 Preliminary Findings of the Minimally. Invasive Surgery Plus rt. PA for Intracerebral Hemorrhage Evacuation (MISTIE) Clinical Trial T. Morgan, M. Zuccarello, R. Narayan, P. Keyl, K. Lane, D. Hanley Journal Club Presentation: Alvin P. Penalosa, MD Neurosurgery Senior House Officer Newcastle General Hospital
Preliminary Findings of the Minimally. Invasive Surgery Plus rt. PA for Intracerebral Hemorrhage Evacuation (MISTIE) Clinical Trial T. Morgan, M. Zuccarello, R. Narayan, P. Keyl, K. Lane, D. Hanley Journal Club Presentation: Alvin P. Penalosa, MD Neurosurgery Senior House Officer Newcastle General Hospital
 Intracerebral Hemorrhage 15 -30% of strokes in the UK, but the most deadly smaller bleeds (<20 cc) lower mortality and better outcome Current standard: medical treatment, craniotomy in the most severe cases.
Intracerebral Hemorrhage 15 -30% of strokes in the UK, but the most deadly smaller bleeds (<20 cc) lower mortality and better outcome Current standard: medical treatment, craniotomy in the most severe cases.
 Objective To determine the safety of using a combination of minimally invasive surgery and clot lysis with rt-PA to remove intracerebral hemorrhage (ICH) to test the safety of this intervention assess ability of this technique to remove blood clot from brain tissue
Objective To determine the safety of using a combination of minimally invasive surgery and clot lysis with rt-PA to remove intracerebral hemorrhage (ICH) to test the safety of this intervention assess ability of this technique to remove blood clot from brain tissue
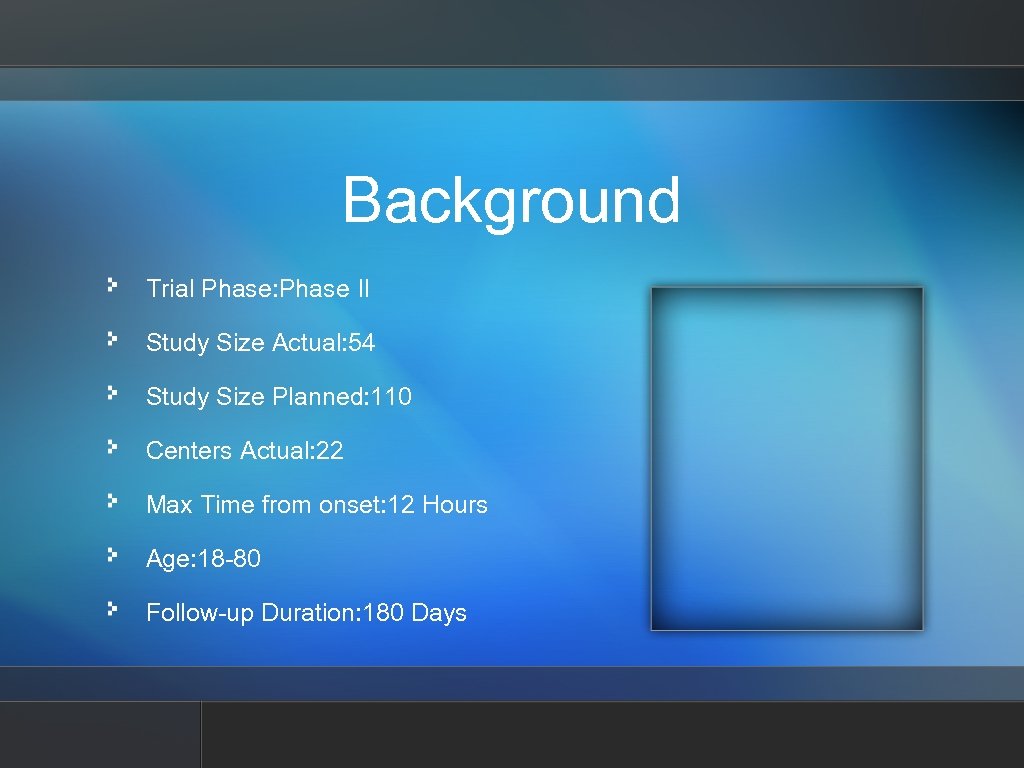 Background Trial Phase: Phase II Study Size Actual: 54 Study Size Planned: 110 Centers Actual: 22 Max Time from onset: 12 Hours Age: 18 -80 Follow-up Duration: 180 Days
Background Trial Phase: Phase II Study Size Actual: 54 Study Size Planned: 110 Centers Actual: 22 Max Time from onset: 12 Hours Age: 18 -80 Follow-up Duration: 180 Days
 Primary Endpoints 30 -day mortality procedure related mortality incidence of cerebritis, meningitis rate of rebleeding
Primary Endpoints 30 -day mortality procedure related mortality incidence of cerebritis, meningitis rate of rebleeding
 Secondary Endpoints Rate of clot size reduction at Days 4 -5 determined by CT scans 90 & 180 day GOS, Rankin, Stroke Impact Scale
Secondary Endpoints Rate of clot size reduction at Days 4 -5 determined by CT scans 90 & 180 day GOS, Rankin, Stroke Impact Scale
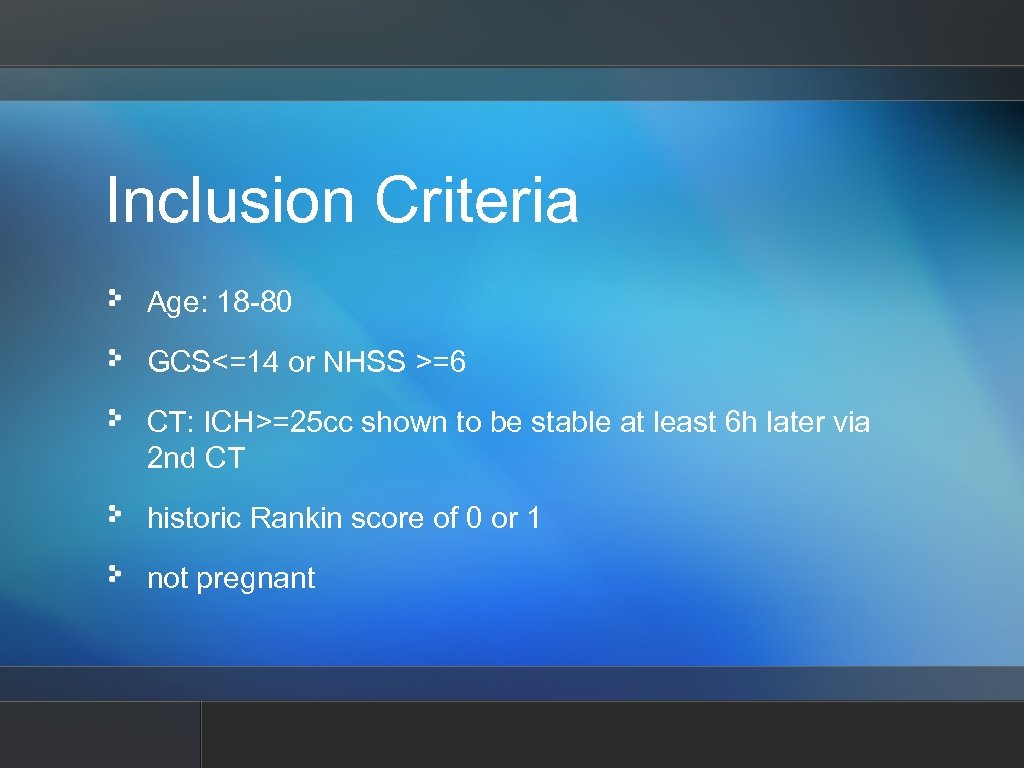 Inclusion Criteria Age: 18 -80 GCS<=14 or NHSS >=6 CT: ICH>=25 cc shown to be stable at least 6 h later via 2 nd CT historic Rankin score of 0 or 1 not pregnant
Inclusion Criteria Age: 18 -80 GCS<=14 or NHSS >=6 CT: ICH>=25 cc shown to be stable at least 6 h later via 2 nd CT historic Rankin score of 0 or 1 not pregnant
 Exclusion Criteria any infratentorial hemorrhage IVH requiring EVD coagulopathy vascular abnormality proven by MRA or CTA
Exclusion Criteria any infratentorial hemorrhage IVH requiring EVD coagulopathy vascular abnormality proven by MRA or CTA
 Methodology
Methodology
 14 -French cannula steriotactically placed in center of the parenchymal clot 2/3 the length of long axis and within the middle 1/3 of the clot aspiration using 10 cc syringe until first resistance to free hand suction soft ventriculostomy catheter is passed through the rigid cannula remove rigid cannula
14 -French cannula steriotactically placed in center of the parenchymal clot 2/3 the length of long axis and within the middle 1/3 of the clot aspiration using 10 cc syringe until first resistance to free hand suction soft ventriculostomy catheter is passed through the rigid cannula remove rigid cannula
 CT: position, rebleed 0. 3 mg rt. PA followed by sterile flush; close system for 1 hour repeat every 8 hours for a total of 9 doses or until a clinical endpoint is reached
CT: position, rebleed 0. 3 mg rt. PA followed by sterile flush; close system for 1 hour repeat every 8 hours for a total of 9 doses or until a clinical endpoint is reached
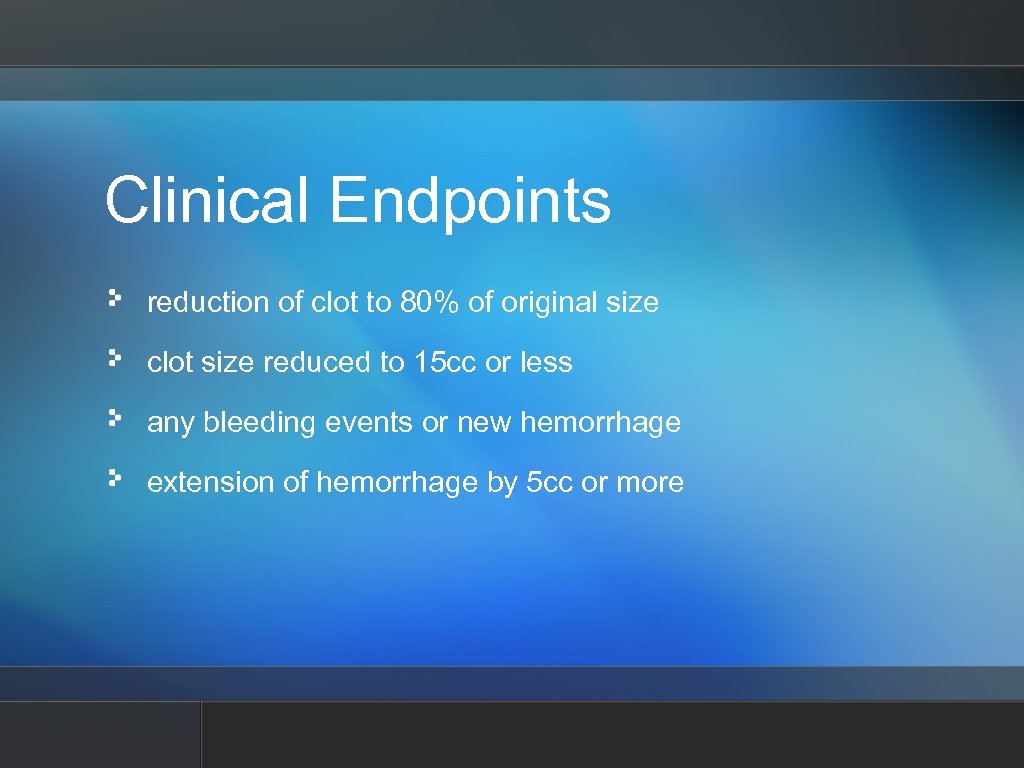 Clinical Endpoints reduction of clot to 80% of original size clot size reduced to 15 cc or less any bleeding events or new hemorrhage extension of hemorrhage by 5 cc or more
Clinical Endpoints reduction of clot to 80% of original size clot size reduced to 15 cc or less any bleeding events or new hemorrhage extension of hemorrhage by 5 cc or more
 Results
Results
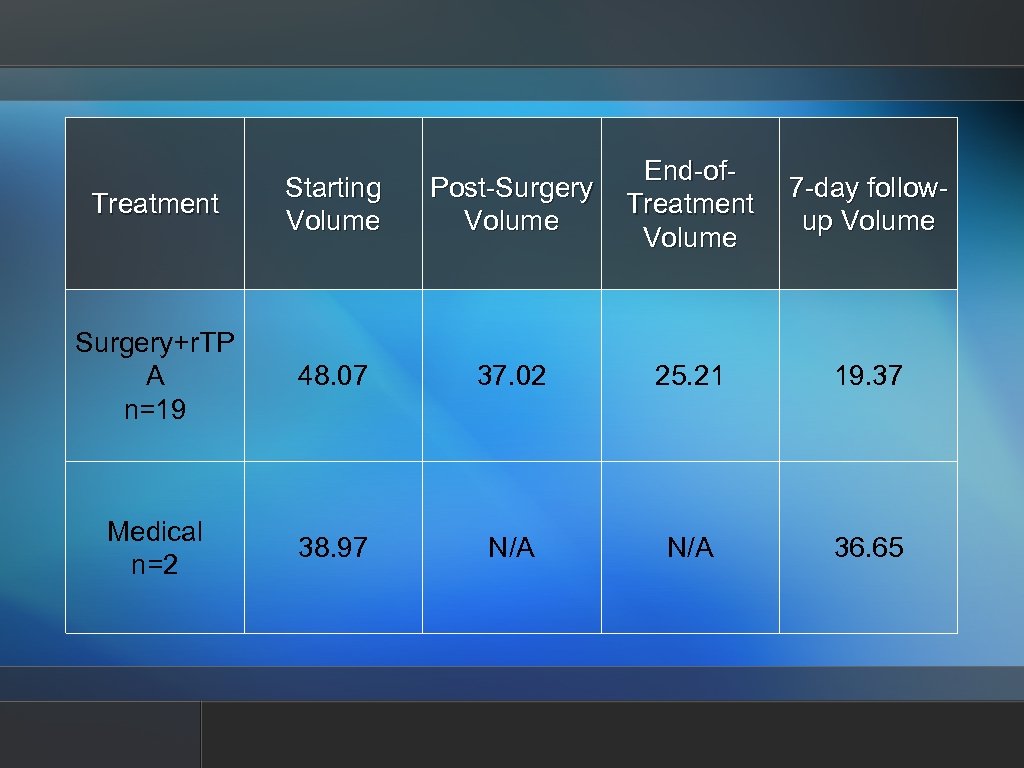 Treatment Starting Volume Post-Surgery Volume End-of. Treatment Volume Surgery+r. TP A n=19 48. 07 37. 02 25. 21 19. 37 Medical n=2 38. 97 N/A 36. 65 7 -day followup Volume
Treatment Starting Volume Post-Surgery Volume End-of. Treatment Volume Surgery+r. TP A n=19 48. 07 37. 02 25. 21 19. 37 Medical n=2 38. 97 N/A 36. 65 7 -day followup Volume
 Summary of Results Aspiration alone: 20% (n=4) After treatment: 50% of starting volume vs 6% reduction (medical management) O doses=4, 9 doses=3 8% symptomatic rebleed bacterial ventriculitis= 0%
Summary of Results Aspiration alone: 20% (n=4) After treatment: 50% of starting volume vs 6% reduction (medical management) O doses=4, 9 doses=3 8% symptomatic rebleed bacterial ventriculitis= 0%
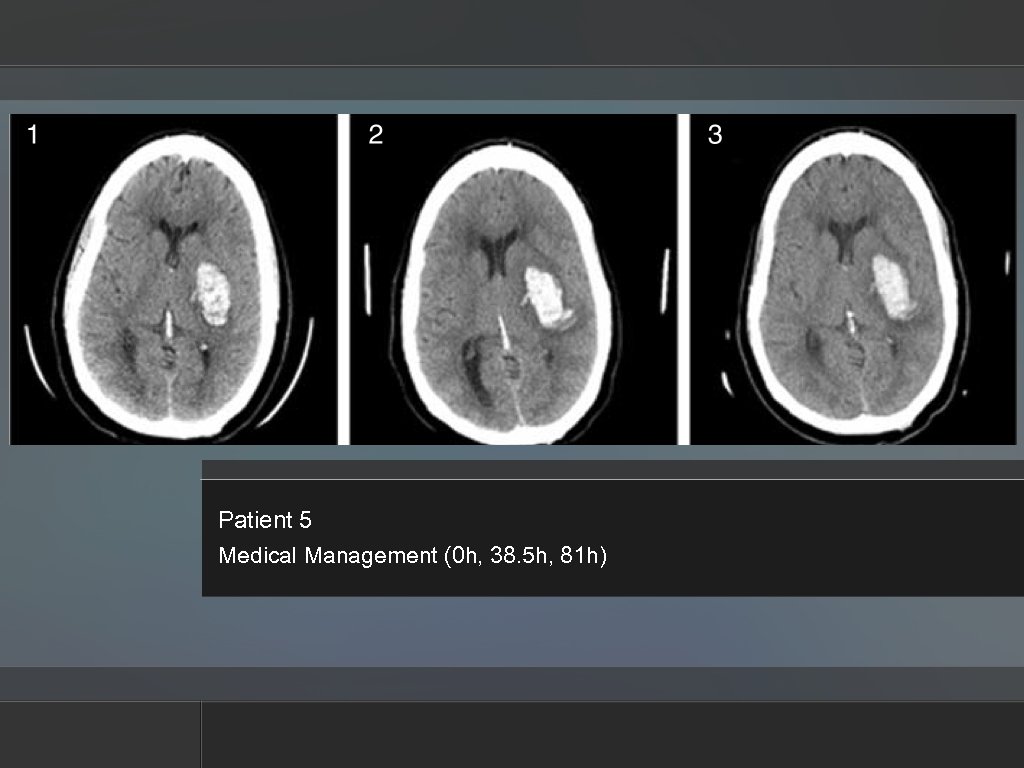 Patient 5 Medical Management (0 h, 38. 5 h, 81 h)
Patient 5 Medical Management (0 h, 38. 5 h, 81 h)
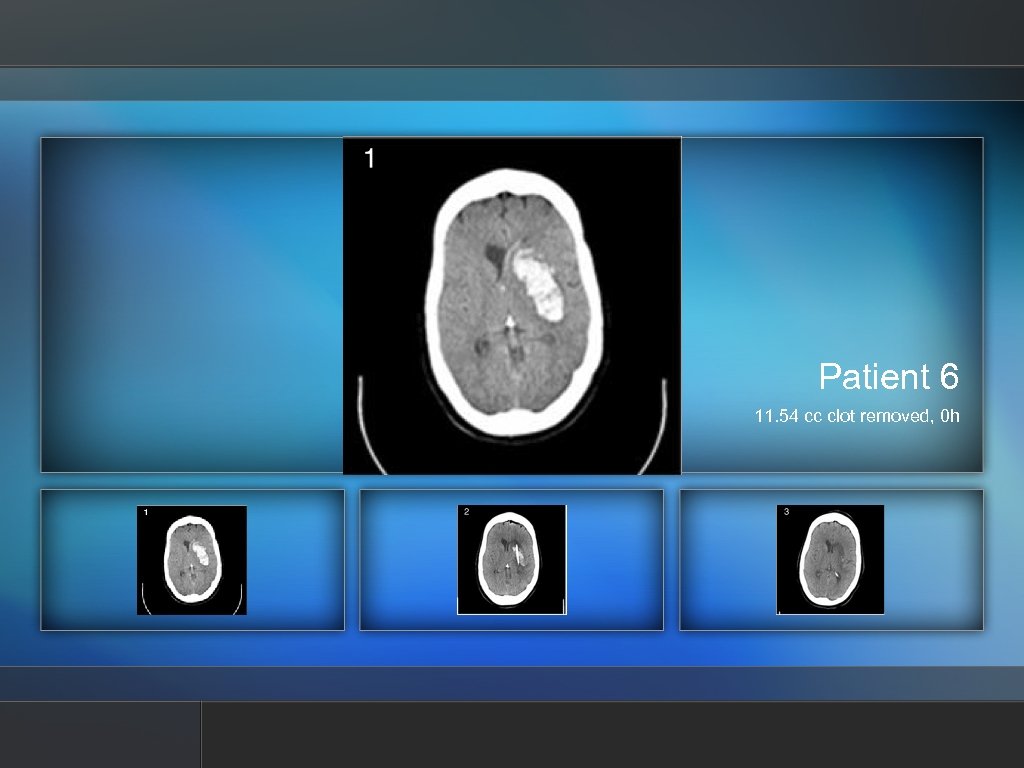 Patient 6 11. 54 cc clot removed, 0 h
Patient 6 11. 54 cc clot removed, 0 h
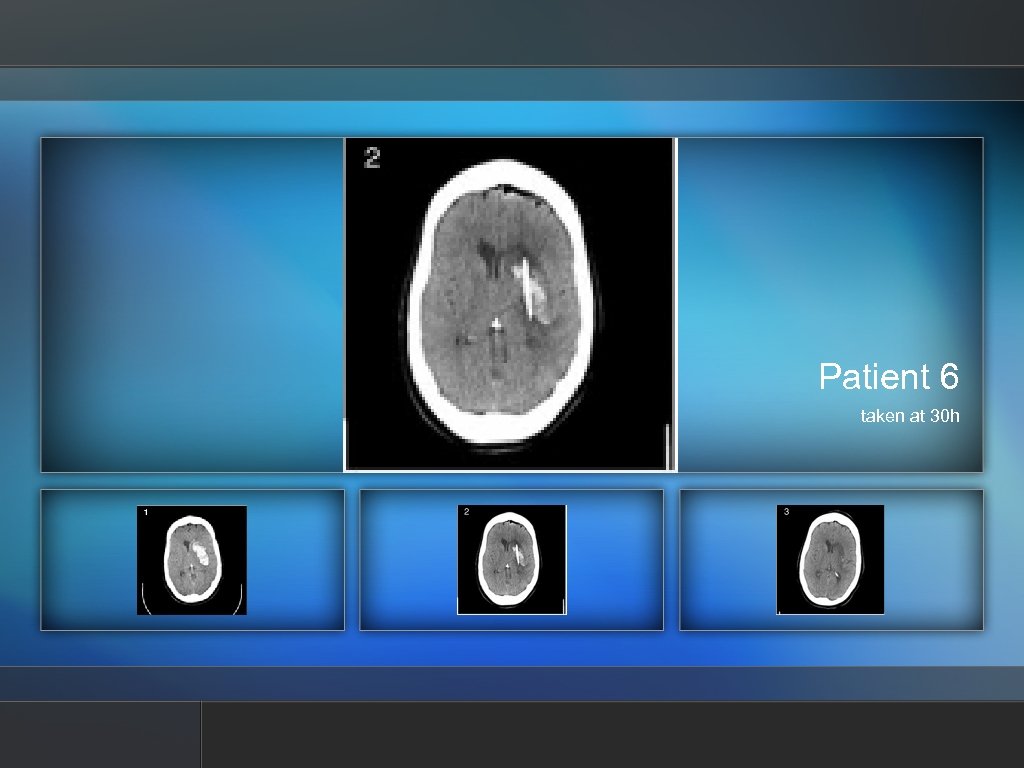 Patient 6 taken at 30 h
Patient 6 taken at 30 h
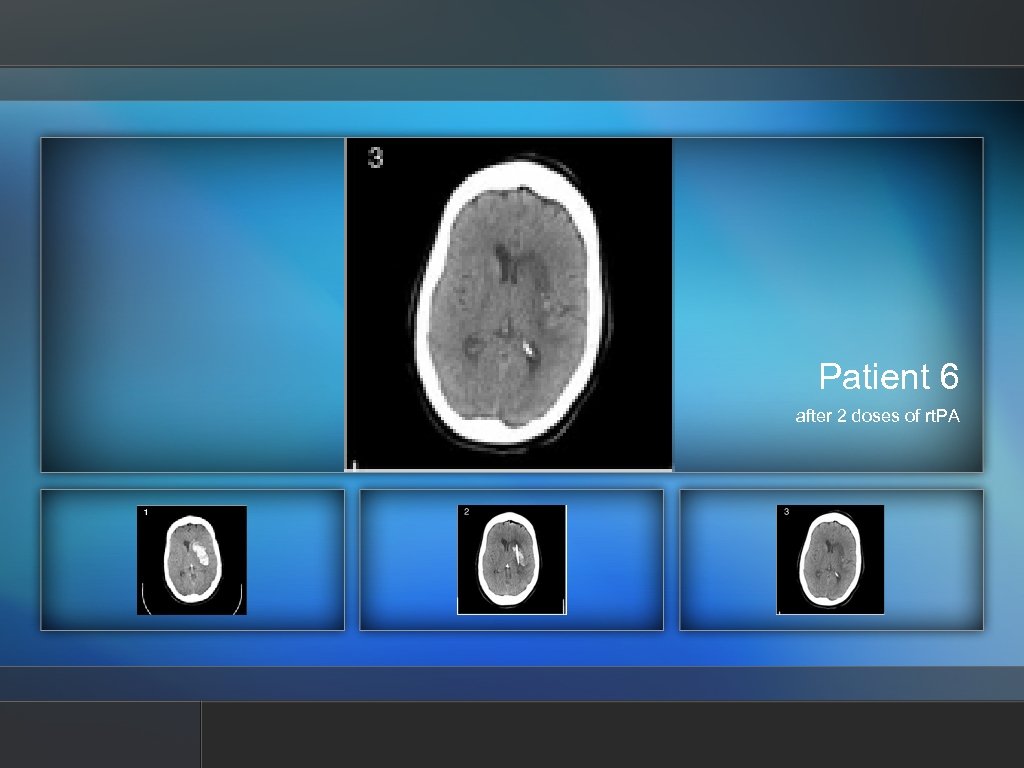 Patient 6 after 2 doses of rt. PA
Patient 6 after 2 doses of rt. PA