimens for the immunohistochemical detection of markers of stemness (c-kit, MDR -1, Isl-1), hematopoietic origin (CD 45), mast cells (tryptase), endothelial cells (CD 105), and cardiac lineage (Nkx 2. 5). These data raise a cautionary note on therapeutic exploitation of cardiac stem cells in patients with ischemic cardiomyopathy, who may be the elective candidates for regenerative therapy. J Pouly, P Menasché et al. , JTCS 2008; 135: 673 (Paris)
3 a) Cardiac Stem Cells in the Real World We prospectively screened 32 endomyocardial biopsies harvested from heart transplant recipients (off rejection episodes) and 18 right appendage biopsies collected during coronary artery bypass surgery, and processed the tissue specimens for the immunohistochemical detection of markers of stemness (c-kit, MDR -1, Isl-1), hematopoietic origin (CD 45), mast cells (tryptase), endothelial cells (CD 105), and cardiac lineage (Nkx 2. 5). These data raise a cautionary note on therapeutic exploitation of cardiac stem cells in patients with ischemic cardiomyopathy, who may be the elective candidates for regenerative therapy. J Pouly, P Menasché et al. , JTCS 2008; 135: 673 (Paris)
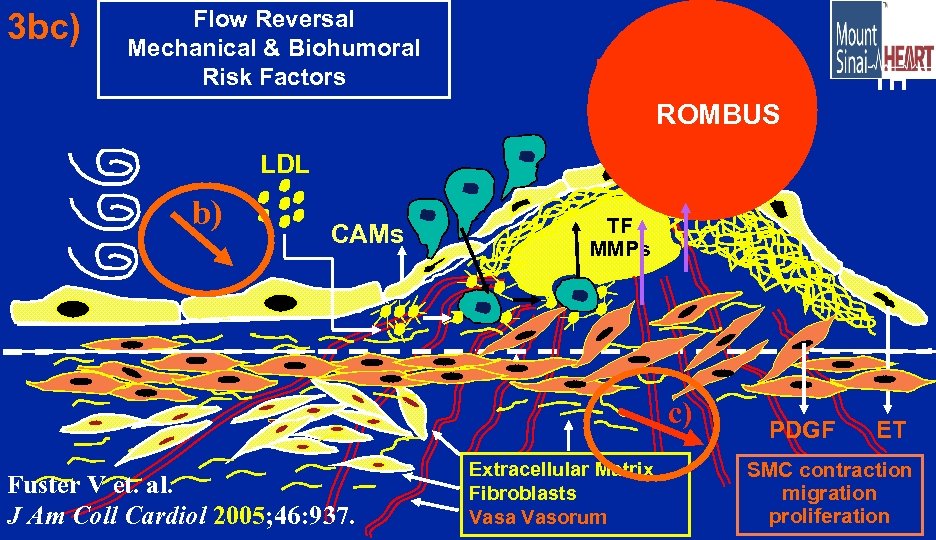 3 bc) Flow Reversal Mechanical & Biohumoral Risk Factors TH ROMBUS LDL b) CAMs TF MMPs c) Fuster V et. al. J Am Coll Cardiol 2005; 46: 937. Extracellular Matrix Fibroblasts Vasa Vasorum PDGF ET SMC contraction migration proliferation
3 bc) Flow Reversal Mechanical & Biohumoral Risk Factors TH ROMBUS LDL b) CAMs TF MMPs c) Fuster V et. al. J Am Coll Cardiol 2005; 46: 937. Extracellular Matrix Fibroblasts Vasa Vasorum PDGF ET SMC contraction migration proliferation
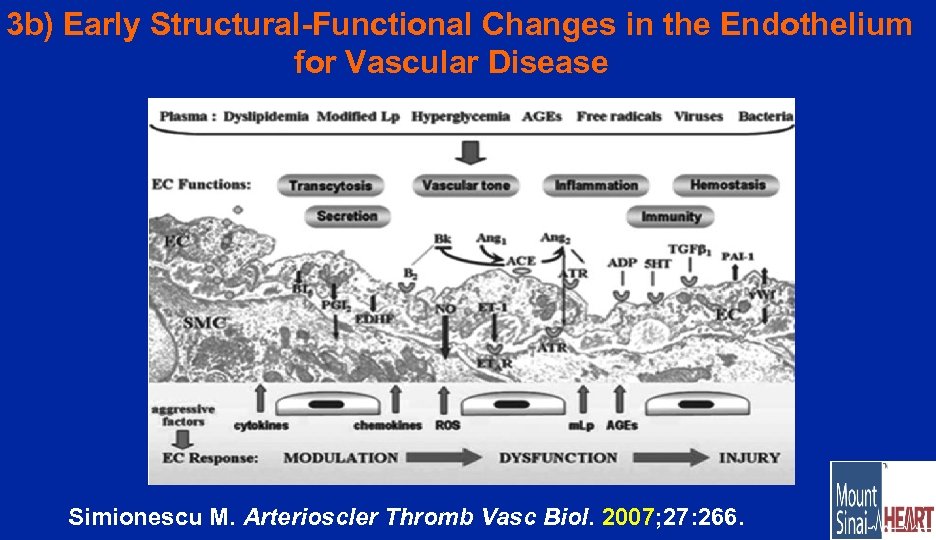 3 b) Early Structural-Functional Changes in the Endothelium for Vascular Disease Simionescu M. Arterioscler Thromb Vasc Biol. 2007; 27: 266.
3 b) Early Structural-Functional Changes in the Endothelium for Vascular Disease Simionescu M. Arterioscler Thromb Vasc Biol. 2007; 27: 266.
 3 b) Rapid Endothelial Turnover in Atherosclerosis-Prone Areas Coincides With Stem Cell Repair in Apolipoprotein E-Deficient Mice Our findings provide the first quantitative data on endothelial turnover and repair (Evans Blue, Brd U) by progenitor cells that are, at least in part, derived from bone marrow (D 31, CD 144) during development of atherosclerosis in apo. E-/- mice. G Foteinos, Q Xu et al. , Circ 2008; 117: 1856 (Insbr. , Lond)
3 b) Rapid Endothelial Turnover in Atherosclerosis-Prone Areas Coincides With Stem Cell Repair in Apolipoprotein E-Deficient Mice Our findings provide the first quantitative data on endothelial turnover and repair (Evans Blue, Brd U) by progenitor cells that are, at least in part, derived from bone marrow (D 31, CD 144) during development of atherosclerosis in apo. E-/- mice. G Foteinos, Q Xu et al. , Circ 2008; 117: 1856 (Insbr. , Lond)
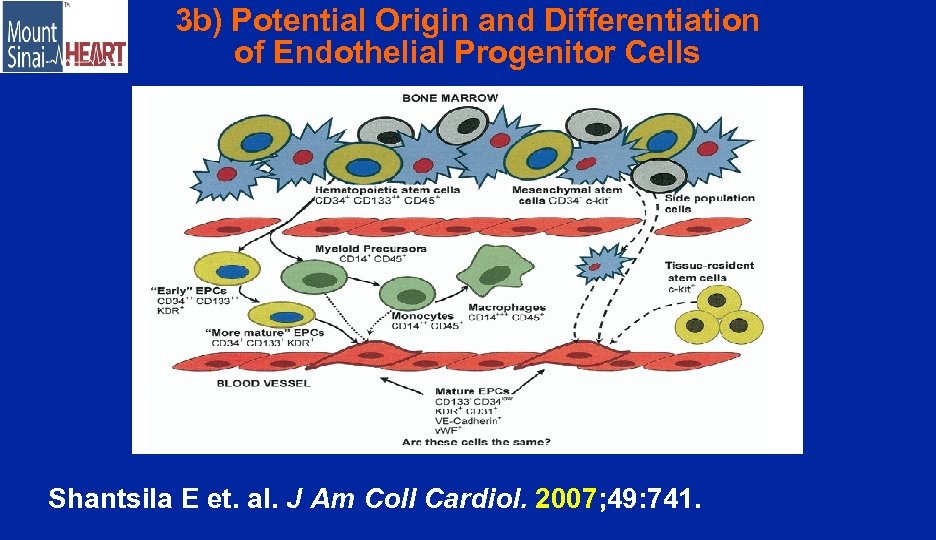 3 b) Potential Origin and Differentiation of Endothelial Progenitor Cells Shantsila E et. al. J Am Coll Cardiol. 2007; 49: 741.
3 b) Potential Origin and Differentiation of Endothelial Progenitor Cells Shantsila E et. al. J Am Coll Cardiol. 2007; 49: 741.
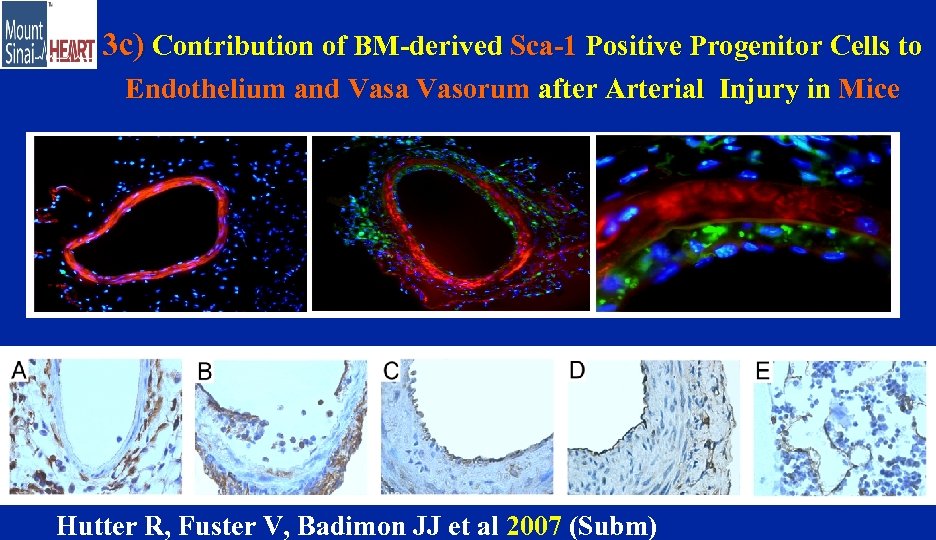 3 c) Contribution of BM-derived Sca-1 Positive Progenitor Cells to Endothelium and Vasa Vasorum after Arterial Injury in Mice Hutter R, Fuster V, Badimon JJ et al 2007 (Subm)
3 c) Contribution of BM-derived Sca-1 Positive Progenitor Cells to Endothelium and Vasa Vasorum after Arterial Injury in Mice Hutter R, Fuster V, Badimon JJ et al 2007 (Subm)
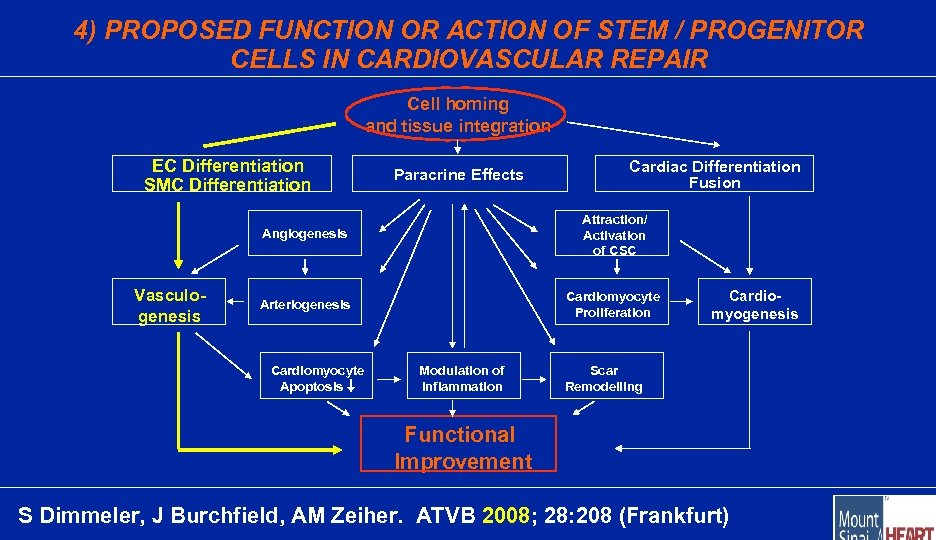 4) PROPOSED FUNCTION OR ACTION OF STEM / PROGENITOR CELLS IN CARDIOVASCULAR REPAIR Cell homing and tissue integration EC Differentiation SMC Differentiation Paracrine Effects Cardiac Differentiation Fusion Angiogenesis Vasculogenesis Attraction/ Activation of CSC Arteriogenesis Cardiomyocyte Proliferation Cardiomyocyte Apoptosis Modulation of Inflammation Cardiomyogenesis Scar Remodelling Functional Improvement S Dimmeler, J Burchfield, AM Zeiher. ATVB 2008; 28: 208 (Frankfurt)
4) PROPOSED FUNCTION OR ACTION OF STEM / PROGENITOR CELLS IN CARDIOVASCULAR REPAIR Cell homing and tissue integration EC Differentiation SMC Differentiation Paracrine Effects Cardiac Differentiation Fusion Angiogenesis Vasculogenesis Attraction/ Activation of CSC Arteriogenesis Cardiomyocyte Proliferation Cardiomyocyte Apoptosis Modulation of Inflammation Cardiomyogenesis Scar Remodelling Functional Improvement S Dimmeler, J Burchfield, AM Zeiher. ATVB 2008; 28: 208 (Frankfurt)
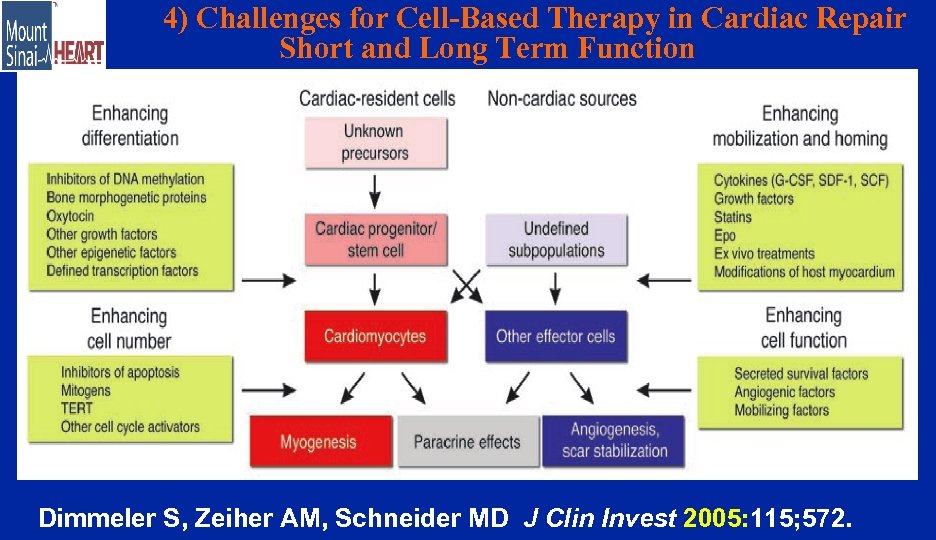 4) Challenges for Cell-Based Therapy in Cardiac Repair Short and Long Term Function Dimmeler S, Zeiher AM, Schneider MD J Clin Invest 2005: 115; 572.
4) Challenges for Cell-Based Therapy in Cardiac Repair Short and Long Term Function Dimmeler S, Zeiher AM, Schneider MD J Clin Invest 2005: 115; 572.
 CARDIOVASCULAR CELL AND GENE THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
CARDIOVASCULAR CELL AND GENE THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
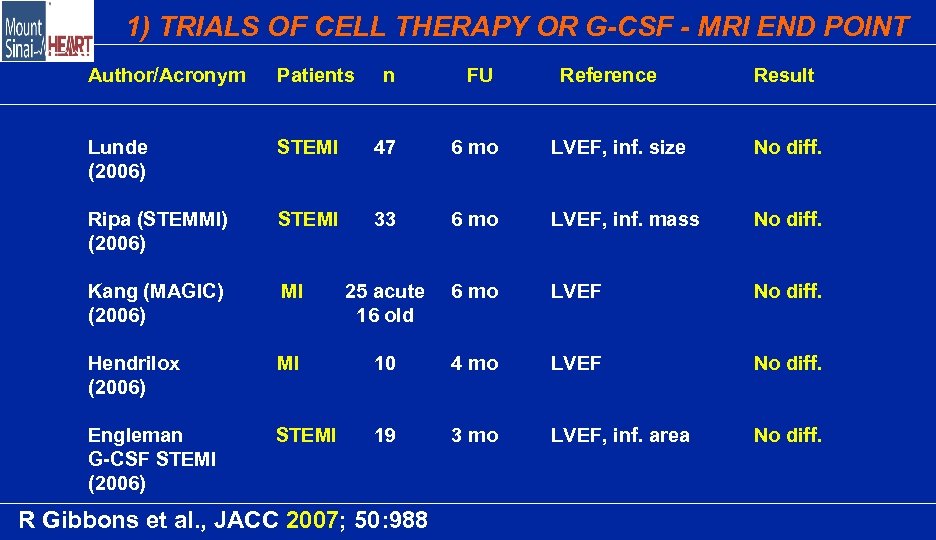 1) TRIALS OF CELL THERAPY OR G-CSF - MRI END POINT Author/Acronym Patients n FU Lunde (2006) STEMI 47 6 mo LVEF, inf. size No diff. Ripa (STEMMI) (2006) STEMI 33 6 mo LVEF, inf. mass No diff. Kang (MAGIC) (2006) MI 25 acute 16 old 6 mo LVEF No diff. Hendrilox (2006) MI 10 4 mo LVEF No diff. Engleman G-CSF STEMI (2006) STEMI 19 3 mo LVEF, inf. area No diff. R Gibbons et al. , JACC 2007; 50: 988 Reference Result
1) TRIALS OF CELL THERAPY OR G-CSF - MRI END POINT Author/Acronym Patients n FU Lunde (2006) STEMI 47 6 mo LVEF, inf. size No diff. Ripa (STEMMI) (2006) STEMI 33 6 mo LVEF, inf. mass No diff. Kang (MAGIC) (2006) MI 25 acute 16 old 6 mo LVEF No diff. Hendrilox (2006) MI 10 4 mo LVEF No diff. Engleman G-CSF STEMI (2006) STEMI 19 3 mo LVEF, inf. area No diff. R Gibbons et al. , JACC 2007; 50: 988 Reference Result
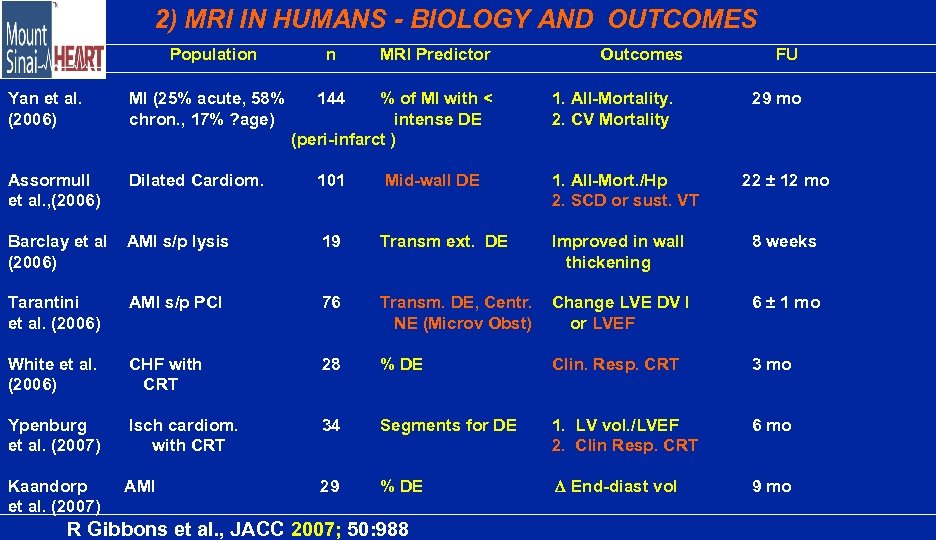 2) MRI IN HUMANS - BIOLOGY AND OUTCOMES Author Population n MRI Predictor 144 % of MI with < intense DE (peri-infarct ) Outcomes Yan et al. (2006) MI (25% acute, 58% chron. , 17% ? age) Assormull et al. , (2006) Dilated Cardiom. 101 Mid-wall DE 1. All-Mort. /Hp 2. SCD or sust. VT Barclay et al (2006) AMI s/p lysis 19 Transm ext. DE Improved in wall thickening 8 weeks Tarantini et al. (2006) AMI s/p PCI 76 Transm. DE, Centr. NE (Microv Obst) Change LVE DV I or LVEF 6 ± 1 mo White et al. (2006) CHF with CRT 28 % DE Clin. Resp. CRT 3 mo Ypenburg et al. (2007) Isch cardiom. with CRT 34 Segments for DE 1. LV vol. /LVEF 2. Clin Resp. CRT 6 mo Kaandorp et al. (2007) AMI 29 % DE End-diast vol 9 mo R Gibbons et al. , JACC 2007; 50: 988 1. All-Mortality. 2. CV Mortality FU 29 mo 22 ± 12 mo
2) MRI IN HUMANS - BIOLOGY AND OUTCOMES Author Population n MRI Predictor 144 % of MI with < intense DE (peri-infarct ) Outcomes Yan et al. (2006) MI (25% acute, 58% chron. , 17% ? age) Assormull et al. , (2006) Dilated Cardiom. 101 Mid-wall DE 1. All-Mort. /Hp 2. SCD or sust. VT Barclay et al (2006) AMI s/p lysis 19 Transm ext. DE Improved in wall thickening 8 weeks Tarantini et al. (2006) AMI s/p PCI 76 Transm. DE, Centr. NE (Microv Obst) Change LVE DV I or LVEF 6 ± 1 mo White et al. (2006) CHF with CRT 28 % DE Clin. Resp. CRT 3 mo Ypenburg et al. (2007) Isch cardiom. with CRT 34 Segments for DE 1. LV vol. /LVEF 2. Clin Resp. CRT 6 mo Kaandorp et al. (2007) AMI 29 % DE End-diast vol 9 mo R Gibbons et al. , JACC 2007; 50: 988 1. All-Mortality. 2. CV Mortality FU 29 mo 22 ± 12 mo
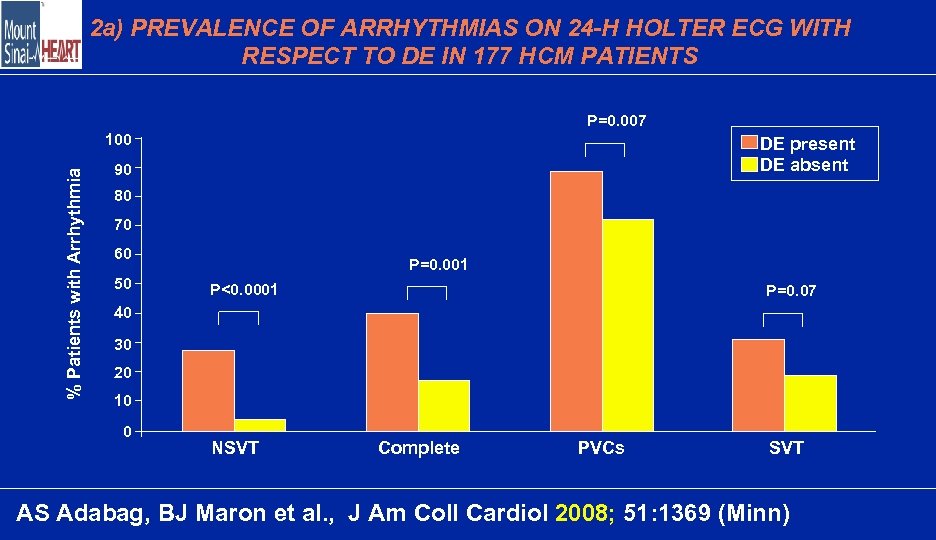 2 a) PREVALENCE OF ARRHYTHMIAS ON 24 -H HOLTER ECG WITH RESPECT TO DE IN 177 HCM PATIENTS P=0. 007 % Patients with Arrhythmia 100 DE present DE absent 90 80 70 60 50 P=0. 001 P<0. 0001 P=0. 07 40 30 20 10 0 NSVT Complete PVCs SVT AS Adabag, BJ Maron et al. , J Am Coll Cardiol 2008; 51: 1369 (Minn)
2 a) PREVALENCE OF ARRHYTHMIAS ON 24 -H HOLTER ECG WITH RESPECT TO DE IN 177 HCM PATIENTS P=0. 007 % Patients with Arrhythmia 100 DE present DE absent 90 80 70 60 50 P=0. 001 P<0. 0001 P=0. 07 40 30 20 10 0 NSVT Complete PVCs SVT AS Adabag, BJ Maron et al. , J Am Coll Cardiol 2008; 51: 1369 (Minn)
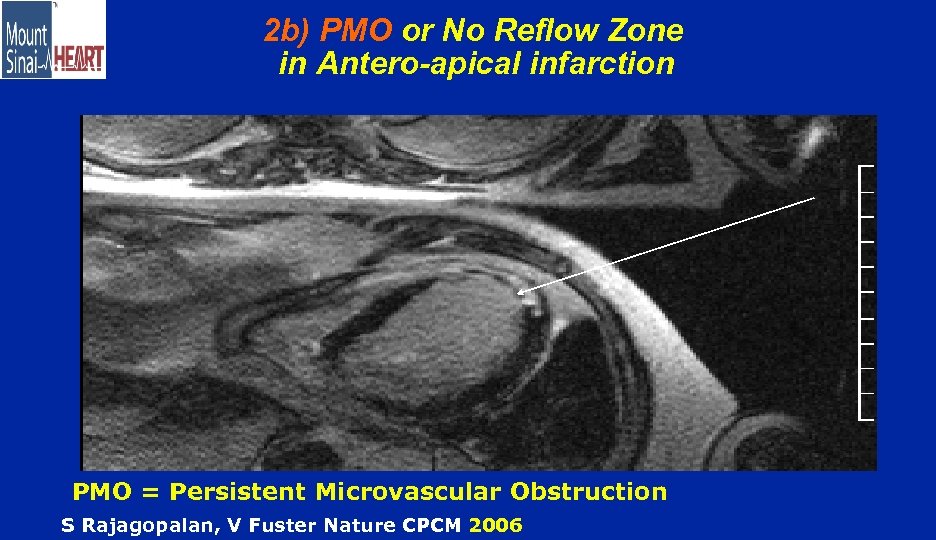 2 b) PMO or No Reflow Zone in Antero-apical infarction PMO = Persistent Microvascular Obstruction S Rajagopalan, V Fuster Nature CPCM 2006
2 b) PMO or No Reflow Zone in Antero-apical infarction PMO = Persistent Microvascular Obstruction S Rajagopalan, V Fuster Nature CPCM 2006
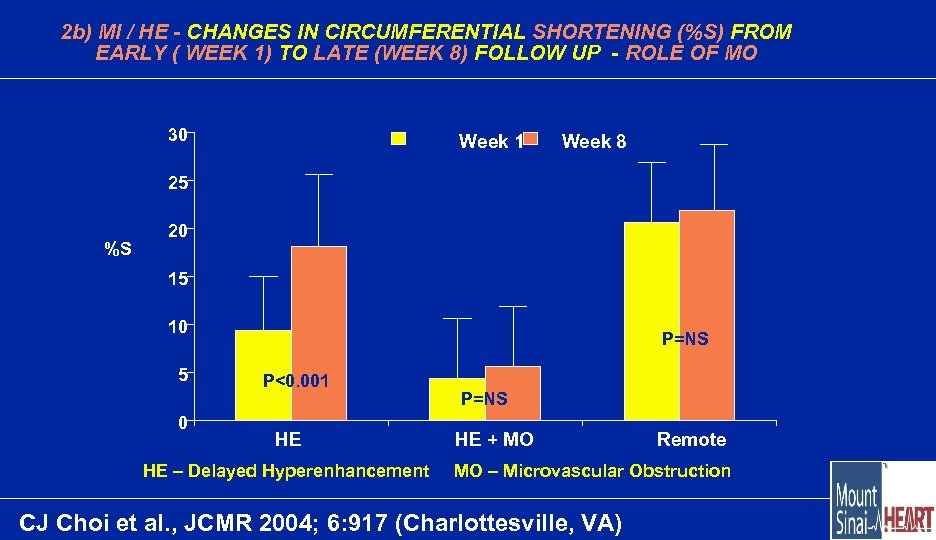 2 b) MI / HE - CHANGES IN CIRCUMFERENTIAL SHORTENING (%S) FROM EARLY ( WEEK 1) TO LATE (WEEK 8) FOLLOW UP - ROLE OF MO 30 Week 1 Week 8 25 %S 20 15 10 5 0 P=NS P<0. 001 HE HE – Delayed Hyperenhancement P=NS HE + MO Remote MO – Microvascular Obstruction CJ Choi et al. , JCMR 2004; 6: 917 (Charlottesville, VA)
2 b) MI / HE - CHANGES IN CIRCUMFERENTIAL SHORTENING (%S) FROM EARLY ( WEEK 1) TO LATE (WEEK 8) FOLLOW UP - ROLE OF MO 30 Week 1 Week 8 25 %S 20 15 10 5 0 P=NS P<0. 001 HE HE – Delayed Hyperenhancement P=NS HE + MO Remote MO – Microvascular Obstruction CJ Choi et al. , JCMR 2004; 6: 917 (Charlottesville, VA)
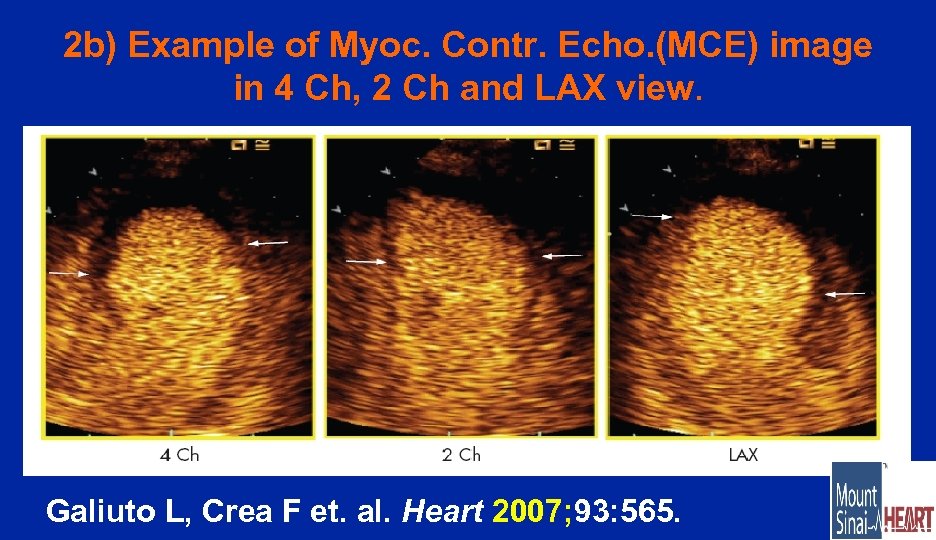 2 b) Example of Myoc. Contr. Echo. (MCE) image in 4 Ch, 2 Ch and LAX view. Galiuto L, Crea F et. al. Heart 2007; 93: 565.
2 b) Example of Myoc. Contr. Echo. (MCE) image in 4 Ch, 2 Ch and LAX view. Galiuto L, Crea F et. al. Heart 2007; 93: 565.
 2 b) Reversible Microvascular Dysfunction Coupled With Persistent Myocardial Dysfunction: Implications For Post. Infarct Left Ventricular Remodelling In 39 patients with a first MI who underwent successful PCI, microvascular dysfunction was studied by myocardial contrast echocardiography (MCE) at 24 h, 1 week and 3 months after the procedure. Improvement in microvascular dysfunction occurs early after MI, although it is not associated with a parallel improvement in wall motion but is beneficial in preventing left ventricular remodelling. Accordingly, 1 -week microvascular dysfunction is a powerful and independent predictor of left ventricular remodelling. L Galiuto, F Crea et al. , Heart 2007; 93: 565 (Rome)
2 b) Reversible Microvascular Dysfunction Coupled With Persistent Myocardial Dysfunction: Implications For Post. Infarct Left Ventricular Remodelling In 39 patients with a first MI who underwent successful PCI, microvascular dysfunction was studied by myocardial contrast echocardiography (MCE) at 24 h, 1 week and 3 months after the procedure. Improvement in microvascular dysfunction occurs early after MI, although it is not associated with a parallel improvement in wall motion but is beneficial in preventing left ventricular remodelling. Accordingly, 1 -week microvascular dysfunction is a powerful and independent predictor of left ventricular remodelling. L Galiuto, F Crea et al. , Heart 2007; 93: 565 (Rome)
 2 c) Impact of Collagen Type I Turnover on the Long-Term Response to Cardiac Resynchronization Rx Collagen type I turnover influences the long-term response to CRT. In addition, the ability of CRT to restore the balance between collagen type I synthesis and degradation is associated with a beneficial response. I García-Bolao, J Diez et al. , Eur Heart J 2008; 29: 898 (Pamplona, Spain)
2 c) Impact of Collagen Type I Turnover on the Long-Term Response to Cardiac Resynchronization Rx Collagen type I turnover influences the long-term response to CRT. In addition, the ability of CRT to restore the balance between collagen type I synthesis and degradation is associated with a beneficial response. I García-Bolao, J Diez et al. , Eur Heart J 2008; 29: 898 (Pamplona, Spain)
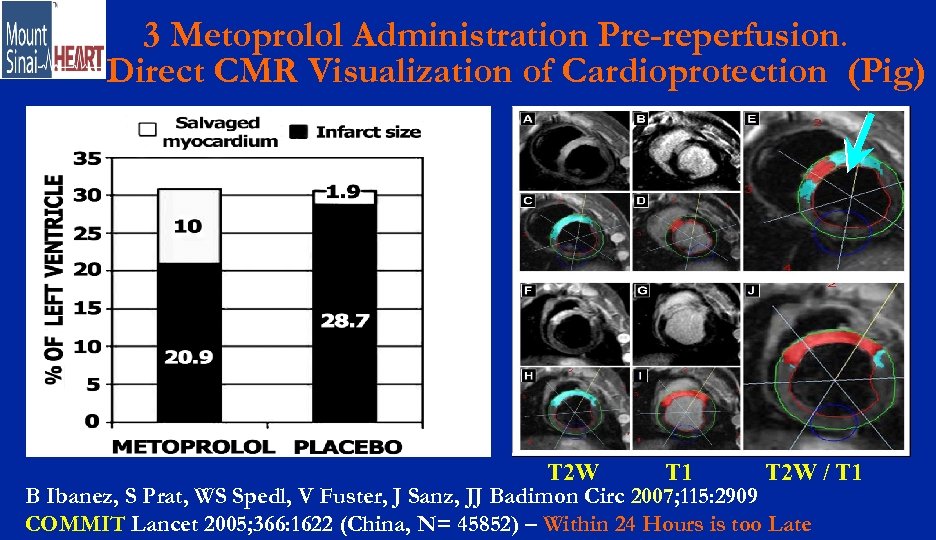 3 Metoprolol Administration Pre-reperfusion. Direct CMR Visualization of Cardioprotection (Pig) T 2 W T 1 T 2 W / T 1 B Ibanez, S Prat, WS Spedl, V Fuster, J Sanz, JJ Badimon Circ 2007; 115: 2909 COMMIT Lancet 2005; 366: 1622 (China, N= 45852) – Within 24 Hours is too Late
3 Metoprolol Administration Pre-reperfusion. Direct CMR Visualization of Cardioprotection (Pig) T 2 W T 1 T 2 W / T 1 B Ibanez, S Prat, WS Spedl, V Fuster, J Sanz, JJ Badimon Circ 2007; 115: 2909 COMMIT Lancet 2005; 366: 1622 (China, N= 45852) – Within 24 Hours is too Late
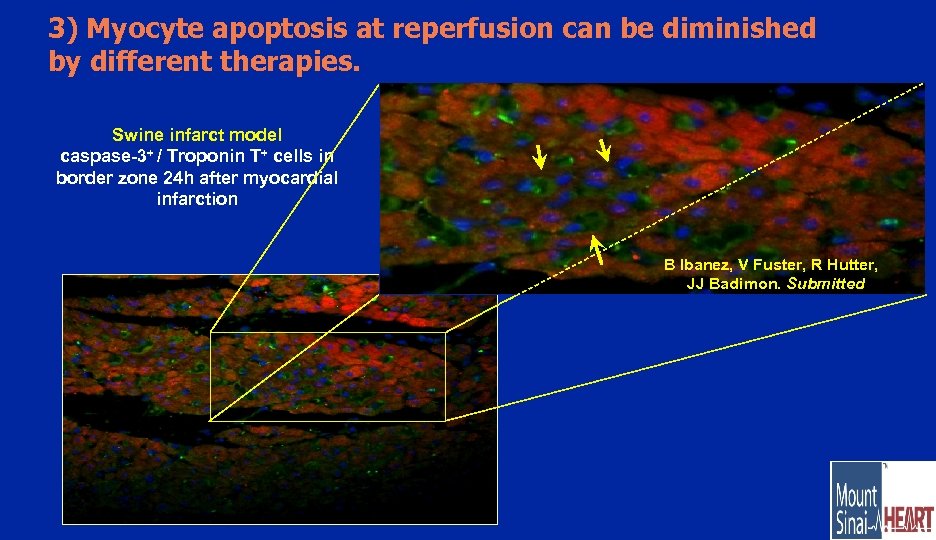 3) Myocyte apoptosis at reperfusion can be diminished by different therapies. Swine infarct model caspase-3+ / Troponin T+ cells in border zone 24 h after myocardial infarction B Ibanez, V Fuster, R Hutter, JJ Badimon. Submitted Focus on saving what is not already dead at reperfusion (but at risk of).
3) Myocyte apoptosis at reperfusion can be diminished by different therapies. Swine infarct model caspase-3+ / Troponin T+ cells in border zone 24 h after myocardial infarction B Ibanez, V Fuster, R Hutter, JJ Badimon. Submitted Focus on saving what is not already dead at reperfusion (but at risk of).
 CARDIOVASCULAR CELL AND GENE THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
CARDIOVASCULAR CELL AND GENE THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
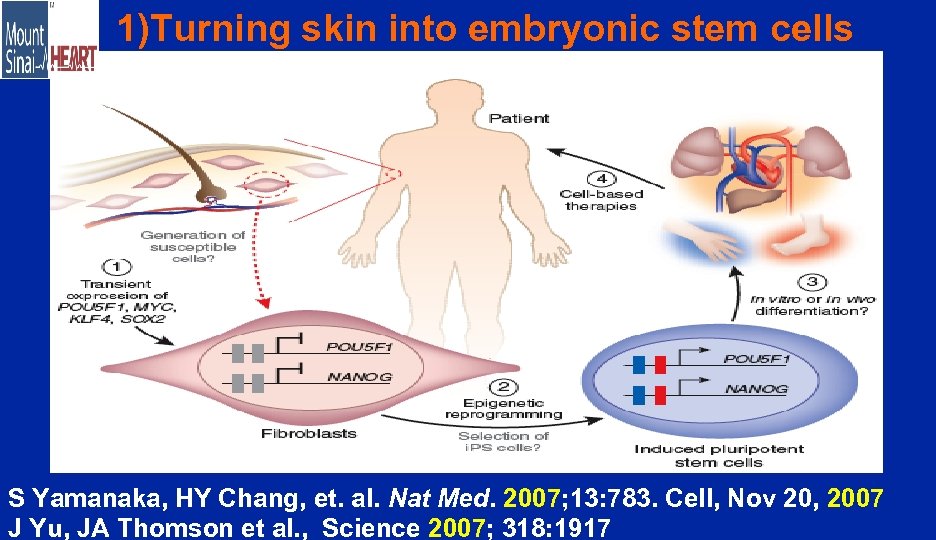 1)Turning skin into embryonic stem cells S Yamanaka, HY Chang, et. al. Nat Med. 2007; 13: 783. Cell, Nov 20, 2007 J Yu, JA Thomson et al. , Science 2007; 318: 1917
1)Turning skin into embryonic stem cells S Yamanaka, HY Chang, et. al. Nat Med. 2007; 13: 783. Cell, Nov 20, 2007 J Yu, JA Thomson et al. , Science 2007; 318: 1917
 Anyone can do it ? “Expertise in human embryonic stem- cell culture is absolutely critical. ” Everyone can have their own custom-tailored cells ? To use custom-made cells “would take a ridiculous amount of money” Very expensive— a desire that some companies will no doubt try to capitalize on. Cyranoski D. Nature 2008; 452: 406.
Anyone can do it ? “Expertise in human embryonic stem- cell culture is absolutely critical. ” Everyone can have their own custom-tailored cells ? To use custom-made cells “would take a ridiculous amount of money” Very expensive— a desire that some companies will no doubt try to capitalize on. Cyranoski D. Nature 2008; 452: 406.
 The cures are on their way ? Status: Too soon to tell Embryonic stem cells are the same as i. PS cells ? i. PS cells, no ethical issues ? Cyranoski D. Nature 2008; 452: 406. “If you can’t tell a difference between i. PS & embryonic stem cells, these will be a historical anomaly. ” “maybe worse ones. Someone might use i. PS cells to derive gametes— human reproductive cells”.
The cures are on their way ? Status: Too soon to tell Embryonic stem cells are the same as i. PS cells ? i. PS cells, no ethical issues ? Cyranoski D. Nature 2008; 452: 406. “If you can’t tell a difference between i. PS & embryonic stem cells, these will be a historical anomaly. ” “maybe worse ones. Someone might use i. PS cells to derive gametes— human reproductive cells”.
 2) Presence of Bona fide Cardiac Progenitors from Embryonic Stem Cells Is this the Ideal Cell For Regeneration?
2) Presence of Bona fide Cardiac Progenitors from Embryonic Stem Cells Is this the Ideal Cell For Regeneration?
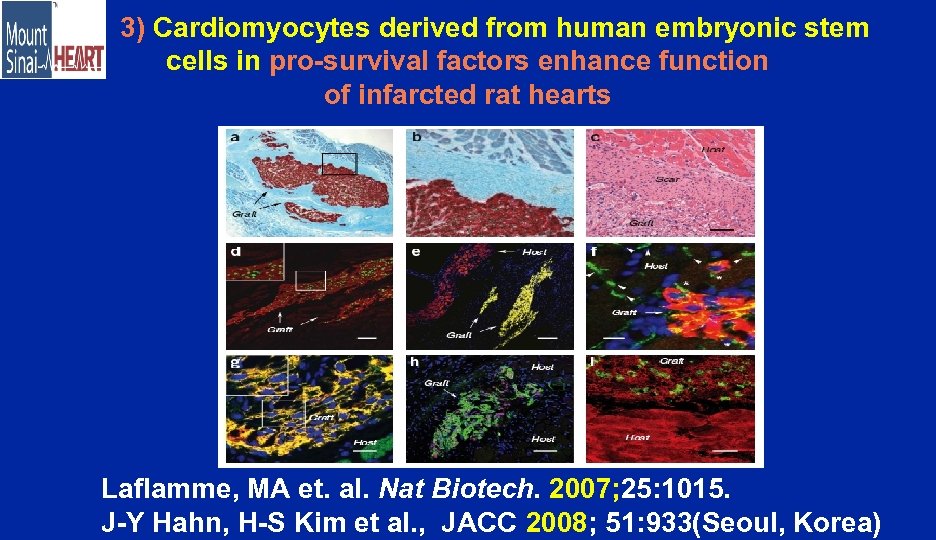 3) Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts Laflamme, MA et. al. Nat Biotech. 2007; 25: 1015. J-Y Hahn, H-S Kim et al. , JACC 2008; 51: 933(Seoul, Korea)
3) Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts Laflamme, MA et. al. Nat Biotech. 2007; 25: 1015. J-Y Hahn, H-S Kim et al. , JACC 2008; 51: 933(Seoul, Korea)
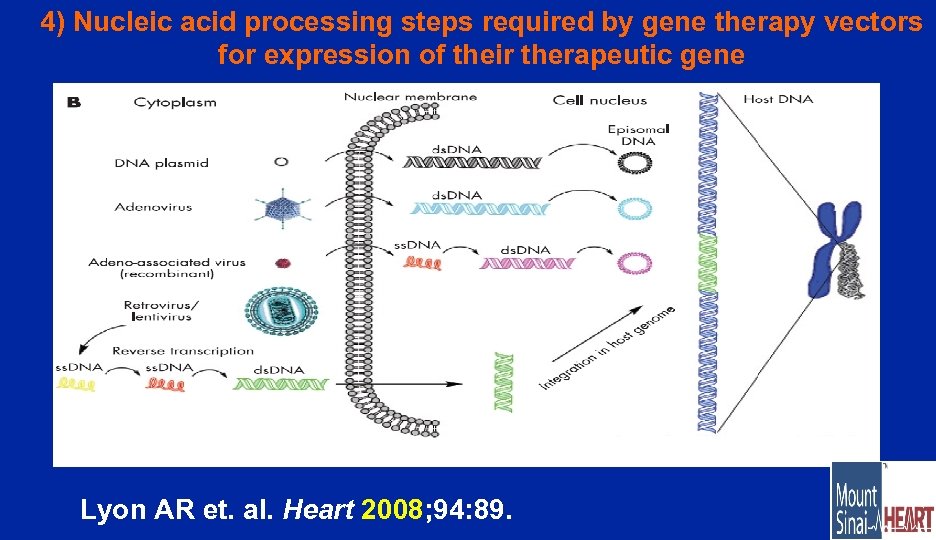 4) Nucleic acid processing steps required by gene therapy vectors for expression of their therapeutic gene Lyon AR et. al. Heart 2008; 94: 89.
4) Nucleic acid processing steps required by gene therapy vectors for expression of their therapeutic gene Lyon AR et. al. Heart 2008; 94: 89.
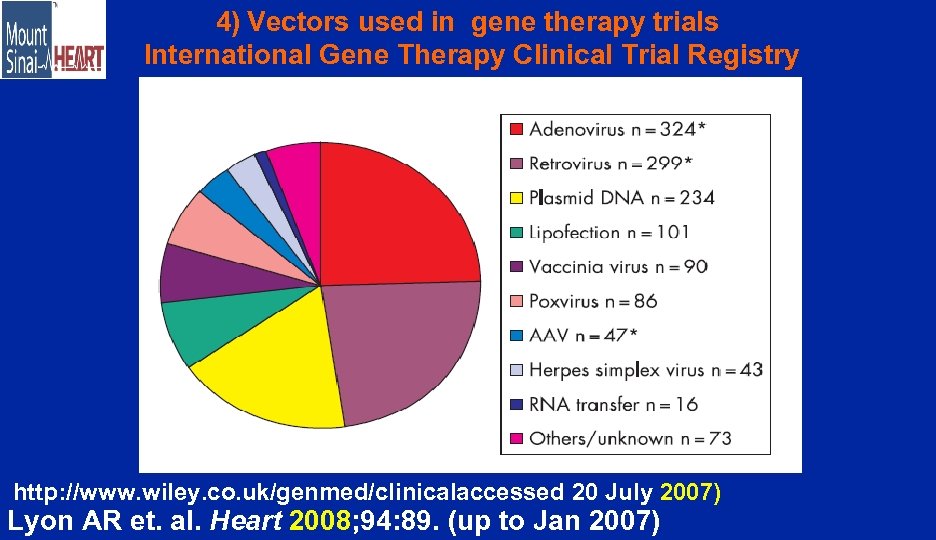 4) Vectors used in gene therapy trials International Gene Therapy Clinical Trial Registry http: //www. wiley. co. uk/genmed/clinicalaccessed 20 July 2007) Lyon AR et. al. Heart 2008; 94: 89. (up to Jan 2007)
4) Vectors used in gene therapy trials International Gene Therapy Clinical Trial Registry http: //www. wiley. co. uk/genmed/clinicalaccessed 20 July 2007) Lyon AR et. al. Heart 2008; 94: 89. (up to Jan 2007)
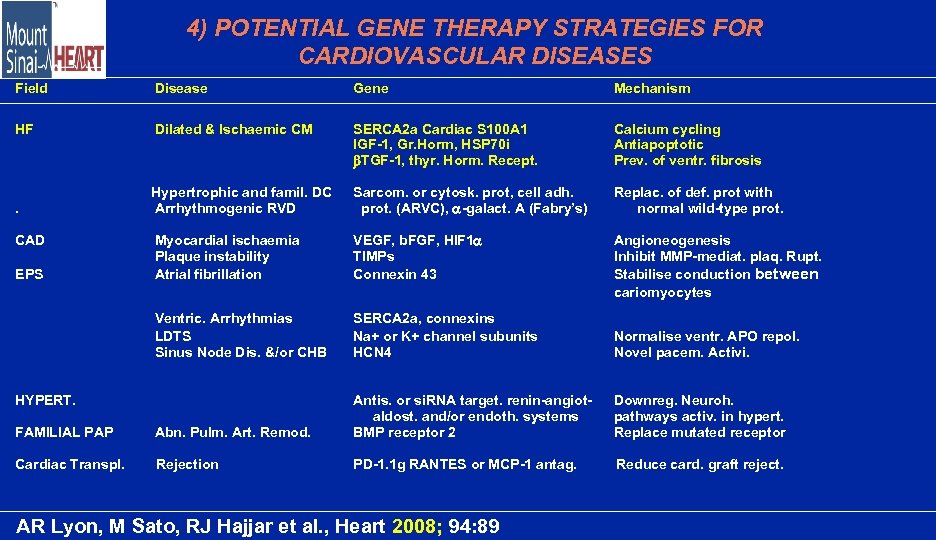 4) POTENTIAL GENE THERAPY STRATEGIES FOR CARDIOVASCULAR DISEASES Field Disease Gene Mechanism HF Dilated & Ischaemic CM SERCA 2 a Cardiac S 100 A 1 IGF-1, Gr. Horm, HSP 70 i TGF-1, thyr. Horm. Recept. Calcium cycling Antiapoptotic Prev. of ventr. fibrosis . Hypertrophic and famil. DC Arrhythmogenic RVD Sarcom. or cytosk. prot, cell adh. prot. (ARVC), -galact. A (Fabry’s) Replac. of def. prot with normal wild-type prot. Myocardial ischaemia Plaque instability Atrial fibrillation VEGF, b. FGF, HIF 1 TIMPs Connexin 43 Angioneogenesis Inhibit MMP-mediat. plaq. Rupt. Stabilise conduction between cariomyocytes Ventric. Arrhythmias LDTS Sinus Node Dis. &/or CHB SERCA 2 a, connexins Na+ or K+ channel subunits HCN 4 Normalise ventr. APO repol. Novel pacem. Activi. FAMILIAL PAP Abn. Pulm. Art. Remod. Antis. or si. RNA target. renin-angiotaldost. and/or endoth. systems BMP receptor 2 Downreg. Neuroh. pathways activ. in hypert. Replace mutated receptor Cardiac Transpl. Rejection PD-1. 1 g RANTES or MCP-1 antag. Reduce card. graft reject. CAD EPS HYPERT. AR Lyon, M Sato, RJ Hajjar et al. , Heart 2008; 94: 89
4) POTENTIAL GENE THERAPY STRATEGIES FOR CARDIOVASCULAR DISEASES Field Disease Gene Mechanism HF Dilated & Ischaemic CM SERCA 2 a Cardiac S 100 A 1 IGF-1, Gr. Horm, HSP 70 i TGF-1, thyr. Horm. Recept. Calcium cycling Antiapoptotic Prev. of ventr. fibrosis . Hypertrophic and famil. DC Arrhythmogenic RVD Sarcom. or cytosk. prot, cell adh. prot. (ARVC), -galact. A (Fabry’s) Replac. of def. prot with normal wild-type prot. Myocardial ischaemia Plaque instability Atrial fibrillation VEGF, b. FGF, HIF 1 TIMPs Connexin 43 Angioneogenesis Inhibit MMP-mediat. plaq. Rupt. Stabilise conduction between cariomyocytes Ventric. Arrhythmias LDTS Sinus Node Dis. &/or CHB SERCA 2 a, connexins Na+ or K+ channel subunits HCN 4 Normalise ventr. APO repol. Novel pacem. Activi. FAMILIAL PAP Abn. Pulm. Art. Remod. Antis. or si. RNA target. renin-angiotaldost. and/or endoth. systems BMP receptor 2 Downreg. Neuroh. pathways activ. in hypert. Replace mutated receptor Cardiac Transpl. Rejection PD-1. 1 g RANTES or MCP-1 antag. Reduce card. graft reject. CAD EPS HYPERT. AR Lyon, M Sato, RJ Hajjar et al. , Heart 2008; 94: 89
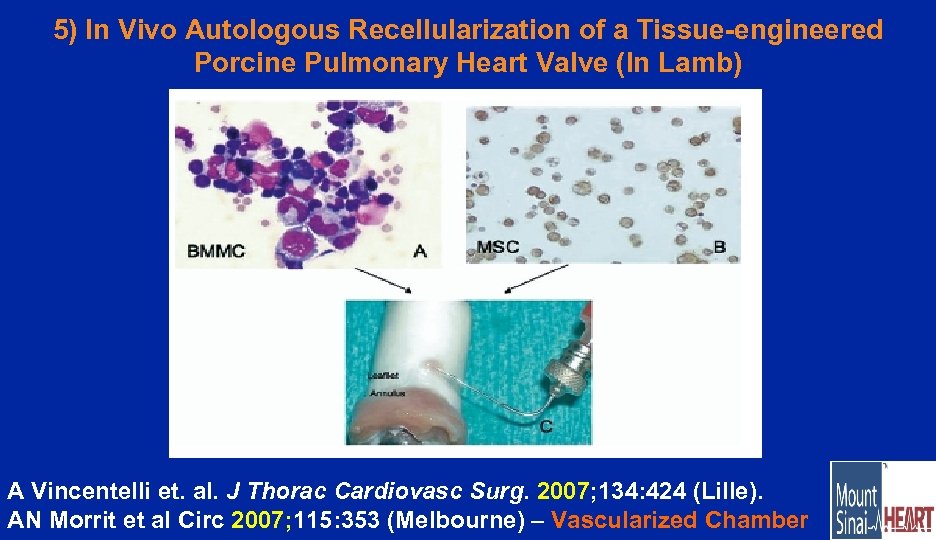 5) In Vivo Autologous Recellularization of a Tissue-engineered Porcine Pulmonary Heart Valve (In Lamb) A Vincentelli et. al. J Thorac Cardiovasc Surg. 2007; 134: 424 (Lille). AN Morrit et al Circ 2007; 115: 353 (Melbourne) – Vascularized Chamber
5) In Vivo Autologous Recellularization of a Tissue-engineered Porcine Pulmonary Heart Valve (In Lamb) A Vincentelli et. al. J Thorac Cardiovasc Surg. 2007; 134: 424 (Lille). AN Morrit et al Circ 2007; 115: 353 (Melbourne) – Vascularized Chamber
 CARDIOVASCULAR GENE AND CELL THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
CARDIOVASCULAR GENE AND CELL THERAPY “RISKY” AND “EXCITING” • Historical Notes – Feasibility, Disappoitments • Observing the Protocols – Heterogeneity, End Points • Stem Cells – Origin, Release, Homing, Target Function • Imaging Technology - Large Experimental Animals • Stimulating Future - Integration of gene / Cell Therapy • Issues for Caution - Tumors, Ethics, Media
 1) Long-Term Caution (Animals) Most preclinical studies only examined a limited time window of events in the foster millieu and hence may fail to detect insidious, potentially prohibitive side effects (i. e. , Teratomas). Careful monitoring of transdifferentiation and fusion events as well as disruptive or neoplastic growth patterns will be critically important before ES cell-derived regenerative treatments can be realistically considered. S Janssens. Heart 2007; 93: 1173 J Leor et al. , Heart 2007; 93: 1278 F Cao et al. , Circ 2006; 113: 1005
1) Long-Term Caution (Animals) Most preclinical studies only examined a limited time window of events in the foster millieu and hence may fail to detect insidious, potentially prohibitive side effects (i. e. , Teratomas). Careful monitoring of transdifferentiation and fusion events as well as disruptive or neoplastic growth patterns will be critically important before ES cell-derived regenerative treatments can be realistically considered. S Janssens. Heart 2007; 93: 1173 J Leor et al. , Heart 2007; 93: 1278 F Cao et al. , Circ 2006; 113: 1005
 2) ETHICS AND ANIMAL-HUMAN HYBRID-EMBRYO RESEARCH • There is a great difference between creating stem-cell lines for research or using cytoplasmic-hybrid embryos and bringing true animal-human hybrids to term. • The promise of this research is significant. Somatic-cell nuclear transfer will allow the production of stem cells that will enable us to develop new treatments, but the ethical questions are inmense and deserve open discussion. The Lancet 2007; 370: 909
2) ETHICS AND ANIMAL-HUMAN HYBRID-EMBRYO RESEARCH • There is a great difference between creating stem-cell lines for research or using cytoplasmic-hybrid embryos and bringing true animal-human hybrids to term. • The promise of this research is significant. Somatic-cell nuclear transfer will allow the production of stem cells that will enable us to develop new treatments, but the ethical questions are inmense and deserve open discussion. The Lancet 2007; 370: 909
 3) Stem Cell Therapy is Available Now The Institute of Cellular Medicine (ICM) is currently accepting patients with the following conditions for stem cell therapy: 1) ALS 2)Autism 3) Autoimmune Diseases 4) Cardiovascular Disease 5) Cerebral Palsy 6) Diabetes Type 2 7) Multiple Sclerosis 8) Parkinson's Disease 9) Rheumatoid Arthritis 10)Stroke
3) Stem Cell Therapy is Available Now The Institute of Cellular Medicine (ICM) is currently accepting patients with the following conditions for stem cell therapy: 1) ALS 2)Autism 3) Autoimmune Diseases 4) Cardiovascular Disease 5) Cerebral Palsy 6) Diabetes Type 2 7) Multiple Sclerosis 8) Parkinson's Disease 9) Rheumatoid Arthritis 10)Stroke


