7876ed36e2d4524e240f1f1bb38a51a3.ppt
- Количество слайдов: 36

Presented by: Alex Shneider, Ph. D. , Founder and CEO Gene Froelich, CFO/COO Jeffery East, EVP of Business Development http: //curelaboncology. com/ +1 -609 -841 -1201 +7 -905 -570 -3725 s

2

Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995. In accordance with the safe harbor provisions of the Private Securities Litigation reform Act of 1995, the Company notes that statements in this web site, and elsewhere, that look forward in time, which include everything other than historical information, involve risks and uncertainties that may affect the Company's actual results of operations. The following important factors could cause actual results to differ materially from those set forth in the forward-looking statements: project financing; new scientific findings; our products may not be accepted by the market; and we may have difficulty in hiring and retaining key personnel. 3

Cure Lab Oncology Mission To overcome the worldwide problem of cancer by developing and utilizing novel anti-cancer vaccines and oncolytic viruses 4
Cure Lab’s Executive Summary Competitive advantage Ø Novel oncolytic virus and product pipeline Ø Novel anticancer immunostimulant and the product pipeline Ø Novel technologies to increase vaccine and oncolytic virus efficiency Ø Anti-cancer vaccine and oncolytic virus may eventually merge into a single product Anticipated Investment and Results by 2015 Ø $10 M of investment Ø Finalize clinical testing in the Former USSR Ø Licensing to big pharma for >$1 B Valuation of the Company by mid-2014 Ø >1 B Ø Multiple of 100 X 5

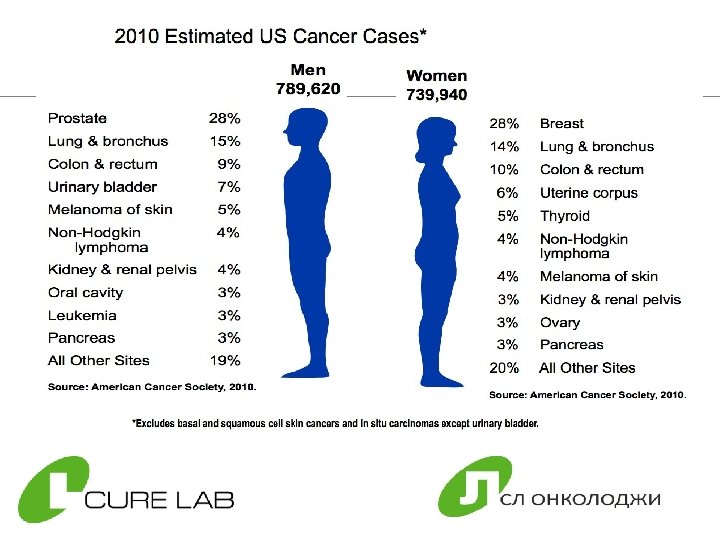
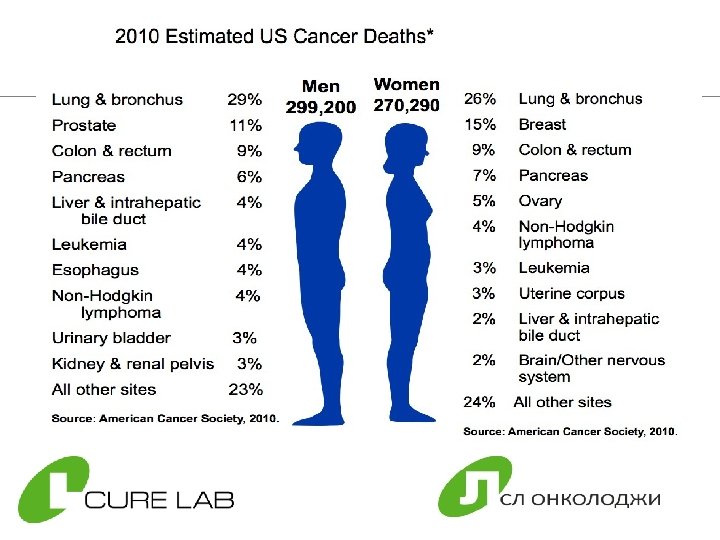
Cancer in Russia Ø 300, 000 people die each year due to cancer Ø 2, 500, 000 people are diagnosed with cancer ØFrom 1997 to 2007 the number of cancer patients has increased 13% 6

Oncolytic Viruses – viruses that selectively destroy tumors without impacting normal tissue Ø Benefits § § Selectivity Effective tumor removal Limited or no side-effects Cost-efficiency Ø For some cancers, oncolytic viruses are the only life-saving option Ø China has approved world's first oncolytic virus therapy for cancer treatment 9
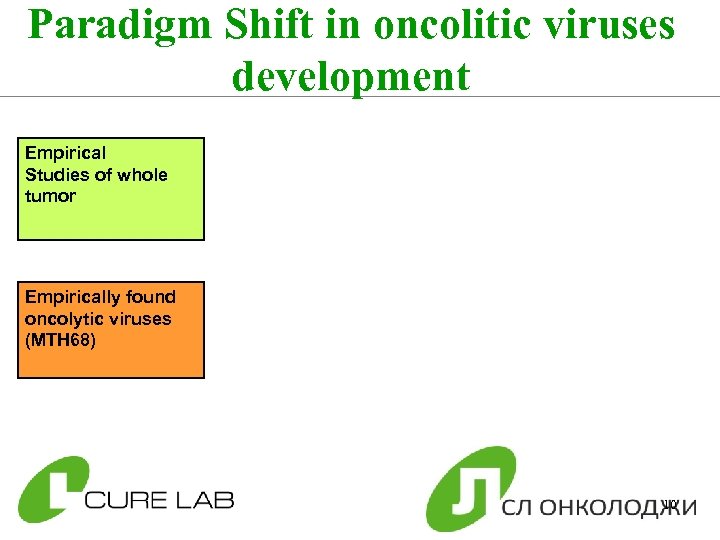
Paradigm Shift in oncolitic viruses development Empirical Studies of whole tumor Empirically found oncolytic viruses (MTH 68) 10
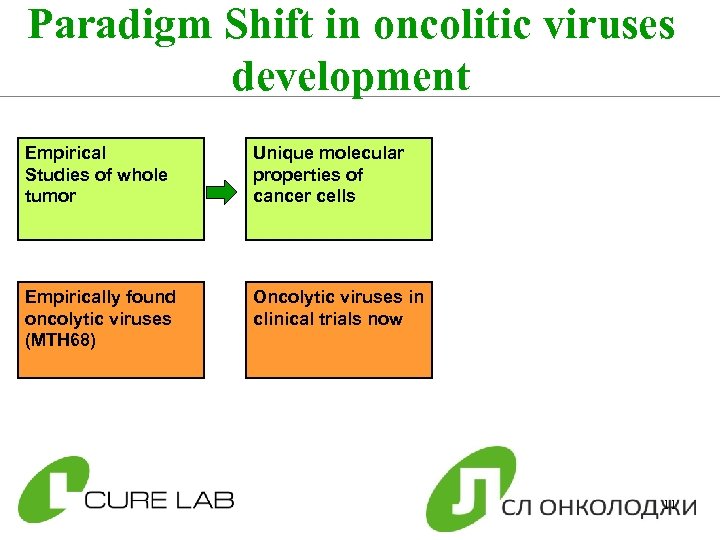
Paradigm Shift in oncolitic viruses development Empirical Studies of whole tumor Unique molecular properties of cancer cells Empirically found oncolytic viruses (MTH 68) Oncolytic viruses in clinical trials now 11
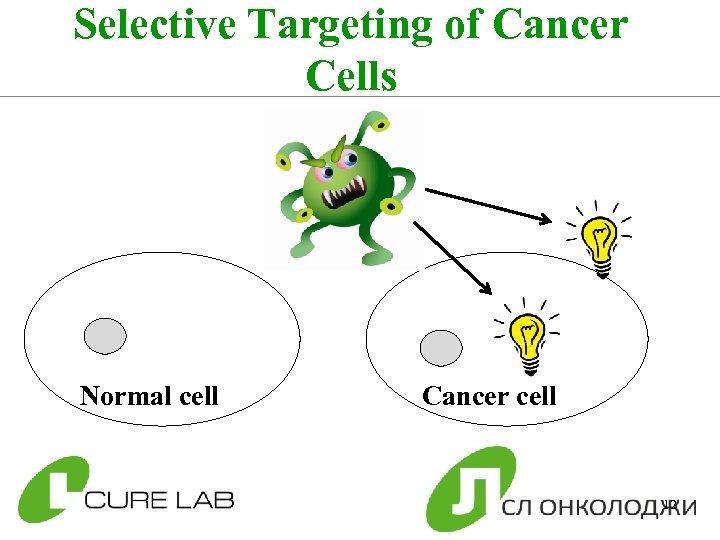
Selective Targeting of Cancer Cells Normal cell Cancer cell 12
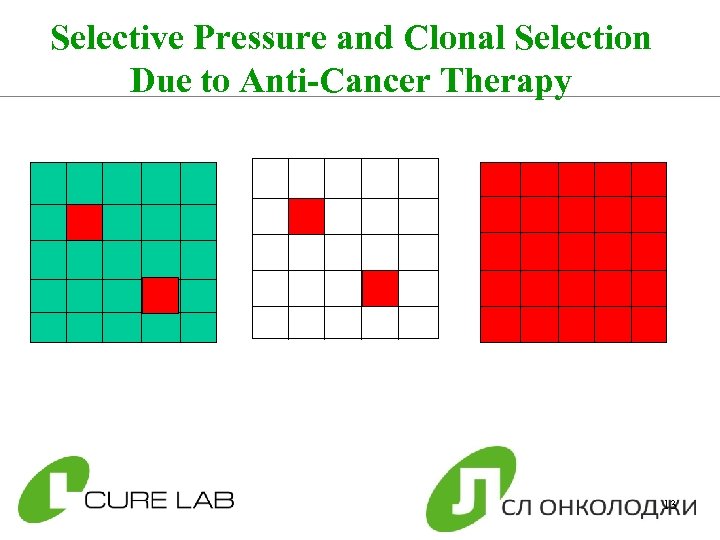
Selective Pressure and Clonal Selection Due to Anti-Cancer Therapy 13
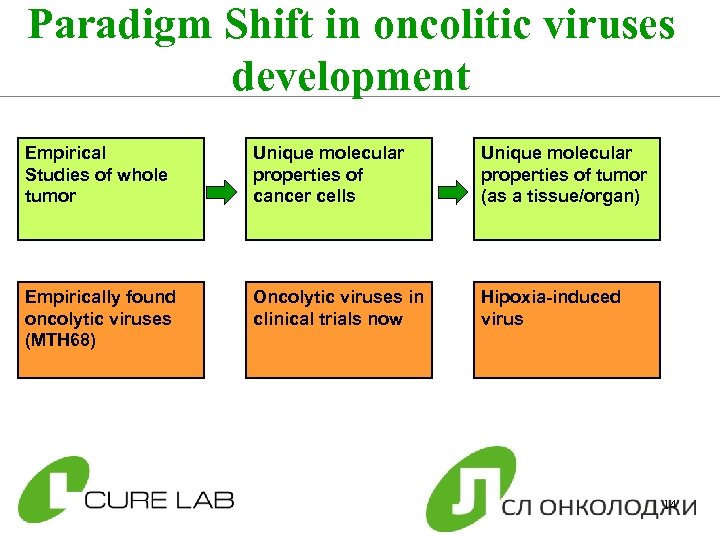
Paradigm Shift in oncolitic viruses development Empirical Studies of whole tumor Unique molecular properties of cancer cells Unique molecular properties of tumor (as a tissue/organ) Empirically found oncolytic viruses (MTH 68) Oncolytic viruses in clinical trials now Hipoxia-induced virus 14

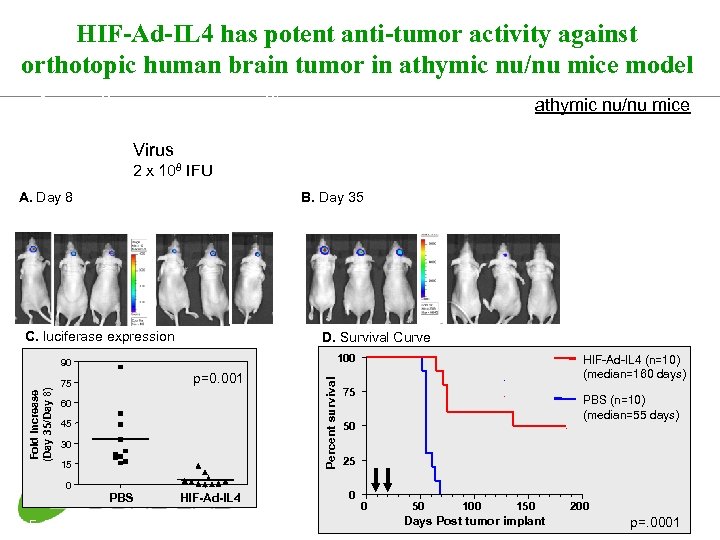
HIF-Ad-IL 4 has potent anti-tumor activity against orthotopic human brain tumor in athymic nu/nu mice model 1 8 implant tumor i. c. image 35 Virus B. Day 35 HIF-Ad-IL 4 C. luciferase expression PBS HIF-Ad-IL 4 D. Survival Curve p=0. 001 75 60 45 30 15 PBS HIF-Ad-IL 4 D. E. Post et al. , unpublished data Percent survival 100 90 Fold Increase (Day 35/Day 8) survival image 2 x 108 IFU A. Day 8 PBS 0 athymic nu/nu mice HIF-Ad-IL 4 (n=10) (median=160 days) 75 PBS (n=10) (median=55 days) 50 25 0 0 50 100 150 Days Post tumor implant 200 p=. 0001

Kidney Caner in Russia Ø 17, 600 diagnosed cases in 2008 Ø 47% increase compared with 2000 ØSignificant increase in disease is reported for patients starting 35 -39 years old and max at 65 -69 ØHigh level of HIF (hypoxia induced factor), which activated our oncolytic virus. This makes the project of relatively low risk. 17

Prostate Cancer in Russia Ø 1 out of 7 men over 50 suffer prostate cancer Ø>15% lethality rate ØCurrent treatment methods lead to impotency 18

Breast Cancer in Russia Ø 50, 000 women per year suffer breast cancer Ø 41% of the cases are diagnosed at late stages Ø 20, 000 women die each year 19
Cancer Vaccines Induce tumor-specific protective immune response Ø Anti-tumor therapeutic agents Ø Preventive means to preclude tumor relapse after surgical removal Ø Future use: preventive vaccination for high risk groups (genetic markers, family history, occupational hazard) 20
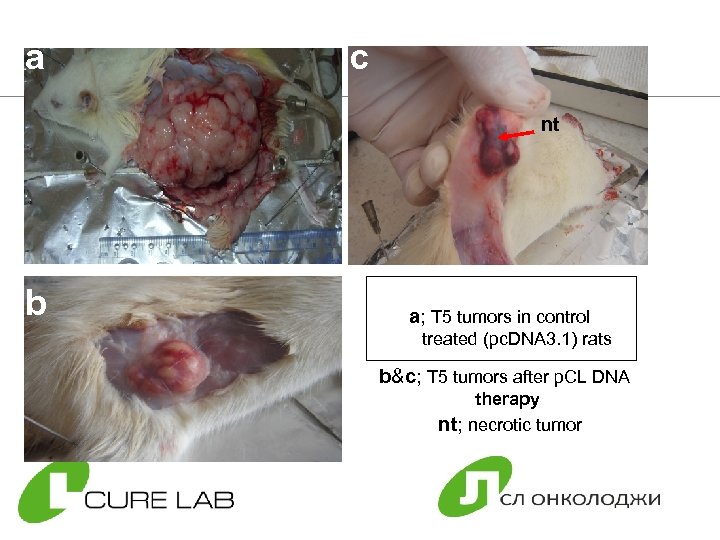
a c nt b a; T 5 tumors in control treated (pc. DNA 3. 1) rats b&c; T 5 tumors after p. CL DNA therapy nt; necrotic tumor

Intellectual Property Ø Issued US, Australian and European patents for the product (both umbrella and application coverage) Ø Russia patent application submitted with Skolkovo’s assistance Ø A pipeline of the follow-up patent applications to be submitted for product modifications Ø Cure Lab’s technological and clinical know-how developed over the years 22

US Market Size for Cancer Drugs Ø$40. 3 B in 1990 Ø$216 B in 2006 23
Market Comparables (Yervoy) Antibody blocking CTLA-4 inactivating lymphocytes Ø CTLA-4 discovered in UC Berkley in the mid 1990 th. No pharm company was interested in “too new” idea. Ø 1998 Berkley licenses it to Ne. Xstar Pharmaceuticals Ø 1999 Ne. Xstar Pharmaceuticals sublicenses it to Medarex for $8. 5 M Ø 2009 Medarex acquired by Bristol-Myers for $2. 4 B based on its anti-CTLA-4 antibody product (no clinical trial results yet) 24
Market Comparables Bevacizumab (Avastin) Ø Monoclonal antibody blocking growth of blood vessels in tumors (Genentech ). Ø Used for colon, breast and lung cancer. Ø $4, 000 -9, 000/moth/patient Ø Insurance paid $100, 000/year per patient Ø 2007 sales: Ø $2. 3 B USA Ø $3. 5 B worldwide Ø Prolongs life for 4. 7 month in average 25

Competition Ø Ø Developers of chemical anti-cancer drugs Developers of biological anti-cancer drugs Anti-cancer vaccines Other companies working on oncolytic viruses (Israel, Japan, USA, EU) “Cancer” is a composite name for multiple diseases (different mechanisms with similar clinical manifestations). Thus, there are several applications for our approach with no real competition but with compelling market need. Major pharmaceutical companies run out of patent protection on their anti-cancer drugs. Thus, demand for new anti-cancer medicine greatly exceeds supply. Different treatments are typically used in combination, thus they do not compete but complement each other 26
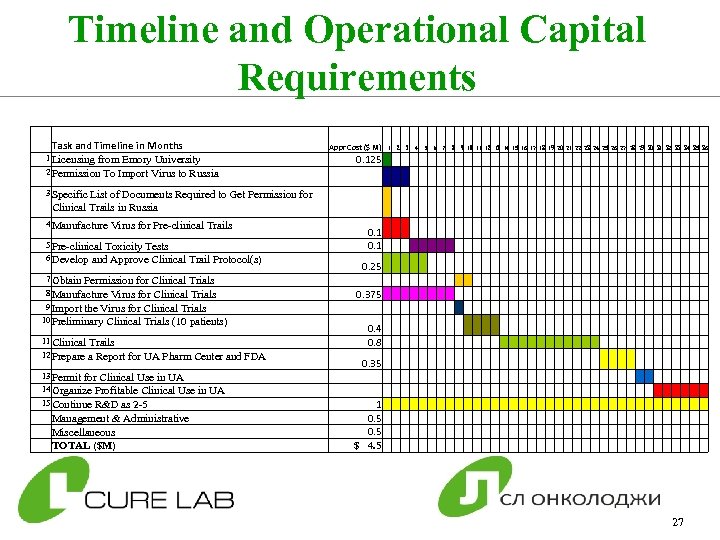
Timeline and Operational Capital Requirements Task and Timeline in Months 1 Licensing from Emory University 2 Permission To Import Virus to Russia Appr Cost ($ M) 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 0. 125 3 Specific List of Documents Required to Get Permission for Clinical Trails in Russia 4 Manufacture Virus for Pre-clinical Trails 5 Pre-clinical Toxicity Tests 6 Develop and Approve Clinical Trail Protocol(s) 7 Obtain Permission for Clinical Trials 8 Manufacture Virus for Clinical Trials 9 Import the Virus for Clinical Trials 10 Preliminary Clinical Trials (10 patients) 11 Clinical Trails 12 Prepare a Report for UA Pharm Center and FDA 13 Permit for Clinical Use in UA 14 Organize Profitable Clinical Use in UA 15 Continue R&D as 2 -5 Management & Administrative Miscellaneous TOTAL ($M) 0. 1 0. 25 0. 375 0. 4 0. 8 0. 35 1 0. 5 $ 4. 5 27

Combining The Best Of Both Worlds ØIt is the most feasible to develop future models of the product in the US in active collaboration with institutions in EU and Former USSR ØIt is the most feasible to conduct pre-clinical and clinical testing of the existing product in the Former USSR ØCure Lab has developed an international network allowing to conduct each part of the project in the country it is the most feasible, minimizing expanses and need for capital while maximizing results 28

Collaborations Ø USA: Harvard, Emory University, Boston University, Northeastern University, University of Arkansas, UCSD Ø Europe: Technical University of Munich, University of Camerino (Italy) Ø Former USSR: Ivanovsky Institute of Virology, Institute of Viral Preparations, Institute of Gene Biology , Institute of Influenza and other Viral Desiases, Kavetsky Institute of Oncology 29
Scientific Advisory Board Ø Prof. Aaron Ciechanover – Nobel Prize Laureate in Chemistry, 2004. Technion, Haifa, Director of Cancer Control Center, Israel. Ø Barry Straube, M. D. , Senior Medical Advisor, Cure Lab; Immediate Past Chief Medical Officer, Centers for Medicare and Medicaid Services. Ø Kim Lewis, Ph. D. , Professor of Biology, Northeastern University Ø Michael Sherman, Ph. D. , Professor of Biochemistry, Boston University School of Medicine Ø Stuart Calderwood, Ph. D. , Associate Professor, Beth Israel Deaconess Medical Center, Harvard Medical School. Ø Herbert T. Cohen, M. D. , Professor of Medicine, Boston University School of Medicine Ø Mikhail Blagosklonny, M. D. , Ph. D. , Member/Professor, Roswell Park Cancer Institute, Editor-in-chief of journals Cell Cycle and Aging Ø Valeria Povolotskaya, Ph. D. , Ex-Director of International Licensing Department of La Roche 30

Scientific Advisory Board, Russia Ø Prof. Anatoly Tsib, Director of Medical Radiological Research Center of RAMS, Active member of Russian National Academy of Medical Science. Ø Prof. Valeriy Charushin, Deputy of Russian Federation State Duma, Director of Institute of Organic Synthesis named after I. Ya. Postovsky (http: //www. ios. uran. ru/), Chairman of Board of Ural Branch of Russian Academy of Sciences, member of the board of Russian Academy of Sciences, International Society of Heterocyclic Chemistry and International Society for Antiviral Research, International Union of Pure and Applied Chemistry (IUPAC), American Chemical Society, State Duma Committee on Science and High Technology. Ø Prof. Sergey Kolesnikov, Deputy of Russian Federation State Duma, Chairman of the Board of East-Siberian Scientific Center, Siberian Division of Russian Academy of Medical Sciences, Deputy-Chairman of Committee for Health Care, Distinguished Scientist of Russian Federation, Active member of Russian National Academy of Medical Science. Ø Prof. Oleg Kiselev, Director of Research Institute of Influenza of Ministry of Health and Social Development of Russian Federation (Saint Petersburg) (http: //www. influenza. spb. ru/), member of WHO, Active member of Russian National Academy of Medical Science. Ø Prof. Nikolay Kaverin, Chief of Applied Virology Laboratory of Ivanovsky Institute of Virology (Moscow), Active member of Russian National Academy of Medical Science. 31

Management Profiles, USA Alex Shneider, Ph. D. – Founder and CEO Ø 20 years of experience in fundamental and applied bio-medical research. Ø Section editor of International Review of Immunology Ø Senior Research Fellow at the Center for History and Philosophy of Science, Boston University Ø Member of the Board of Trustees of St. Petersburg Institute of Technology Ø Author of the best selling book on business analysis in Eastern Europe. Ø Ph. D. in life science form Roskilder University, Denmark. 32

Management Profiles, USA Eugene L. “Gene” Froelich, CPA – CFO/COO ØOver 30 years of successful business experience ØEx- CEO MCA Records Group ØEx-CFO and EVP of Maxicare Health Plans Inc ØEx-COO and CFO at Wizshop. com 33
Management Profiles, USA Victor Shifrin, Ph. D. , Director of R&D Ø Over 20 years of experience in academia and biotech industry. Ø Formerly Director of Pharmacology at Surface Logix. Worked in various capacities at Scriptgen (Anadys) and Eisai Research Institute. Ø Led discovery biology at Combinato. Rx, where he studied the combinatorial effects of the approved drugs. Ø Ph. D. in Cell and Molecular Biology from Harvard University. Postdoctoral training at the Harvard-affiliated Dana Farber Cancer Institute, and Brigham and Women’s Hospital, Harvard Medical School. 34

Management Profiles, USA Jeff East - Executive Vice President, Strategy/Business Development Ø 25 years of executive level experience across the healthcare industyr. Ø Former CEO of sister company to New England Journal of Medicine, Masspro. Ø Former leader of $2 billion hospital medical device and pharmaceutical distribution company Ø Former senior executive at Massachusetts Department of Public Health Ø Various health care company board officer and director experience. 35

THANK YOU!
7876ed36e2d4524e240f1f1bb38a51a3.ppt