06c031133002df6f079ae9f543e963d1.ppt
- Количество слайдов: 28

Presented by Georgia Institute of Technology Institutional Review Board Presented by Melanie Clark, CIP Associate Director Office of Research Integrity Assurance All rights reserved GTRC
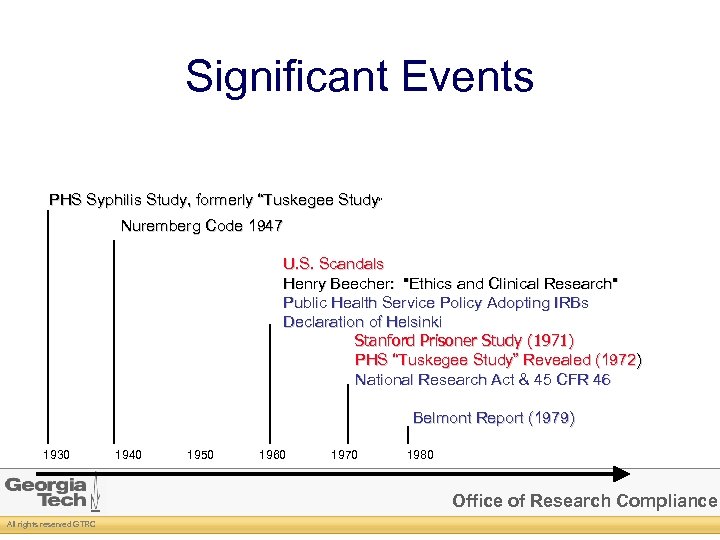
Significant Events PHS Syphilis Study, formerly “Tuskegee Study” Nuremberg Code 1947 U. S. Scandals Henry Beecher: "Ethics and Clinical Research" Public Health Service Policy Adopting IRBs Declaration of Helsinki Stanford Prisoner Study (1971) PHS “Tuskegee Study” Revealed (1972) National Research Act & 45 CFR 46 Belmont Report (1979) 1930 1940 1950 1960 1970 1980 Office of Research Compliance All rights reserved GTRC
Office of Research Compliance All rights reserved GTRC

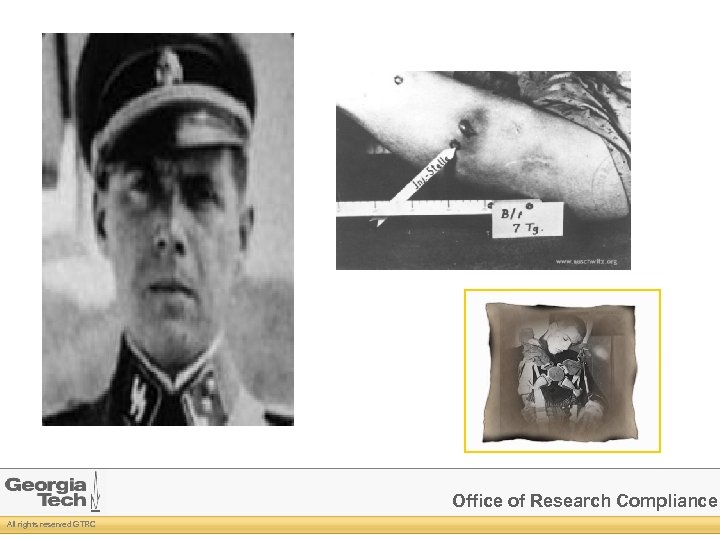
Military Tribunal led to Nuremberg Code Voluntary consent Anticipated scientific benefits Benefits outweigh risks Animal experiments first No intentional death or disability Subjects may withdraw at anytime Investigators must be qualified Investigators will stop if harm occurs Office of Research Compliance All rights reserved GTRC

Office of Research Compliance All rights reserved GTRC

1950 s: Willowbrook State School Staten Island, New York 1960 s: Jewish Chronic Disease Hospital Cancer Experiments Office of Research Compliance All rights reserved GTRC
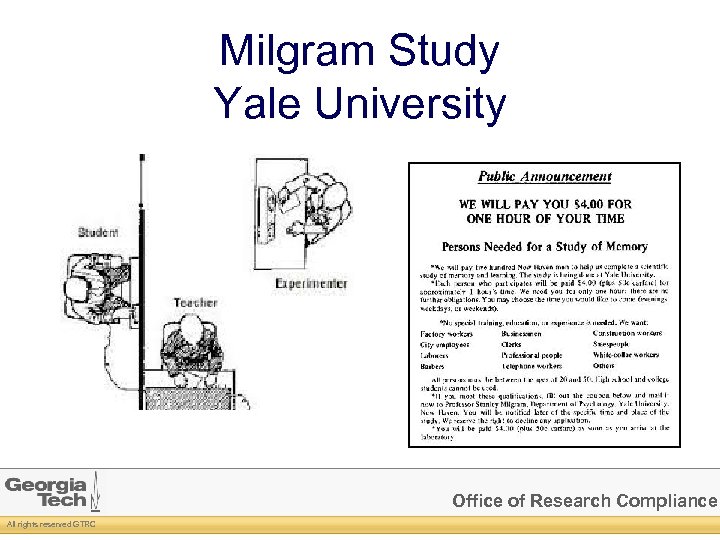
Milgram Study Yale University Office of Research Compliance All rights reserved GTRC

Stanford Prison Experiment www. prisonexp. org Office of Research Compliance All rights reserved GTRC

1932, Public Health Service study, in collaboration with the Tuskegee Institute in Macon County, Alabama, undertook a study of untreated syphilis in hopes of justifying treatment programs for blacks. 600 black men (399 with syphilis, 201 without) were told they had "bad blood, " a local term used to describe syphilis, anemia, and fatigue. Penicillin, treatment of choice, was discovered in 1940 s but was withheld. In exchange for taking part in the study, the men received free medical exams, free meals, and burial insurance. Although originally projected to last 6 months, the study actually went on for 40 years. Office of Research Compliance All rights reserved GTRC

Ethical Principles and Guidelines for the Protection of Human Subjects of Research Respect for Persons Individual autonomy Protection of individuals with reduced autonomy Beneficence Maximize benefits and minimize harms Justice Equitable distribution of research costs and benefits The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research April 18, 1979 Office of Research Compliance All rights reserved GTRC
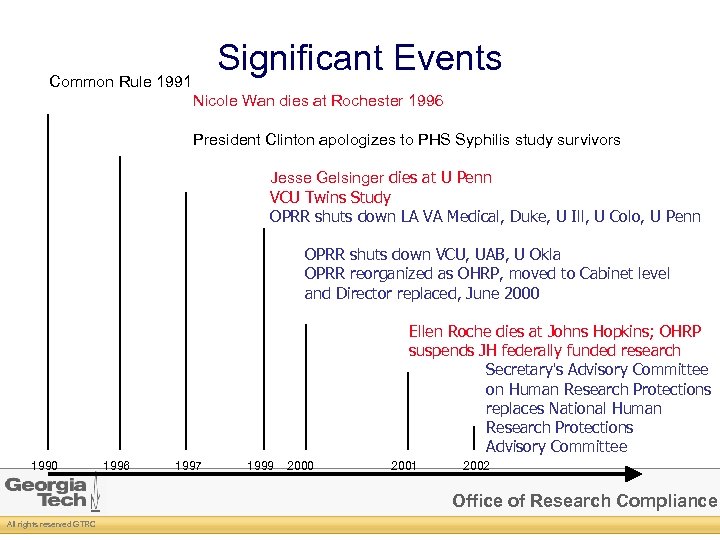
Significant Events Common Rule 1991 Nicole Wan dies at Rochester 1996 President Clinton apologizes to PHS Syphilis study survivors Jesse Gelsinger dies at U Penn VCU Twins Study OPRR shuts down LA VA Medical, Duke, U Ill, U Colo, U Penn OPRR shuts down VCU, UAB, U Okla OPRR reorganized as OHRP, moved to Cabinet level and Director replaced, June 2000 Ellen Roche dies at Johns Hopkins; OHRP suspends JH federally funded research Secretary's Advisory Committee on Human Research Protections replaces National Human Research Protections Advisory Committee 1990 1996 1997 1999 2000 2001 2002 Office of Research Compliance All rights reserved GTRC

1999: Jesse Gelsinger & University of Pennsylvania 18 -year old volunteer in U Penn’s gene therapy study of partial omithine transcarbamylase deficiency (OTC) Office of Research Compliance All rights reserved GTRC

2001: Ellen Roche & Johns Hopkins Healthy lab technician volunteered for asthma study of hexamethonium bromide. Respiratory effects of hexamethonium bromide were not known to investigator prior to study. Previous participant had respiratory problems, solution was changed without IRB being informed. Johns Hopkins forced to halt $300 million in medical research Ellen Roche died. Compensation for her participation: $365. Johns Hopkins settled out of court with her family. Office of Research Compliance All rights reserved GTRC

Office of Research Compliance All rights reserved GTRC

Oh No! The Feds Shut Us Down Office of Research Compliance All rights reserved GTRC

What Happens When the Research Enterprise is Shut Down All federal research funding involving human subjects suspended (payroll, operations, etc. ) All experiments halted (except to treat enrolled subjects on a case-by-case basis) IRB must re-review all protocols Millions of dollars in lost revenue and expenses to bring institution into compliance Office of Research Compliance All rights reserved GTRC

Office of Research Compliance All rights reserved GTRC

What is an IRB? Faculty committee with the purpose of reviewing research activities involving human participants Constituted in accordance with federal law: Minimum of five members Community representation Scientific and non-scientific members Adequate expertise to review the proposed work Consultants, when needed Office of Research Compliance All rights reserved GTRC

What is a human subject? As defined by 45 CFR 46 Is a living individual about whom an investigator obtains either: Data through intervention or interaction with the individual; or Identifiable private information Office of Research Compliance All rights reserved GTRC

What is research? Research: As defined by 45 CFR 46, "a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge" Office of Research Compliance All rights reserved GTRC
Categories of Research Exempt Expedited Full Office of Research Compliance All rights reserved GTRC

What you need to submit IRB application IRBWISE Project description Consent/Assent/Parental Permission All recruitment flyers & scripts Human Subjects Training Certificate Questionnaire, surveys or interview guides Office of Research Compliance All rights reserved GTRC

§ 46. 116 General requirements for informed consent Statement that it is research Purpose & procedures Health and financial risk Compensation/costs Confidentiality In case of injury Subjects rights Appropriate reading level Office of Research Compliance All rights reserved GTRC

Anything Else? All Amendments must be submitted to IRB for review and approval prior to commencement. Any change to the protocol or consent form All Adverse Events must be submitted for IRB review even if they are not study related. Office of Research Compliance All rights reserved GTRC

Principal Investigator’s RESPONSIBILITIES Obtain approval prior to start of research Inform IRB of: Adverse events Changes in protocol Violations Continuing review request Obtain consent prior to enrolling subjects Office of Research Compliance All rights reserved GTRC

Case Study “Check List” Johns Hopkins University Intensive Care Units in State of Michigan Office for Human Research Protections Office of Research Compliance All rights reserved GTRC

Important Information http: //www. researchintegrity. gatech. edu IRBWISE information Human Subjects Training (CITI) Policy and Procedures Consent templates Office of Research Compliance All rights reserved GTRC

Contact Information Melanie Clark, Associate Director melanie. clark@gtrc. gatech. edu 404 -894 -6942 Kelly Winn, Research Associate Kelly. winn@gtrc. gatech. edu 404 -385 -2175 Barbara Henry, Director barbara. henry@gtrc. gatech. edu 404 -894 -6949 Dennis Folds, IRB Chair dennis. folds@gtri. gatech. edu 404 -407 -7262 Office of Research Compliance All rights reserved GTRC
06c031133002df6f079ae9f543e963d1.ppt