f62d76d0283b83661f3f7e89446a6513.ppt
- Количество слайдов: 46
 Pregnancy & Renal Transplantation Alicia Notkin May 20, 2008
Pregnancy & Renal Transplantation Alicia Notkin May 20, 2008
 Case A 30 year old female w/ ESRD, s/p LDRT from her mother 3 years prior, comes to clinic for f/u. She is fully compliant with her regimen of prednisone 5 mg daily, tacro 3 mg q 12 h, and MMF 1 g q 12 h. Her renal function has been stable, with a Cr ~ 1. 2 mg/dl and a negative UA. She wishes to become pregnant. How should she be advised & managed?
Case A 30 year old female w/ ESRD, s/p LDRT from her mother 3 years prior, comes to clinic for f/u. She is fully compliant with her regimen of prednisone 5 mg daily, tacro 3 mg q 12 h, and MMF 1 g q 12 h. Her renal function has been stable, with a Cr ~ 1. 2 mg/dl and a negative UA. She wishes to become pregnant. How should she be advised & managed?
 Outline • Pregnancy in patients with chronic kidney • • • disease Pregnancy in patients on dialysis Pregnancy in renal transplant patients Transplantation medications in pregnancy Recommendations Other issues: graft dysfunction in pregnancy, donor & pregnancy, male fertility
Outline • Pregnancy in patients with chronic kidney • • • disease Pregnancy in patients on dialysis Pregnancy in renal transplant patients Transplantation medications in pregnancy Recommendations Other issues: graft dysfunction in pregnancy, donor & pregnancy, male fertility
 Pregnancy in patients with chronic kidney disease: patient considerations • Permanent decline in renal function in 0 -10% of • • women with normal to mildly reduced renal function Patients w/ moderate renal insufficiency may initially have decline in Cr, but may rise above baseline over rest of pregnancy (in a small study, 40% of patients w/ a Cr from 1. 4 -1. 9 mg/dl had rise in Cr) Women w/ Cr > 3. 0 mg/dl have menstrual abnormalities & have much lower chance of conception & carrying fetus to term
Pregnancy in patients with chronic kidney disease: patient considerations • Permanent decline in renal function in 0 -10% of • • women with normal to mildly reduced renal function Patients w/ moderate renal insufficiency may initially have decline in Cr, but may rise above baseline over rest of pregnancy (in a small study, 40% of patients w/ a Cr from 1. 4 -1. 9 mg/dl had rise in Cr) Women w/ Cr > 3. 0 mg/dl have menstrual abnormalities & have much lower chance of conception & carrying fetus to term
 Pregnancy in patients with chronic kidney disease: other patient considerations • Proteinuria increases in ~ ½ of the patients • Hypertension develops or worsens in ~ ¼ of the • • patients Significant worsening of edema can occur during pregnancy in women w/ nephrotic syndrome Β-HCG can be increased in patients w/ ESRD, so confirm pregnancy w/ an ultrasound
Pregnancy in patients with chronic kidney disease: other patient considerations • Proteinuria increases in ~ ½ of the patients • Hypertension develops or worsens in ~ ¼ of the • • patients Significant worsening of edema can occur during pregnancy in women w/ nephrotic syndrome Β-HCG can be increased in patients w/ ESRD, so confirm pregnancy w/ an ultrasound
 Pregnancy in patients with chronic kidney disease: fetal outcomes • If blood pressure is controlled, rate of live births • • is > 90% in women w/ normal renal function & is slightly lower in women w/ mild renal insufficiency Lower fetal survival if bp not controlled (10 -fold increase if MAP > 105 at conception) Higher risk of prematurity if Cr > 1. 4 (59% v. 10%) – increased risk of preeclampsia & IUGR
Pregnancy in patients with chronic kidney disease: fetal outcomes • If blood pressure is controlled, rate of live births • • is > 90% in women w/ normal renal function & is slightly lower in women w/ mild renal insufficiency Lower fetal survival if bp not controlled (10 -fold increase if MAP > 105 at conception) Higher risk of prematurity if Cr > 1. 4 (59% v. 10%) – increased risk of preeclampsia & IUGR
 Pregnancy in patients on dialysis • Conception occurs in 0. 3 -1. 5% of women of childbearing • • • age per year (disrupted gonadal function) Live births occur in 40 -50% Prematurity occurs in most (average at delivery is ~ 30. 5 weeks) Increased risk for severe hypertension Similar outcomes in HD & PD patients More intensive dialysis recommended (5 -7 x/wk to keep BUN under 45 -50); more frequent, lower volume exchanges if on PD Avoid hemodynamic instability & monitor the fetus during treatment
Pregnancy in patients on dialysis • Conception occurs in 0. 3 -1. 5% of women of childbearing • • • age per year (disrupted gonadal function) Live births occur in 40 -50% Prematurity occurs in most (average at delivery is ~ 30. 5 weeks) Increased risk for severe hypertension Similar outcomes in HD & PD patients More intensive dialysis recommended (5 -7 x/wk to keep BUN under 45 -50); more frequent, lower volume exchanges if on PD Avoid hemodynamic instability & monitor the fetus during treatment
 Pregnancy in renal transplant patients: outcomes • Fertility returns! • > 90% success after 1 st trimester; slight increase in • • spontaneous abortion IUGR a/o premature delivery in up to 20% & 50%, respectively (some say as much as 1/2 -2/3 cases) US & UK registries suggest ~ 14% spontaneous abortion, high prevalence of hypertension, increased preeclampsia (~ 1/3) Developmental delays related to prematurity Fewer complications & birth abnormalities than dialysis patients
Pregnancy in renal transplant patients: outcomes • Fertility returns! • > 90% success after 1 st trimester; slight increase in • • spontaneous abortion IUGR a/o premature delivery in up to 20% & 50%, respectively (some say as much as 1/2 -2/3 cases) US & UK registries suggest ~ 14% spontaneous abortion, high prevalence of hypertension, increased preeclampsia (~ 1/3) Developmental delays related to prematurity Fewer complications & birth abnormalities than dialysis patients
 Pregnancy in renal transplant patients: outcomes • Increased risk of graft loss if Cr > 1. 5 mg/dl • • before pregnancy No large, long-term controlled studies looking at GFR & proteinuria in graft recipients who have become pregnant (varying results) Birth weight & gestational age seem to be lower in pancreas-kidney transplants than in kidney alone
Pregnancy in renal transplant patients: outcomes • Increased risk of graft loss if Cr > 1. 5 mg/dl • • before pregnancy No large, long-term controlled studies looking at GFR & proteinuria in graft recipients who have become pregnant (varying results) Birth weight & gestational age seem to be lower in pancreas-kidney transplants than in kidney alone
 Pregnancy in renal transplant patients: outcomes • One of the best studies we have: case-control study from 1 • • • center in Israel Included patients transplanted between ’ 83 & ’ 98 Looked at 39 women who became pregnant (44% received CRT, 43. 6% had glomerular disease originally, average 24, most at least 2 years out) Each matched w/ 3 controls from the Collaborative Transplant Study database for 12 factors known to affect graft survival (donor type, ethnic origin, transplant #, year transplanted, donor & recipient ages, IS regimen, CIT, HLA mismatch, PRA, underlying disease, duration of functioning graft from transplant to pregnancy) IS regimen: 26 on Cs. A/AZA/pred, 7 on AZA/pred, 4 on Cs. A/pred, 2 on Cs. A/AZA F/u of 15 years
Pregnancy in renal transplant patients: outcomes • One of the best studies we have: case-control study from 1 • • • center in Israel Included patients transplanted between ’ 83 & ’ 98 Looked at 39 women who became pregnant (44% received CRT, 43. 6% had glomerular disease originally, average 24, most at least 2 years out) Each matched w/ 3 controls from the Collaborative Transplant Study database for 12 factors known to affect graft survival (donor type, ethnic origin, transplant #, year transplanted, donor & recipient ages, IS regimen, CIT, HLA mismatch, PRA, underlying disease, duration of functioning graft from transplant to pregnancy) IS regimen: 26 on Cs. A/AZA/pred, 7 on AZA/pred, 4 on Cs. A/pred, 2 on Cs. A/AZA F/u of 15 years
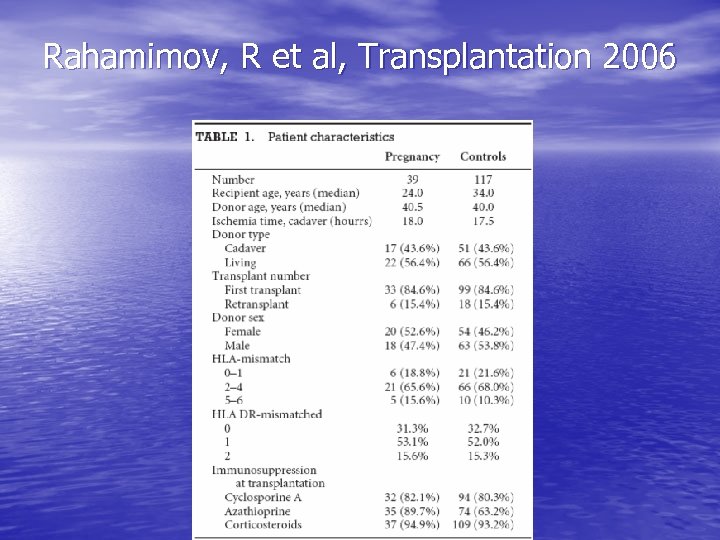 Rahamimov, R et al, Transplantation 2006
Rahamimov, R et al, Transplantation 2006
 Rahamimov, R et al 2006 • Similar graft and patient survival (62 & 85% v. 69 & 79%) • Similar kidney function 1, 5, & 10 years post-transplant • Preterm delivery in 60% • Preeclampsia in 15. 3% • IUGR in 52% • No acute rejection
Rahamimov, R et al 2006 • Similar graft and patient survival (62 & 85% v. 69 & 79%) • Similar kidney function 1, 5, & 10 years post-transplant • Preterm delivery in 60% • Preeclampsia in 15. 3% • IUGR in 52% • No acute rejection
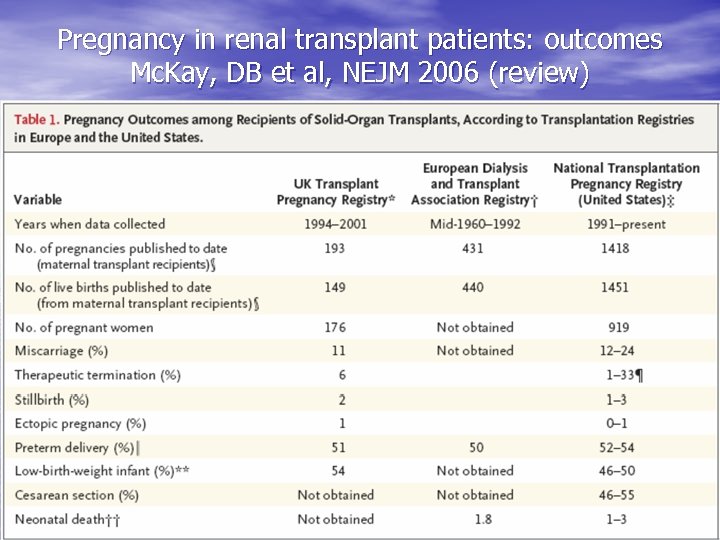 Pregnancy in renal transplant patients: outcomes Mc. Kay, DB et al, NEJM 2006 (review)
Pregnancy in renal transplant patients: outcomes Mc. Kay, DB et al, NEJM 2006 (review)
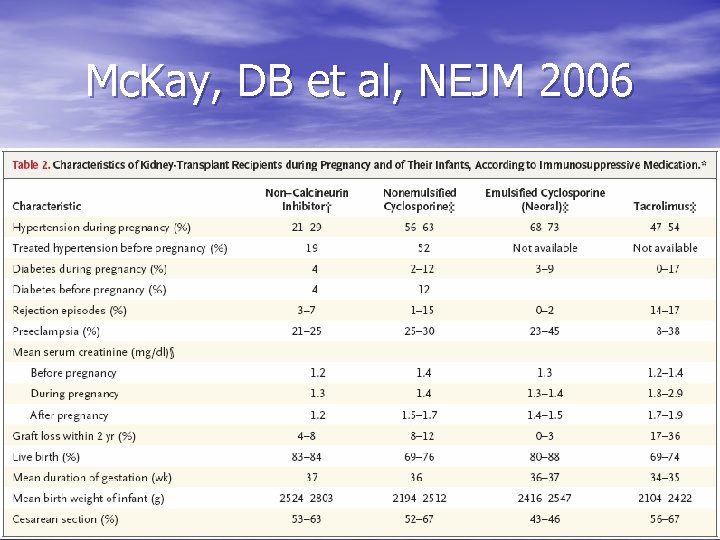 Mc. Kay, DB et al, NEJM 2006
Mc. Kay, DB et al, NEJM 2006
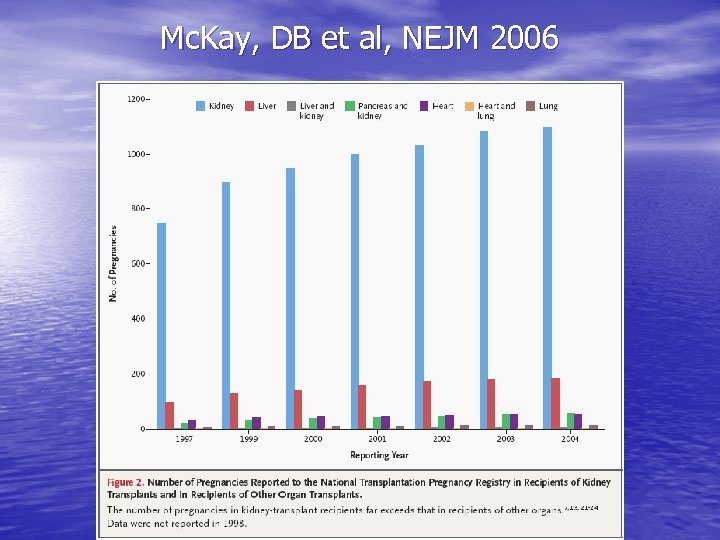 Mc. Kay, DB et al, NEJM 2006
Mc. Kay, DB et al, NEJM 2006
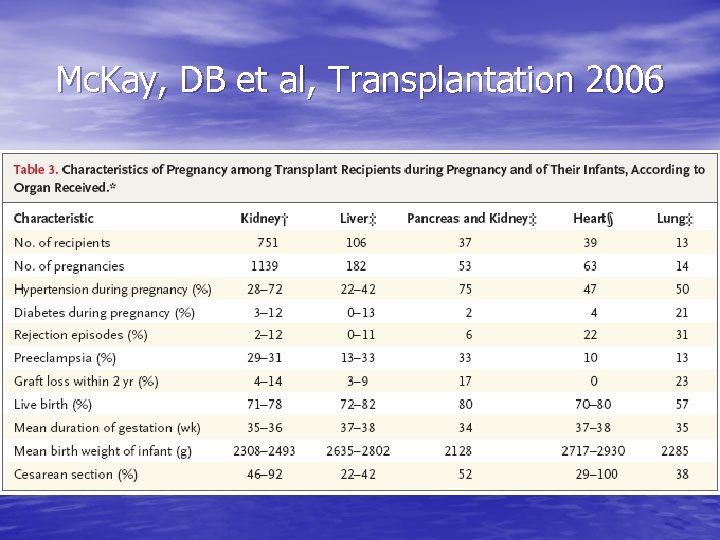 Mc. Kay, DB et al, Transplantation 2006
Mc. Kay, DB et al, Transplantation 2006
 Transplant medications: steroids • Associations noted between prednisone & a • • • variety of birth defects (but mainly @ doses > 20 mg/d) Retrospective data suggest an increased risk of cleft palate w/ glucocorticoids Possible increased risk of PROM & IUGR w/ glucocorticoids Glucocorticoids are excreted in breast milk (small amounts), but considered ok if needed by mother
Transplant medications: steroids • Associations noted between prednisone & a • • • variety of birth defects (but mainly @ doses > 20 mg/d) Retrospective data suggest an increased risk of cleft palate w/ glucocorticoids Possible increased risk of PROM & IUGR w/ glucocorticoids Glucocorticoids are excreted in breast milk (small amounts), but considered ok if needed by mother
 Transplant medications: cyclosporine • Can induce/worsen hypertension • Drug levels may fall during pregnancy • Premature labor and infants that are small for gestational age have been reported (possible confounders)
Transplant medications: cyclosporine • Can induce/worsen hypertension • Drug levels may fall during pregnancy • Premature labor and infants that are small for gestational age have been reported (possible confounders)
 Transplant medications: cyclosporine • 115 renal transplant recipients (154 • • pregnancies): Cs. A v. AZA/pred Cs. A had lower birth weights, more maternal DM/htn/rejection, but complication rate in newborns was slightly lower & congenital malformations were not seen Meta-analysis of 15 studies suggests that it is not a significant teratogen (4. 1% of offspring w/ major malformations – similar to general population); limited by data available, study design, confounders…
Transplant medications: cyclosporine • 115 renal transplant recipients (154 • • pregnancies): Cs. A v. AZA/pred Cs. A had lower birth weights, more maternal DM/htn/rejection, but complication rate in newborns was slightly lower & congenital malformations were not seen Meta-analysis of 15 studies suggests that it is not a significant teratogen (4. 1% of offspring w/ major malformations – similar to general population); limited by data available, study design, confounders…
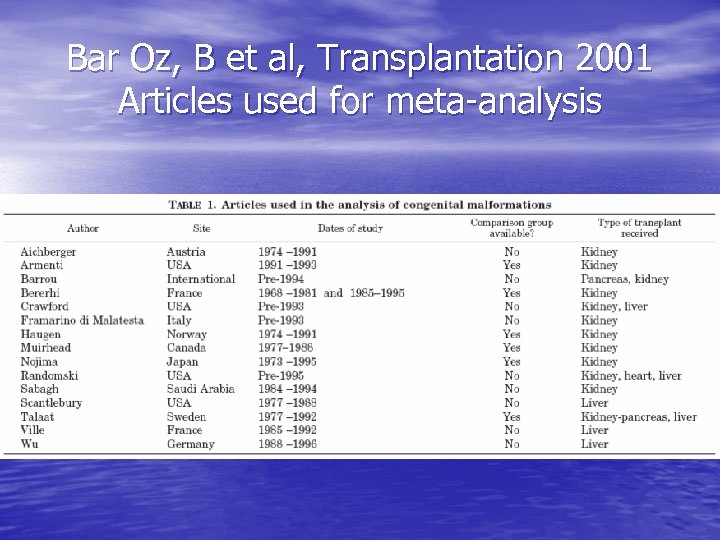 Bar Oz, B et al, Transplantation 2001 Articles used for meta-analysis
Bar Oz, B et al, Transplantation 2001 Articles used for meta-analysis
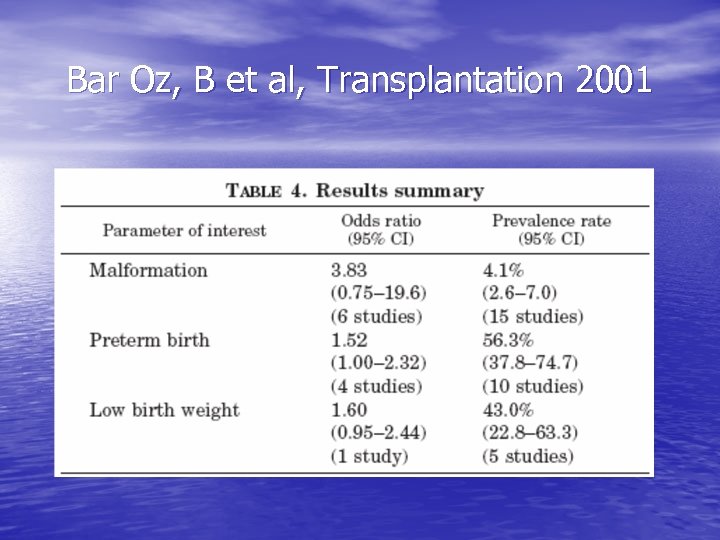 Bar Oz, B et al, Transplantation 2001
Bar Oz, B et al, Transplantation 2001
 Transplant medications: cyclosporine • Conflicting data re. passage across placenta (rodents show little or no transfer) • Excreted in breast milk with even therapeutic levels found in infants • Not recommended for lactating mothers
Transplant medications: cyclosporine • Conflicting data re. passage across placenta (rodents show little or no transfer) • Excreted in breast milk with even therapeutic levels found in infants • Not recommended for lactating mothers
 Transplant medications: tacrolimus • Again, limited data • 84 women (100 pregnancies – 27% of them in • • • renal transplant recipients) Live birth in 68 60% of deliveries premature 4 babies w/ malformations (no pattern) Dose remained reasonably stable Levels in breast milk similar to that in maternal serum; not recommended during lactation
Transplant medications: tacrolimus • Again, limited data • 84 women (100 pregnancies – 27% of them in • • • renal transplant recipients) Live birth in 68 60% of deliveries premature 4 babies w/ malformations (no pattern) Dose remained reasonably stable Levels in breast milk similar to that in maternal serum; not recommended during lactation
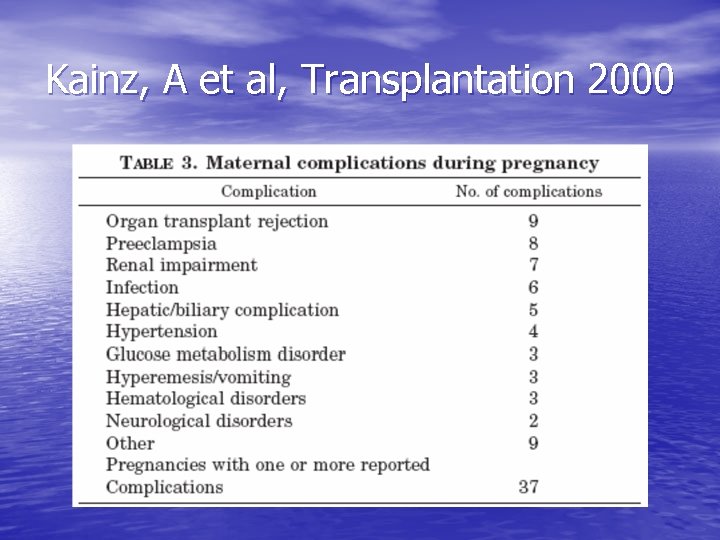 Kainz, A et al, Transplantation 2000
Kainz, A et al, Transplantation 2000
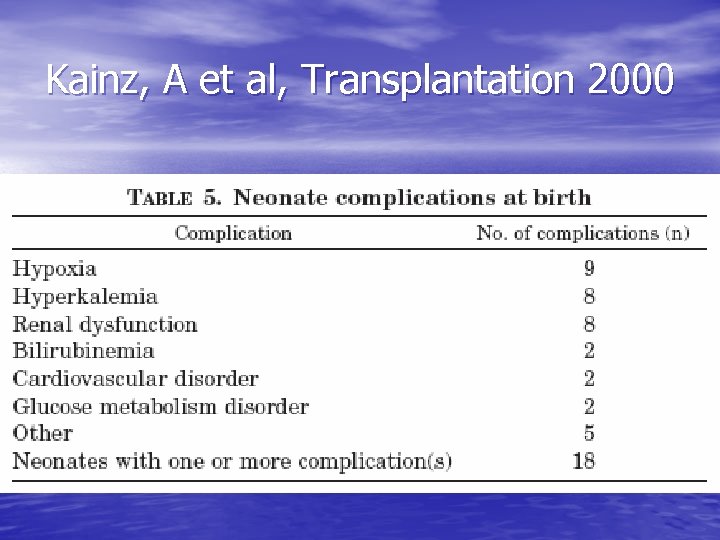 Kainz, A et al, Transplantation 2000
Kainz, A et al, Transplantation 2000
 Transplant medications: sirolimus • Should be discontinued >/= 12 weeks before conception • Recommend switch to cyclosporine if planning to conceive • Can switch back following delivery • Case series in 2006 – 7 pregnancies w/ exposure: 4 live births (1 w/ structural malformations), 3 spontaneous abortions
Transplant medications: sirolimus • Should be discontinued >/= 12 weeks before conception • Recommend switch to cyclosporine if planning to conceive • Can switch back following delivery • Case series in 2006 – 7 pregnancies w/ exposure: 4 live births (1 w/ structural malformations), 3 spontaneous abortions
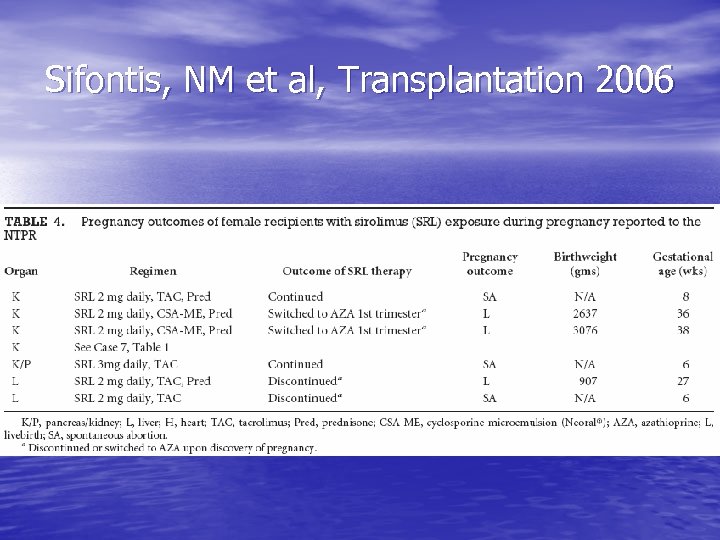 Sifontis, NM et al, Transplantation 2006
Sifontis, NM et al, Transplantation 2006
 Transplant medications: mycophenolate mofetil • Adverse effects seen in lab animals at lower doses than those used in humans • Increases 1 st trimester pregnancy loss & congenital malformations (cleft lip/palate, anomalies of distal limbs, heart, esophagus, kidneys)
Transplant medications: mycophenolate mofetil • Adverse effects seen in lab animals at lower doses than those used in humans • Increases 1 st trimester pregnancy loss & congenital malformations (cleft lip/palate, anomalies of distal limbs, heart, esophagus, kidneys)
 Transplant medications: mycophenolate mofetil • Same case series from 2006: 18 renal transplant • • • recipients (26 pregnancies) exposed to MMF 11 spontaneous abortions 15 live births 4/15 live births had structural malformations: hypoplastic nails, shortened 5 th finger, microtia w/ & w/o cleft lip & palate, neonatal death w/ multiple malformations
Transplant medications: mycophenolate mofetil • Same case series from 2006: 18 renal transplant • • • recipients (26 pregnancies) exposed to MMF 11 spontaneous abortions 15 live births 4/15 live births had structural malformations: hypoplastic nails, shortened 5 th finger, microtia w/ & w/o cleft lip & palate, neonatal death w/ multiple malformations
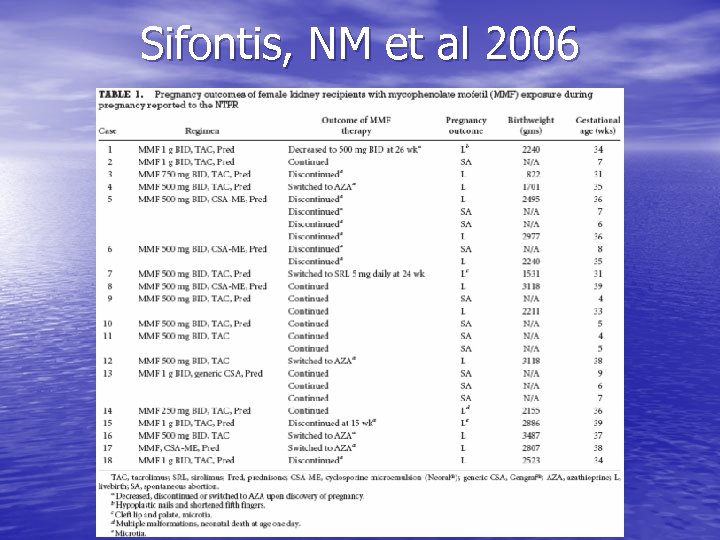 Sifontis, NM et al 2006
Sifontis, NM et al 2006
 Sifontis, NM et al, Transplantation 2006
Sifontis, NM et al, Transplantation 2006
 Transplant medications: mycophenolate mofetil • 2 forms of contraception should be used a few • • • weeks before & after therapy, as well as during therapy If planning pregnancy, should switch to azathioprine Should be off of MMF >/= 6 weeks before conception Excreted into breast milk – lactating mothers should avoid
Transplant medications: mycophenolate mofetil • 2 forms of contraception should be used a few • • • weeks before & after therapy, as well as during therapy If planning pregnancy, should switch to azathioprine Should be off of MMF >/= 6 weeks before conception Excreted into breast milk – lactating mothers should avoid
 Transplant medications: azathioprine • AZA is metabolized to thiouric acid (inactive) by • • • the fetus (a large percent of AZA given to mothers appears as inactive metabolites in fetal blood) Suggests that fetus lacks inosinate pyrophosphorylase which converts AZA to 6 -MP 146 renal transplant recipients: 90% given AZA/pred, 2% given AZA, 8% given pred AZA groups showed more problems w/ low birthweight, prematurity, jaundice, respiratory distress syndrome, & aspiration
Transplant medications: azathioprine • AZA is metabolized to thiouric acid (inactive) by • • • the fetus (a large percent of AZA given to mothers appears as inactive metabolites in fetal blood) Suggests that fetus lacks inosinate pyrophosphorylase which converts AZA to 6 -MP 146 renal transplant recipients: 90% given AZA/pred, 2% given AZA, 8% given pred AZA groups showed more problems w/ low birthweight, prematurity, jaundice, respiratory distress syndrome, & aspiration
 Transplant medications: azathioprine • Lactation: 31 breast milk samples – 29 had no 6 -MP & 2 had minimal • 6 -MP & 6 -thioguanine were not detectable in neonatal blood • Preferable to MMF
Transplant medications: azathioprine • Lactation: 31 breast milk samples – 29 had no 6 -MP & 2 had minimal • 6 -MP & 6 -thioguanine were not detectable in neonatal blood • Preferable to MMF
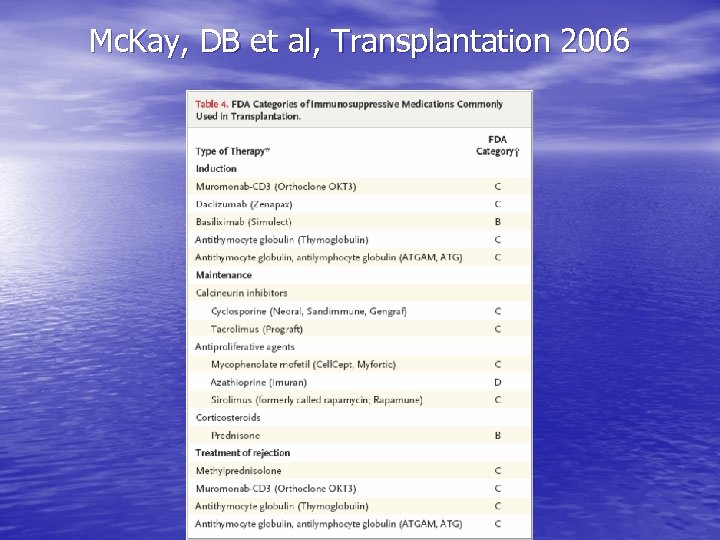 Mc. Kay, DB et al, Transplantation 2006
Mc. Kay, DB et al, Transplantation 2006
 Recommendations… & our patient
Recommendations… & our patient
 AST Consensus Conference on Reproductive Issues & Transplantation 2005
AST Consensus Conference on Reproductive Issues & Transplantation 2005
 AST Consensus Conference on Reproductive Issues & Transplantation 2005
AST Consensus Conference on Reproductive Issues & Transplantation 2005
 Mc. Kay, DB et al, CJASN 2008
Mc. Kay, DB et al, CJASN 2008
 Recommendations: key points • Preferable to wait >/= 1 year following LDRT & >/= 2 years following CRT to avoid rejection-related complications (drug doses are lower & doses are stable) • Graft should preferably be functioning well (stable Cr < 1. 5 mg/dl, proteinuria < 500 mg/d) • Frequent monitoring
Recommendations: key points • Preferable to wait >/= 1 year following LDRT & >/= 2 years following CRT to avoid rejection-related complications (drug doses are lower & doses are stable) • Graft should preferably be functioning well (stable Cr < 1. 5 mg/dl, proteinuria < 500 mg/d) • Frequent monitoring
 Recommendations • Aggressive treatment of hypertension (goal is normalization of bp) • Close monitoring for preeclampsia • Evidence suggests that pregnancy is not an immunosuppressed state & transplant medications should not be reduced based on that notion
Recommendations • Aggressive treatment of hypertension (goal is normalization of bp) • Close monitoring for preeclampsia • Evidence suggests that pregnancy is not an immunosuppressed state & transplant medications should not be reduced based on that notion
 Recommendations • In case cesarian section is necessary, • • obstetrician should know graft and ureter location Careful wound closure & prophylactic antibiotics to avoid infection Contraception: theoretical problems with hormonal methods, IUDs less effective & increased risk of infection, barrier methods traditionally preferred
Recommendations • In case cesarian section is necessary, • • obstetrician should know graft and ureter location Careful wound closure & prophylactic antibiotics to avoid infection Contraception: theoretical problems with hormonal methods, IUDs less effective & increased risk of infection, barrier methods traditionally preferred
 Graft dysfunction in pregnancy • Rejection is difficult to diagnose since Cr falls somewhat • • • during pregnancy Methylprednisolone is the recommended treatment of rejection IVIg has been used a fair amount without problems Need to include causes specific to transplant as well as causes specific to pregnancy Ureteral obstruction from a gravid uterus is not common, but has been reported TTP-HUS from AHR or from cyclosporine/tacro occur peri -transplant, so a TTP-HUS picture in a pregnant patient is likely pregnancy-related
Graft dysfunction in pregnancy • Rejection is difficult to diagnose since Cr falls somewhat • • • during pregnancy Methylprednisolone is the recommended treatment of rejection IVIg has been used a fair amount without problems Need to include causes specific to transplant as well as causes specific to pregnancy Ureteral obstruction from a gravid uterus is not common, but has been reported TTP-HUS from AHR or from cyclosporine/tacro occur peri -transplant, so a TTP-HUS picture in a pregnant patient is likely pregnancy-related
 OK to biopsy? ? • Data for native kidneys • Can be done safely in women with well- controlled blood pressure • Biopsy after 32 weeks is not recommended (? if applies to transplant patients? )
OK to biopsy? ? • Data for native kidneys • Can be done safely in women with well- controlled blood pressure • Biopsy after 32 weeks is not recommended (? if applies to transplant patients? )
 Issues for donor & male recipient • Little data re. hyperfiltration in donor who • • becomes pregnant; fertility & complications do not seem to be affected Sexual function & sperm motility (but not sperm counts or morphology) improve after transplantation Several reports of male infertility associated w/ sirolimus (CNIs & AZA seem ok)
Issues for donor & male recipient • Little data re. hyperfiltration in donor who • • becomes pregnant; fertility & complications do not seem to be affected Sexual function & sperm motility (but not sperm counts or morphology) improve after transplantation Several reports of male infertility associated w/ sirolimus (CNIs & AZA seem ok)
 References • Bar Oz, B et al. Pregnancy outcome after cyclosporine therapy during • • • pregnancy: a meta-analysis. Transplantation 2001; 71: 1051. Kainz, A et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation 2000; 70: 1718. Mc. Kay, DB et al. Pregnancy after kidney transplantation. CJASN 2008; 3: S 117. Mc. Kay, DB et al. Pregnancy in recipients of solid organs – effects on mother and child. N Engl J Med 2006; 354: 1281. Mc. Kay, DB et al. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5: 1592. Rahamimov, R et al. Pregnancy in renal transplant recipients: long-term effect on patient and graft survival. A single-center experience. Transplantation 2006; 81: 660. Salmela, KT et al. Impaired renal function after pregnancy in renal transplant recipients. Transplantation 1993; 56: 1372. Sifontis, NM et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006; 82: 1698. Sturgiss, SN et al. Effect of pregnancy on long-term function in renal allografts: an update. Am J Kidney Dis 1995; 26: 54. www. uptodate. com
References • Bar Oz, B et al. Pregnancy outcome after cyclosporine therapy during • • • pregnancy: a meta-analysis. Transplantation 2001; 71: 1051. Kainz, A et al. Review of the course and outcome of 100 pregnancies in 84 women treated with tacrolimus. Transplantation 2000; 70: 1718. Mc. Kay, DB et al. Pregnancy after kidney transplantation. CJASN 2008; 3: S 117. Mc. Kay, DB et al. Pregnancy in recipients of solid organs – effects on mother and child. N Engl J Med 2006; 354: 1281. Mc. Kay, DB et al. Reproduction and transplantation: report on the AST Consensus Conference on Reproductive Issues and Transplantation. Am J Transplant 2005; 5: 1592. Rahamimov, R et al. Pregnancy in renal transplant recipients: long-term effect on patient and graft survival. A single-center experience. Transplantation 2006; 81: 660. Salmela, KT et al. Impaired renal function after pregnancy in renal transplant recipients. Transplantation 1993; 56: 1372. Sifontis, NM et al. Pregnancy outcomes in solid organ transplant recipients with exposure to mycophenolate mofetil or sirolimus. Transplantation 2006; 82: 1698. Sturgiss, SN et al. Effect of pregnancy on long-term function in renal allografts: an update. Am J Kidney Dis 1995; 26: 54. www. uptodate. com


