c148fd9f19a3c0ab64e81e93ca0b0362.ppt
- Количество слайдов: 78

Practice Basics Chapter 16: Aseptic Technique, Sterile Compounding, and IV Admixture Programs

Learning Outcomes Describe basics of intravenous drug therapy Describe key elements of working in laminar airflow workbenches List types of contamination in a laminar flow hood & describe how to minimize their risks Perform basic manipulations needed to prepare a sterile product by using aseptic technique Describe the risks of handling cytotoxic & hazardous drugs

Learning Outcomes List steps in drug preparation & handling that are unique to cytotoxic & hazardous drugs List typical ingredients of total parenteral nutrition solutions Describe manual & automated means of preparing total parenteral nutrition solutions Describe benefits of having a formal intravenous admixture program Describe how USP 797 has impacted preparation of sterile products
Key Terms Aseptic technique Biological safety cabinet Coring Free –flow protection HEPA filter Laminar airflow workbench (LAFW) Large volume parenteral (LVP) Total parenteral nutrition (TPN) Small volume parenteral (SVP)

Parenteral Drug Administration Parenteral – “not through digestive tract” Intravenous (IV) Intramuscular (IM) Intrathecal (IT) Epidural Intraarticular Intraarterial Intraocular Intraperitoneal Subcutaneous (SQ, SC, Sub. Q)

Risks of Intravenous Therapy Infection Air embolus Bleeding Allergic reaction Incompatibilities Extravasation Particulate Matter Pyrogens Phlebitis

Types of IV Administration Infusions Continuous Intermittent

IV Containers Large Volume Parenterals (LVPs) Small Volume Parenterals or “Piggyback” Systems Add-Vantage® Vial Spike Systems Flexible Plastic Bags Glass Containers
Basic Continuous IV Therapy Large volume parenteral (LVP) hung on an IV pole 36 inches above patient’s bed flow maintained by gravity Sterile tubing attached to LVP primary IV set Catheter in patient’s vein

LVP Usually a simple solution of dilute dextrose sodium chloride or combination of both Additives swab rubber stopper with alcohol & let dry inject drug into fluid remove bottle vacuum
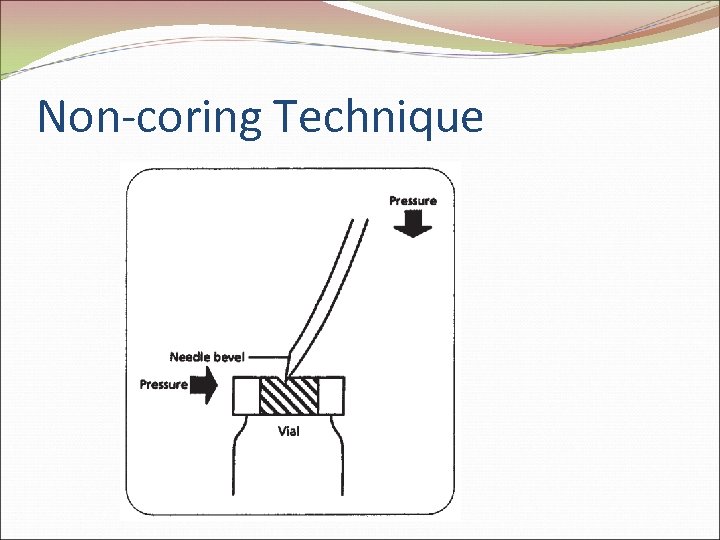
Non-coring Technique

Administration Systems Continuous Infusions more effective & less toxic than when given intermittently basic fluid & electrolyte therapy blood products drugs that require tight administration control Intermittent Injections periodic administration increases efficacy reduces toxicity
Pre-Mixed Admixtures Manufactured LVPs with additives stable in solution for longer periods of time available in many of sizes (250 m. L, 500 m. L, 1000 m. L) Examples lidocaine potassium nitroglycerin dopamine aminophylline

RTU Advantages Reduce handling by pharmacy Reduce potential for contamination Emergency situations-stocked in patient care area Standard concentrations of IV medications decrease potential medication errors in compounding & administration

Pharmacy Prepared Admixtures Volumes (100 m. L, 250 m. L, 500 m. L, or 1000 m. L) Containers (glass, plastic, bag, bottle or syringe) Syringe Systems syringe pumps volume control chambers gravity feed intravenous push systems

Syringe Systems Pharmacy fills syringes with drugs & labels stability in syringes related to drug concentration Syringe Pumps adjusted to administer volume over given period of time pumps are operated by battery or compressed spring may administer single dose or pre-programmed intervals doses must be sent from pharmacy in standard syringe sizes & concentrations
Electronic Infusion Devices Electronic infusion devices increase precision & accuracy in fluid restricted patients when drug must be administered at precise rate “Smart pumps” alert user to problems infusion settings outside recommended range updates may be sent to pumps pump log data may be sent to information system
Volume Control Chambers Buretrol or Volutrol Syringes used to administer drugs through volumetric chamber drug injected through port on top of chamber solution added from primary LVP minimal amounts of fluid can be given per dose beneficial in fluid-restricted or pediatric patients important that medication is followed by IV flush

Gravity Feed Syringes can use gravity to administer drugs vented set allows air to enter syringe inexpensive & requires no other special equipment Intravenous Push injected directly into IV tubing primary IV set is usually clamped off Drug delivered directly to patient Rapid onset of effects of drug

Patient Controlled Analgesia Very effective in managing pain Patient administers dose as soon as pain felt Reduces nursing time Pump programmed Basal rate Bolus when patient pushes button Example: max 1 mg of morphine every 15 minutes If patient pushes button in 10 minutes, drug not released but attempt recorded so that pump tracks if pain not controlled
Unique Infusion Devices Implanted pump drug reservoir for continuous low-dose chemotherapy administration Elastomeric infusion device (EID) acts as its own pump pressure of container forces drug through tubing

Administration Sets Primary IV Set attached to the LVP can be one of several varieties Drip chamber-estimate administration rate by counting drops as they fall through chamber Drip chamber macrodrip or minidrip based on size of drop tubing is labeled according to number of drops it produces from 1 milliliter of solution

Drip Sets Macro-drip sets deliver 10 -20 drops per 1 m. L Minidrip sets deliver sixty drops per 1 m. L Rate controlled by roller clamp or electronic infusion device Drugs injected through ports either Y-sites or flashballs

Venous Access Devices Peripheral insertion most common Peripheral catheters-limitations on what can be infused & at what rate Central catheter more complicated riskier to insert & maintain fewer restrictions concentration of drug rate of administration time venous access can remain in place
Peripheral Catheters Plastic-flexible & most comfortable for patient Steel needle with short end of tubing scalp vein or butterfly may be left in the patient’s vein if flushed Central catheters temporary or permanent access vein with high blood flow
Catheter Examples Permanent catheters Hickman® Broviac® Port-a-cath® Peripheral catheter “peripherally inserted central catheter” (PICC) PICC inserted peripherally flexible catheter threaded through venous system & its tip ends near heart high volume of blood flow

IV Miscellaneous Information Heparin Lock maintain catheter access to vein resealable rubber diaphragm provide port for intermittent use concentration of heparin used in heparin locks is usually 10 units/m. L or 100 units/m. L Needleless Systems reduce risks of needle sticks required in some states & some healthcare systems
IV Misc. Information Continued Final Filters located in the tubing used to remove particles in IV solution used with drugs that have a risk of particulate matter or crystals in final solution examples of drugs requiring filters phenytoin mannitol

Aseptic Preparation Admixture preparation program includes: 1. Development & maintenance of good aseptic technique in personnel who prepare & administerile products 2. Development & maintenance of sterile compounding area, complete with sterilized equipment & supplies 3. Development & maintenance of skills needed to properly use laminar airflow workbench (LAFW) or laminar airflow hood
Aseptic Technique Manipulating sterile products without compromising their sterility proper use of LAFW strict aseptic technique Conscientious work habits

Sterile Compounding Area Compounded sterile products (CSPs) must be free of living microorganisms pyrogens visible particles Reduce number of particles in air no cardboard in clean room Clean work surfaces & floors daily Clean walls, ceilings, & shelving monthly

Sterile Compounding Area Segregate compounding area minimize traffic in sterile compounding area remove trash d frequently & regularly Filter incoming air Ultraviolet irradiation Air-lock entry portals Sticky mats
Sterile Compounding Area Use anteroom for non-aseptic activities order processing gowning handling of stock ISO Class 5 environment no more than 100 particles per cubic foot that are 0. 5 micron or larger in size LAFWs are used to achieve an ISO Class 5 environment

Laminar Airflow Workbenches Principle of LAFWs twice-filtered laminar layers of aseptic air continuously sweep work area inside hood prevents entry of contaminated room air 2 common types of LAFWs horizontal flow vertical flow
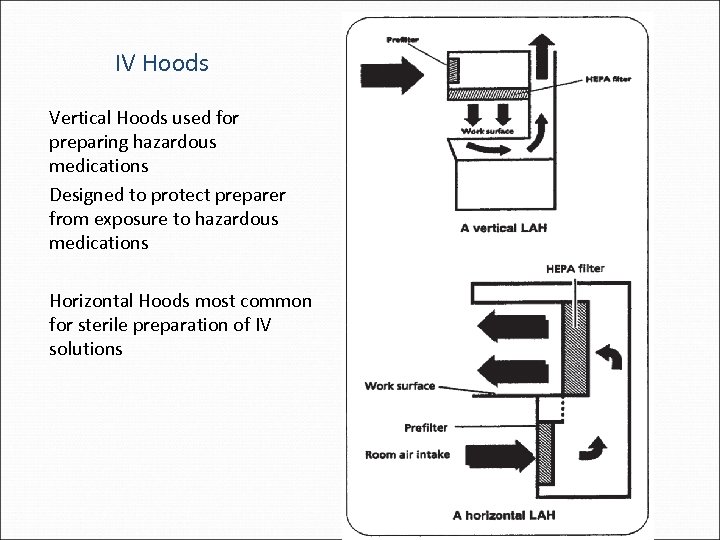
IV Hoods Vertical Hoods used for preparing hazardous medications Designed to protect preparer from exposure to hazardous medications Horizontal Hoods most common for sterile preparation of IV solutions
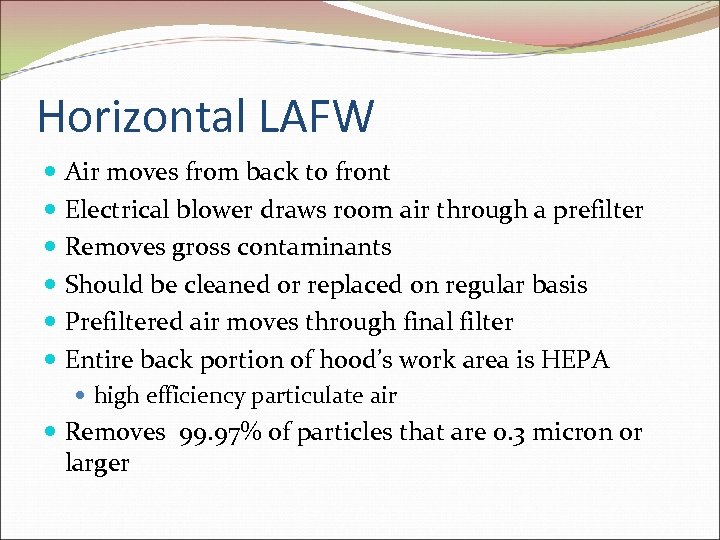
Horizontal LAFW Air moves from back to front Electrical blower draws room air through a prefilter Removes gross contaminants Should be cleaned or replaced on regular basis Prefiltered air moves through final filter Entire back portion of hood’s work area is HEPA high efficiency particulate air Removes 99. 97% of particles that are 0. 3 micron or larger
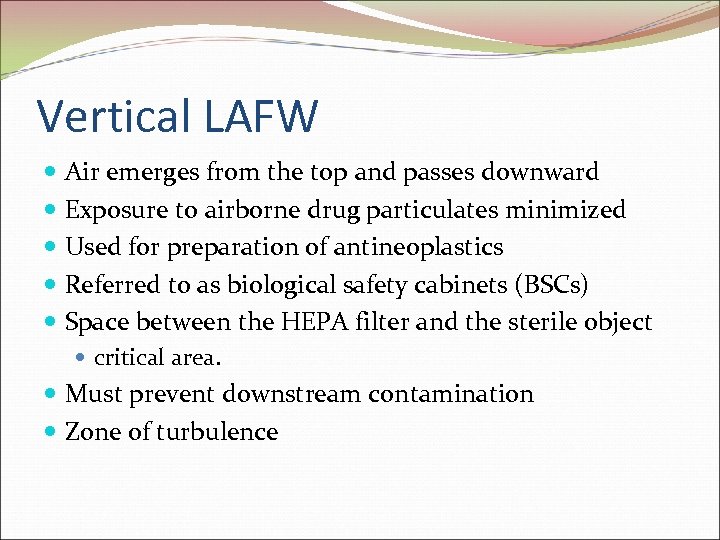
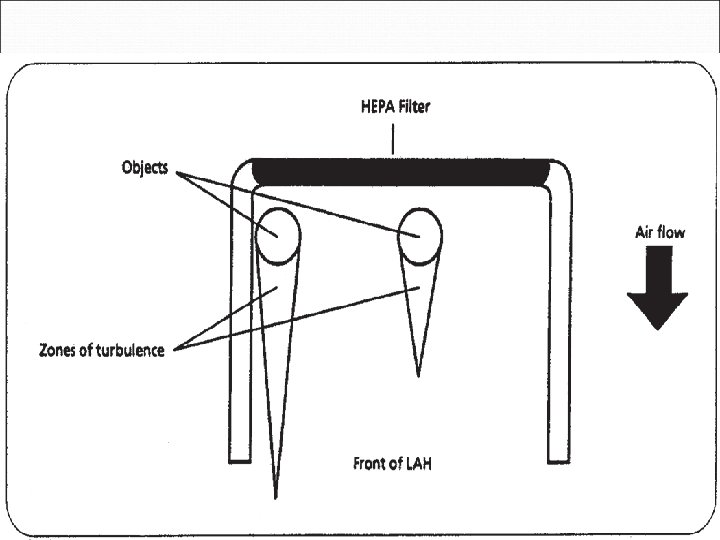
Vertical LAFW Air emerges from the top and passes downward Exposure to airborne drug particulates minimized Used for preparation of antineoplastics Referred to as biological safety cabinets (BSCs) Space between the HEPA filter and the sterile object critical area. Must prevent downstream contamination Zone of turbulence

LAFW Principles Position away from excess traffic, doors, air vents, etc. Must run for 15 -3 o minutes if turned off & back on All interior working surfaces should be cleaned 70% isopropyl alcohol/other disinfecting agent clean, lint-free cloth

Cleaning LAFWs Clean sides of hoods using up & down direction start at HEPA work toward outer edge of hood Order of cleaning walls 1 st floor of hood 2 nd

Cleaning LAFWs Frequency beginning of each shift before each batch not longer than 30 minutes following previous surface disinfection when ongoing compounding activities are occurring after spills when surface contamination is known or suspected

Cleaning LAFWs If materials not soluble in alcohol, initially use water follow with alcohol Do not use spray bottles of alcohol in hood Let alcohol air dry Clean Plexiglas sides -warm, soapy water Alcohol will dry out Plexiglas clouds & cracks

Additional LAFW Instructions Nothing should come in contact with HEPA filter Nothing in hood that is not essential IV preparation no paper, pens, labels, or trays No jewelry on hands or wrists Talk & cough away from LAFW No smoking, eating, drinking in aseptic area Manipulations at least six inches within hood

Additional LAFW Instructions Must test LAFWs at least every 6 months Also test if hood moved, or if filter damage suspected Specific tests airflow velocity HEPA filter integrity Strict aseptic technique must be used

Aseptic Environment Personal Attire -Cover Shoes, head & facial hair, use face masks/eye shields cover scrub suits when leaving pharmacy Handwashing touch is most common source of contamination scrub hands, nails, wrists, forearms to elbows for at least 30 seconds with a brush, warm water, & appropriate bactericidal soap Gloving only sterile until they touch something unsterile
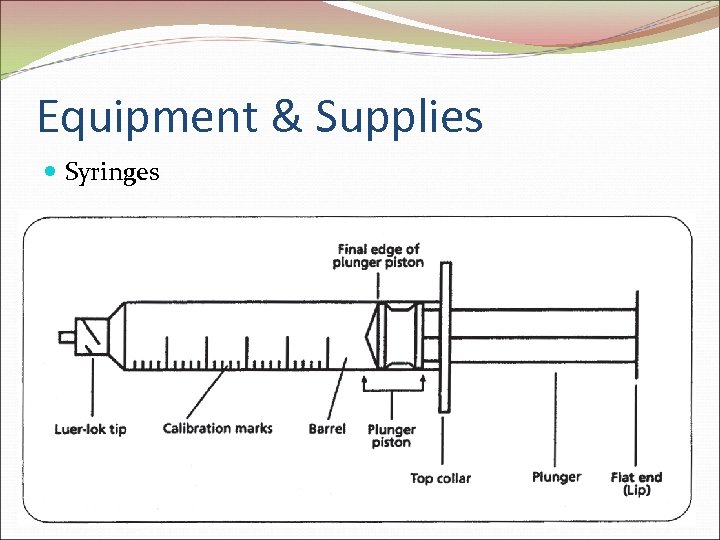
Equipment & Supplies Syringes
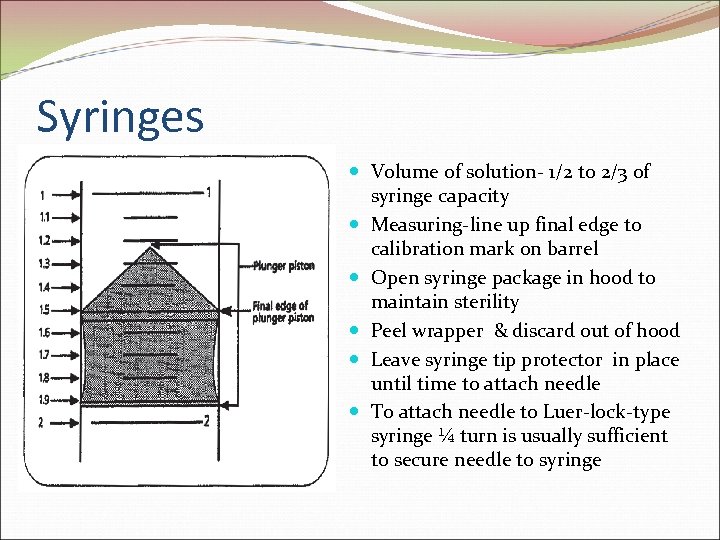
Syringes Volume of solution- 1/2 to 2/3 of syringe capacity Measuring-line up final edge to calibration mark on barrel Open syringe package in hood to maintain sterility Peel wrapper & discard out of hood Leave syringe tip protector in place until time to attach needle To attach needle to Luer-lock-type syringe ¼ turn is usually sufficient to secure needle to syringe
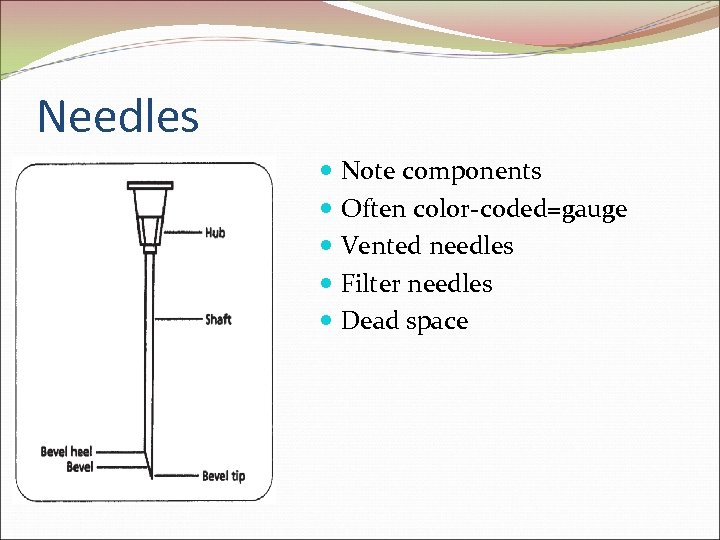
Needles Note components Often color-coded=gauge Vented needles Filter needles Dead space

Vials Rubber stopper Powders or liquids 70% isopropyl alcohol Avoid coring Normalize pressure Reconstitution SDV or MDV Preservative considerations
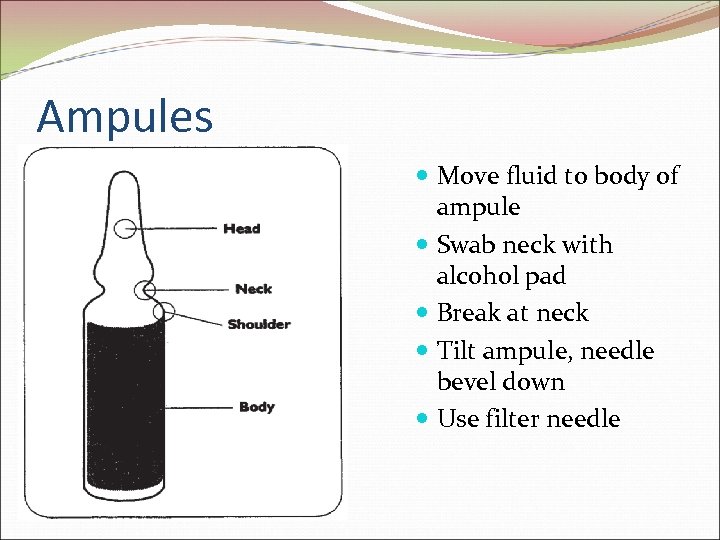
Ampules Move fluid to body of ampule Swab neck with alcohol pad Break at neck Tilt ampule, needle bevel down Use filter needle

Prefilled Syringes Manufactured ready-to-inject syringes Commonly given IM, IV, or subcutaneously Convenient for administration emergency situations Most likely to be kept in patient care areas

Preparation of IV Admixtures Pharmacist inputs order into computer system Assemble all materials & visually inspect Clean hood-only needed products in hood Disinfect all injection surfaces Withdraw & measure drug fluid Remove air bubbles from syringe Discard syringes & uncapped needles Recapping needles is generally unsafe practice use one-handed scoop method if recap needed

Closures & Seals Luer Tips for syringes when final product being dispensed is not intended for injection oral topical IV port seals Tamperproof caps
Automated Compounding Sterile product preparation is technically complex Verification challenging Automation can eliminate preparation errors Enclosed IV preparation environments & robotics used in high volume situations or may prepare patient specific doses

Labeling of IV Preparations 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Patient name, identification #, room # Bottle or bag sequence number Name & amount of drug(s) added Name & volume of admixture solution Final total volume of admixture Prescribed flow rate (in milliliters per hour) Date & time of scheduled administration Date & time of preparation Expiration date Initials of person who prepared/checked IV admixture Auxiliary labeling Bar coding

Beyond Use Date (Exp Date) Label & final sterile product- validated by registered pharmacist Label with beyond use date (BUD) stability sterility Policies & procedures substantiated by references published literature reasonable professional judgment

Cytotoxic & Hazardous Drugs Hazardous agents special procedures for labeling, storage, transport special clothing Biological Safety Cabinets (BSCs) special handling of spills & waste Additional information is available from ASHP Technical Assistance Bulletin on Handling of Cytotoxic and Hazardous Drugs

Protective Apparel Disposable coveralls 0 r or solid front gown Low-permeability, lint-free fabric Long sleeves & tight-fitting elastic or knit cuffs Wash hands before putting on the gloves & after removing them One or two pairs of gloves may be required Tuck one pair under cuffs of gown & place second pair over cuffs

First Aid Eyewash fountain in work area with hazardous drugs Appropriate first aid equipment Follow established first aid procedures Obtain medical attention without delay & document injury

Biological Safety Cabinet (BSC) Type of vertical LAFW Designed to protect workers BSCs must meet standards set by National Sanitation Foundation (NSF Standard 49) Do not use horizontal LAFWs to prepare hazardous drugs

BSC Front air barrier-protects handler from contact with hazardous drug dusts & aerosols Types of Class II BSCs Type A Type B BSCs must be operated continuously, 24/7 Inspected & certified every 6 months Clean work surface, back, side walls with water or cleaner recommended by cabinet manufacturer

BSC Disinfect work surface with 70% isopropyl alcohol Do not to use excessive amounts of alcohol Treat cleaning supplies as hazardous waste Decontaminate on weekly basis/immediately after spill Refer to facility’s procedure on hood maintenance for specific cleaning procedures & schedules

Preparing Hazardous Drugs Same as regular drugs EXCEPT attach & prime IV sets before adding hazardous drug maintain slight negative pressure inside vial or use chemotherapy dispensing pin use syringes & IV sets with locking fittings use oversize syringe for reconstitution apply warnings on IV bag (Hazardous) place IV in sealable bag to contain any leakage

Waste Disposal & Spill Cleanup Spills-use spill kit cleanup should follow established procedures kits contain protective gear, eye protection respirator utility & latex gloves disposable gown or coveralls shoe covers scoop, plastic container for glass fragments, absorbent spill pads, gauze & disposable toweling, absorbent powder, & sealable, thick plastic waste disposal bags
Total Parenteral Nutrition TPNs aka hyperalimentation Contain carbohydrates protein fats water electrolytes vitamins trace elements

TPN Therapy Meets nutritional needs for patients who can’t eat who will not eat who should not eat who cannot eat enough to sustain their needs due to increased nutritional requirements from their medical condition
Components of TPNs Base components dextrose (carbohydrates) amino acids (protein) may also include fat & water Additives electrolytes vitamins trace elements (micronutrients) drugs such as heparin, insulin, H 2 antagonists
Components Dextrose -usually a 50% or 70% final dextrose concentration ~25% if via central vein maximum of 10– 12. 5% for peripheral administration Protein –usually 8. 5%, 10%, or 15% special formulations for pediatric patients, kidney disease, liver disease, high stress situation (ICU pts) Fats (or lipids)-10% or 20% fat emulsions separately through peripheral IV line or may be added to TPN solution: 3 -in-1 solution
Components Water Electrolytes –to meet daily metabolic needs sodium, potassium, chloride, acetate, phosphate, magnesium, calcium administered as a specific salt of product can cause precipitation: wrong sequence or concentrations of electrolytes are added to bag Vitamins- “MVI” for multiple-vitamin infusion Vitamin K (phytonadione) Trace elements for proper enzymatic reactions
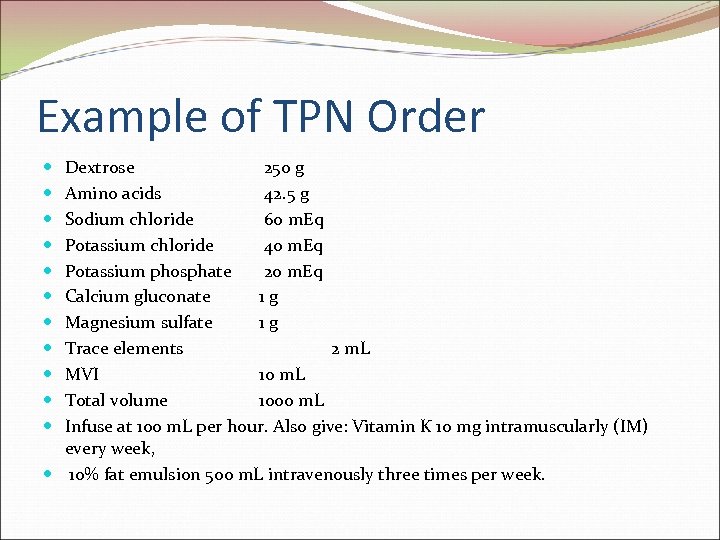
Example of TPN Order Dextrose 250 g Amino acids 42. 5 g Sodium chloride 60 m. Eq Potassium chloride 40 m. Eq Potassium phosphate 20 m. Eq Calcium gluconate 1 g Magnesium sulfate 1 g Trace elements 2 m. L MVI 10 m. L Total volume 1000 m. L Infuse at 100 m. L per hour. Also give: Vitamin K 10 mg intramuscularly (IM) every week, 10% fat emulsion 500 m. L intravenously three times per week.
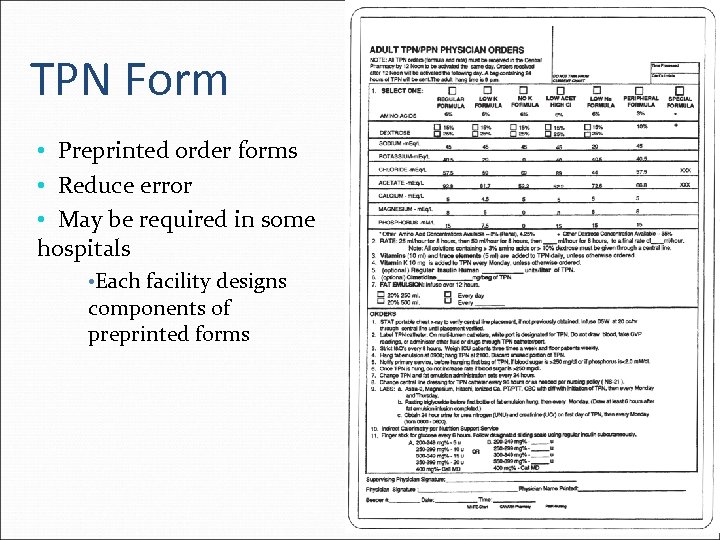
TPN Form • Preprinted order forms • Reduce error • May be required in some hospitals • Each facility designs components of preprinted forms
Preparation of TPN Solutions Automated compounder 2 primary versions of TPN compounders 1 st-provides a separate compounder for base solutions and electrolytes 2 nd -uses one compounder to infuse all compounded ingredients Gravity fill preparation
Administration Central line immediate dilution of administered solution by blood allows use of very concentrated solution Peripheral parenteral nutrition (PPN) same components as TPN not as concentrated may not meet all the patient’s nutritional needs

Pediatric IV Drug Administration Doses individualized calculated based on patients’ body weight Intermittent doses via syringe through volume control chamber or by using syringepump maximize accuracy Minimize amount of fluid Calculations should be checked & double-checked

Epidural Administration Special catheter into epidural space of spine Drug injected at nerve ending-dose greatly reduced All solutions must be free of preservatives Epidural patient controlled analgesia Continuous infusions Bolus injections

Admixture Programs Policies & Procedures Space Training Equipment Standard & Non-Standard Preparations Labeling Handling

Quality Assurance Program ASHP’s Technical Assistance Bulletin on Quality Assurance for Pharmacy-Prepared Sterile Products preparation expiration dating labeling facilities equipment personnel education training evaluation end-product testing

USP Chapter 797 Refer to USP Chapter 797, “Pharmaceutical Compounding—Sterile Preparations” recommendations & regulations regarding IV admixture programs different levels of risk for products fourth class, immediate-use CSPs training policies & procedures garb, aseptic technique, process validation, end-product evaluation
c148fd9f19a3c0ab64e81e93ca0b0362.ppt