d22bf6982bf11cecc1d3f1fe45acb138.ppt
- Количество слайдов: 56

 Practical High Sensitivity LC-MS Fundamentals, Challenges, and Prospects Gary A. Valaskovic, Ph. D. New Objective, Inc.
Practical High Sensitivity LC-MS Fundamentals, Challenges, and Prospects Gary A. Valaskovic, Ph. D. New Objective, Inc.
 Main Topics • • • Anatomy of Electrospray Introduction to Nanospray The Nanobore LC Advantage Flow Splitting and Sample Injection Nanobore LC to MS Interfacing Keys to Success
Main Topics • • • Anatomy of Electrospray Introduction to Nanospray The Nanobore LC Advantage Flow Splitting and Sample Injection Nanobore LC to MS Interfacing Keys to Success
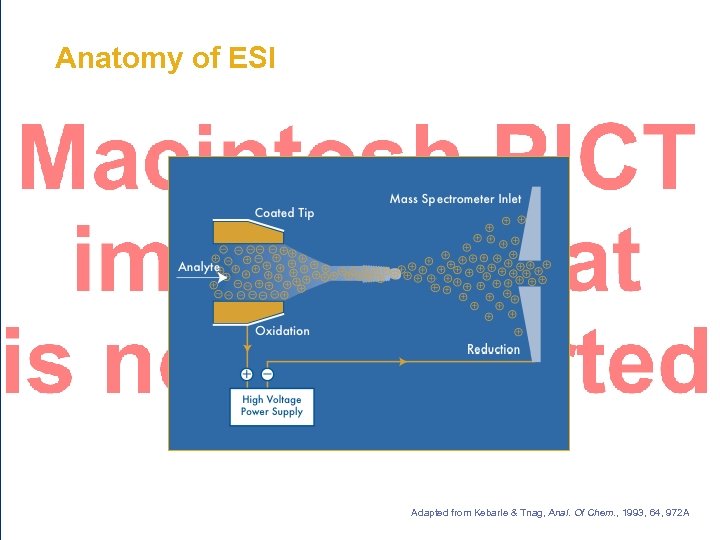 Anatomy of ESI Adapted from Kebarle & Tnag, Anal. Of Chem. , 1993, 64, 972 A
Anatomy of ESI Adapted from Kebarle & Tnag, Anal. Of Chem. , 1993, 64, 972 A
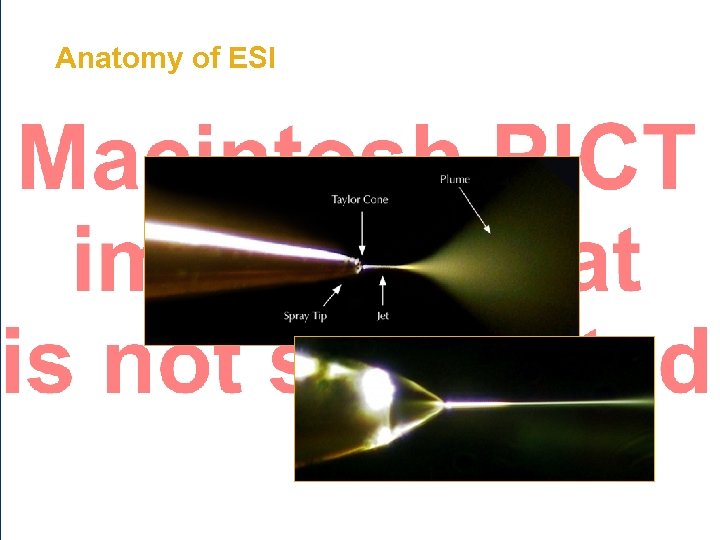 Anatomy of ESI
Anatomy of ESI
 What is Nanospray? Flavor of ESI Flow Rate Sheath Gas Conventional 50 to 1000 µL/min Yes Microspray 0. 1 to 10 µL/min Optional Nanospray <0. 01 to 0. 2 µL/min Not usually
What is Nanospray? Flavor of ESI Flow Rate Sheath Gas Conventional 50 to 1000 µL/min Yes Microspray 0. 1 to 10 µL/min Optional Nanospray <0. 01 to 0. 2 µL/min Not usually
 Why Use Nanospray? ESI-MS (as commonly implemented) is a concentration sensitive detector. There is little or no loss in signal/noise as you reduce the flow rate. You can obtain the same S/N for most compounds from 1 m. L/min to 10 n. L/min (with the right equipment)! Adapted From Cody, Appl. Elec. Mass. Spec. , Pramanik, Ganguly, Gross Eds.
Why Use Nanospray? ESI-MS (as commonly implemented) is a concentration sensitive detector. There is little or no loss in signal/noise as you reduce the flow rate. You can obtain the same S/N for most compounds from 1 m. L/min to 10 n. L/min (with the right equipment)! Adapted From Cody, Appl. Elec. Mass. Spec. , Pramanik, Ganguly, Gross Eds.
 Why Use Nanospray? There are three reasons to use Nanospray: Sensitivity Nanospray is one of the key technologies for MS-based Proteomics
Why Use Nanospray? There are three reasons to use Nanospray: Sensitivity Nanospray is one of the key technologies for MS-based Proteomics
 How Does Nanospray Yield Sensitivity? Two ways to obtain sensitivity with Nanospray: Off-line “Static” Nanospray • Extend the analysis time for a given sample – Sum spectra to increase S/N – Complete MS/MS or MSn possible On-line LC-Nanospray • Analyze a small volume sample (1 µL or much less) – Concentrate your sample into as small a volume as possible
How Does Nanospray Yield Sensitivity? Two ways to obtain sensitivity with Nanospray: Off-line “Static” Nanospray • Extend the analysis time for a given sample – Sum spectra to increase S/N – Complete MS/MS or MSn possible On-line LC-Nanospray • Analyze a small volume sample (1 µL or much less) – Concentrate your sample into as small a volume as possible
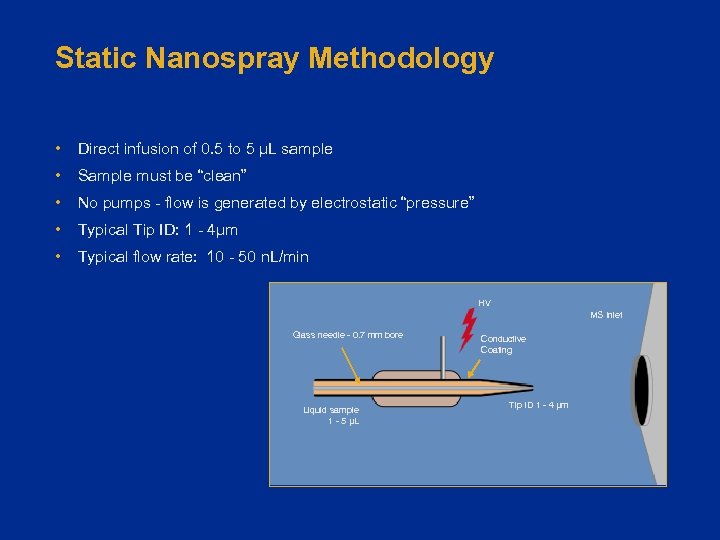 Static Nanospray Methodology • Direct infusion of 0. 5 to 5 µL sample • Sample must be “clean” • No pumps - flow is generated by electrostatic “pressure” • Typical Tip ID: 1 - 4µm • Typical flow rate: 10 - 50 n. L/min HV MS Inlet Glass needle - 0. 7 mm bore Liquid sample 1 - 5 µL Conductive Coating Tip ID 1 - 4 µm
Static Nanospray Methodology • Direct infusion of 0. 5 to 5 µL sample • Sample must be “clean” • No pumps - flow is generated by electrostatic “pressure” • Typical Tip ID: 1 - 4µm • Typical flow rate: 10 - 50 n. L/min HV MS Inlet Glass needle - 0. 7 mm bore Liquid sample 1 - 5 µL Conductive Coating Tip ID 1 - 4 µm
 Static Nanospray Extends Analysis Time Adapted From Corey & Pinto, Appl. Elec. Mass. Spec. , Pramanik, Ganguly, Gross Eds.
Static Nanospray Extends Analysis Time Adapted From Corey & Pinto, Appl. Elec. Mass. Spec. , Pramanik, Ganguly, Gross Eds.
 Static Nanospray Limitations • Sensitivity is good, but inferior to LC methods – Typically 10 -100 fmol proteins and peptides • Sample prep is not integral, sample must be clean and concentrated – Typically 100 n. M to 10 µM • Limited utility on complex mixtures (OK on single bands but unable to handle “shotgun” methods) • Highly dependent on operator skill • Limited throughput • Automation is possible but $$$
Static Nanospray Limitations • Sensitivity is good, but inferior to LC methods – Typically 10 -100 fmol proteins and peptides • Sample prep is not integral, sample must be clean and concentrated – Typically 100 n. M to 10 µM • Limited utility on complex mixtures (OK on single bands but unable to handle “shotgun” methods) • Highly dependent on operator skill • Limited throughput • Automation is possible but $$$
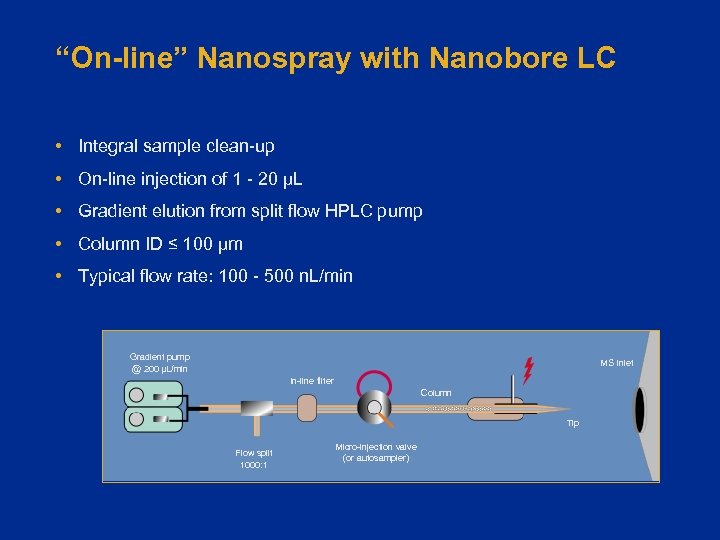 “On-line” Nanospray with Nanobore LC • Integral sample clean-up • On-line injection of 1 - 20 µL • Gradient elution from split flow HPLC pump • Column ID ≤ 100 µm • Typical flow rate: 100 - 500 n. L/min Gradient pump @ 200 µL/min MS Inlet In-line filter Column Tip Flow split 1000: 1 Micro-injection valve (or autosampler)
“On-line” Nanospray with Nanobore LC • Integral sample clean-up • On-line injection of 1 - 20 µL • Gradient elution from split flow HPLC pump • Column ID ≤ 100 µm • Typical flow rate: 100 - 500 n. L/min Gradient pump @ 200 µL/min MS Inlet In-line filter Column Tip Flow split 1000: 1 Micro-injection valve (or autosampler)
 Why Use Nanospray LC? 4. 6 mm 50 µm Elute your sample into the smallest practical volume for the highest S/N!
Why Use Nanospray LC? 4. 6 mm 50 µm Elute your sample into the smallest practical volume for the highest S/N!
![Why Use Nanobore LC? The Concentration Advantage! Column ID Flow Rate Relative [C] Standard Why Use Nanobore LC? The Concentration Advantage! Column ID Flow Rate Relative [C] Standard](https://present5.com/presentation/d22bf6982bf11cecc1d3f1fe45acb138/image-15.jpg) Why Use Nanobore LC? The Concentration Advantage! Column ID Flow Rate Relative [C] Standard 4. 6 mm 1 m. L/min 1 Microbore 1 mm 50 µL/min 21 Capillary 320 µm 5 µL/min 206 Nanobore 75 µm 250 n. L/min 3, 750 Nanobore 50 µm 150 n. L/min 8, 450 Adapted From Tomer & Moseley, Mass. Spec. Rev. , 1994, 13, 431
Why Use Nanobore LC? The Concentration Advantage! Column ID Flow Rate Relative [C] Standard 4. 6 mm 1 m. L/min 1 Microbore 1 mm 50 µL/min 21 Capillary 320 µm 5 µL/min 206 Nanobore 75 µm 250 n. L/min 3, 750 Nanobore 50 µm 150 n. L/min 8, 450 Adapted From Tomer & Moseley, Mass. Spec. Rev. , 1994, 13, 431
 Requirements for LC System Gradient Operation • Binary required; tertiary, quaternary preferred Injection • 1 - 20 µL Typical • Accommodate sample trapping Flow rate ≈ 100 to 1000 n. L/min • Typically pre-column flow split from conventional pump
Requirements for LC System Gradient Operation • Binary required; tertiary, quaternary preferred Injection • 1 - 20 µL Typical • Accommodate sample trapping Flow rate ≈ 100 to 1000 n. L/min • Typically pre-column flow split from conventional pump
 Flow Splitting Methods Simple “T” Splitter (build) • Inexpensive! Easy to do. Split is non-linear but reproducible. Balanced Flow Splitter (build or buy) • Good performance, inexpensive High-Pressure Flow Splitter (buy) • Good performance, $$$ “Active” Mass Flow Control (buy) • Good performance, $$$
Flow Splitting Methods Simple “T” Splitter (build) • Inexpensive! Easy to do. Split is non-linear but reproducible. Balanced Flow Splitter (build or buy) • Good performance, inexpensive High-Pressure Flow Splitter (buy) • Good performance, $$$ “Active” Mass Flow Control (buy) • Good performance, $$$
 Simple Flow Splitting • Use a simple Tee • Use a small bore (20 - 50 µm ID) tubing to create a flow “calibrator” • Adjust split ratio by adjusting the length of the calibrator • Fine tune by setting the pump flow • Ratios from 1: 10 to 1: 1000 are readily obtained
Simple Flow Splitting • Use a simple Tee • Use a small bore (20 - 50 µm ID) tubing to create a flow “calibrator” • Adjust split ratio by adjusting the length of the calibrator • Fine tune by setting the pump flow • Ratios from 1: 10 to 1: 1000 are readily obtained
 Nanospray Source Requirements • Mechanical requirements – XYZ Stage for tip positioning – Tip and spray imaging system – Junction and proximal HV contact • Tip requirements – ID of 10 - 30 µm – Typically fused-silica, 360 µm OD – Uncoated or coated
Nanospray Source Requirements • Mechanical requirements – XYZ Stage for tip positioning – Tip and spray imaging system – Junction and proximal HV contact • Tip requirements – ID of 10 - 30 µm – Typically fused-silica, 360 µm OD – Uncoated or coated
 On-line Nanospray Source Objective Lens Tip Holder HV Contact CCD Camera XYZ Stage Injection Valve www. newobjective. com
On-line Nanospray Source Objective Lens Tip Holder HV Contact CCD Camera XYZ Stage Injection Valve www. newobjective. com
 On-line Nanospray Source Monitor Illuminator Source
On-line Nanospray Source Monitor Illuminator Source
 What About Sample Injection? Gradient elution in reverse phase enables sample stacking: • Large (1 - 20 µL) injection volumes are OK If we ran isocratically, a 75 µm ID column would require a 10 - 20 n. L injection volume!
What About Sample Injection? Gradient elution in reverse phase enables sample stacking: • Large (1 - 20 µL) injection volumes are OK If we ran isocratically, a 75 µm ID column would require a 10 - 20 n. L injection volume!
 Injection Strategies • On-column Injection (Pressure Bomb) – High sensitivity – Zero sample loss or waste – Time consuming (manual) • “Micro” Injection Valve – 0. 1 - 5 µL – Easy to use • Sample Trapping – Faster injection of large volumes (5 - 20 µL) – Trap protects columns for increased lifetime – Some peptides lost during injection and analysis
Injection Strategies • On-column Injection (Pressure Bomb) – High sensitivity – Zero sample loss or waste – Time consuming (manual) • “Micro” Injection Valve – 0. 1 - 5 µL – Easy to use • Sample Trapping – Faster injection of large volumes (5 - 20 µL) – Trap protects columns for increased lifetime – Some peptides lost during injection and analysis
 Bomb Injection To Column Pressure Bomb Gas In Sample Vial
Bomb Injection To Column Pressure Bomb Gas In Sample Vial
 Sample Trapping • Trap Cartridge/Column – 100 - 500 µm ID – 1 - 25 mm in length • Typically C 18 or SCX • Loading rate 1 - 20 µL/min • Enable hundreds/thousands of injections on an analytical column Fused Silica Column
Sample Trapping • Trap Cartridge/Column – 100 - 500 µm ID – 1 - 25 mm in length • Typically C 18 or SCX • Loading rate 1 - 20 µL/min • Enable hundreds/thousands of injections on an analytical column Fused Silica Column
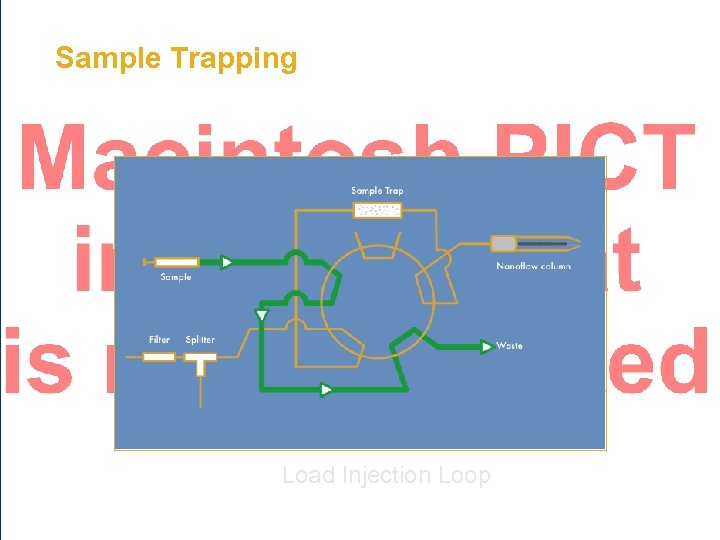 Sample Trapping Load Injection Loop
Sample Trapping Load Injection Loop
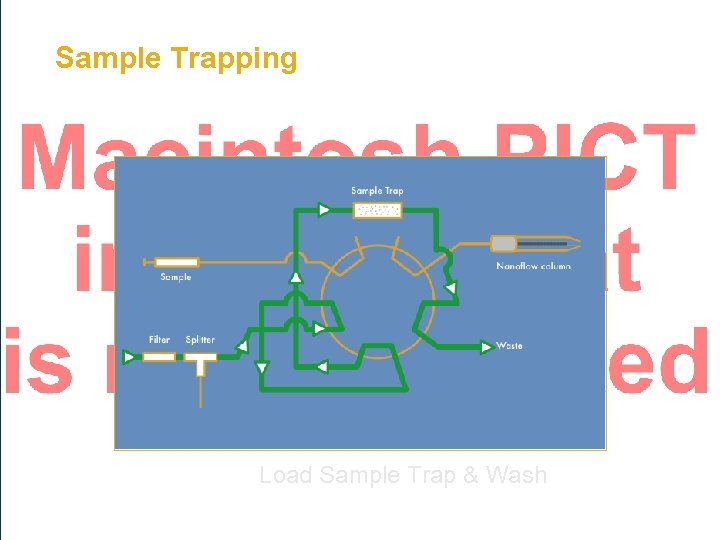 Sample Trapping Load Sample Trap & Wash
Sample Trapping Load Sample Trap & Wash
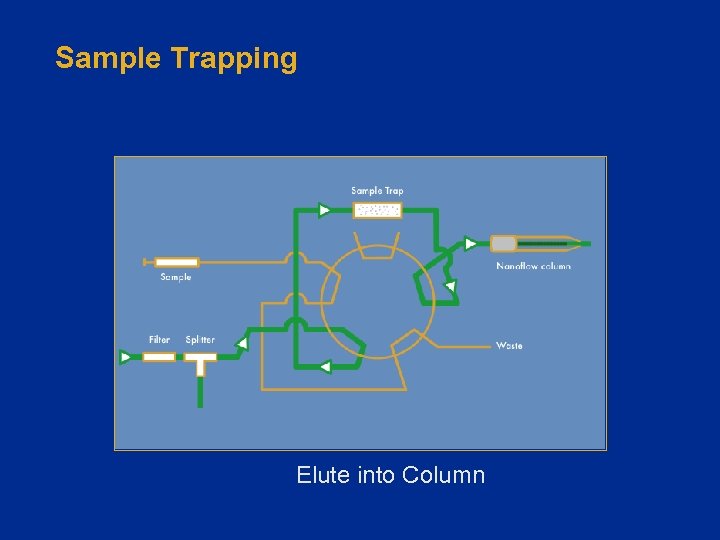 Sample Trapping Elute into Column
Sample Trapping Elute into Column
 How Do We Interface? • Liquid sheath for make-up flow (The Early Days) – Generally not used, compromised sensitivity • “Direct Connect” interface with fused-silica tip – No “make-up” or sheath liquid – Reasonable sensitivity – Plumbing can be a challenge • Integration of LC column with emitter – Highest sensitivity – Robust interface – Greater ease of use
How Do We Interface? • Liquid sheath for make-up flow (The Early Days) – Generally not used, compromised sensitivity • “Direct Connect” interface with fused-silica tip – No “make-up” or sheath liquid – Reasonable sensitivity – Plumbing can be a challenge • Integration of LC column with emitter – Highest sensitivity – Robust interface – Greater ease of use
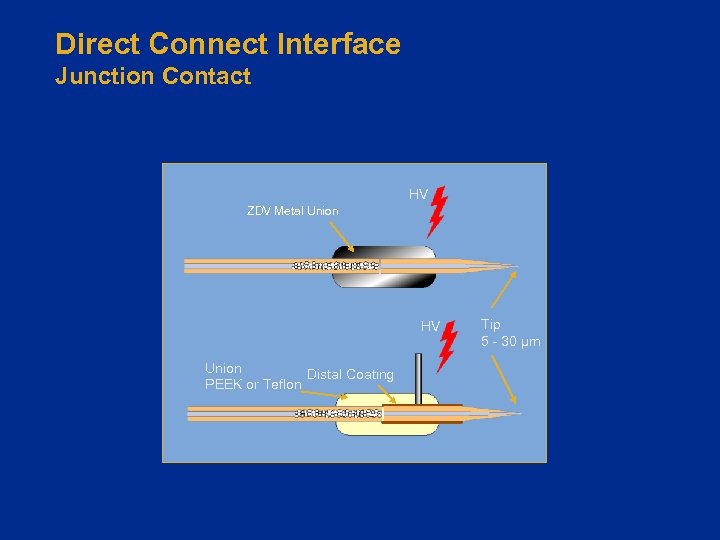 Direct Connect Interface Junction Contact HV ZDV Metal Union HV Union Distal Coating PEEK or Teflon Tip 5 - 30 µm
Direct Connect Interface Junction Contact HV ZDV Metal Union HV Union Distal Coating PEEK or Teflon Tip 5 - 30 µm
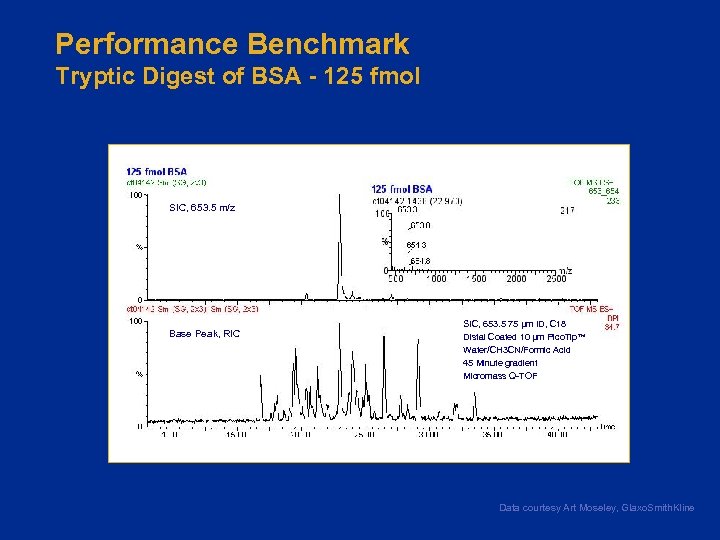 Performance Benchmark Tryptic Digest of BSA - 125 fmol SIC, 653. 5 m/z Base Peak, RIC SIC, 653. 5 75 µm ID, C 18 Distal Coated 10 µm Pico. Tip™ Water/CH 3 CN/Formic Acid 45 Minute gradient Micromass Q-TOF Data courtesy Art Moseley, Glaxo. Smith. Kline
Performance Benchmark Tryptic Digest of BSA - 125 fmol SIC, 653. 5 m/z Base Peak, RIC SIC, 653. 5 75 µm ID, C 18 Distal Coated 10 µm Pico. Tip™ Water/CH 3 CN/Formic Acid 45 Minute gradient Micromass Q-TOF Data courtesy Art Moseley, Glaxo. Smith. Kline
 Direct Connect Interface Common Problems Poor peak shape • Difficult post-column plumbing, requiring a “perfect” connection Impractical with columns smaller than ≈75 µm • Clogged tips and columns • Difficult to distinguish point of plug - is it the column or the tip? Air bubbles in line • Out-gassing, leaks, electrolysis, etc.
Direct Connect Interface Common Problems Poor peak shape • Difficult post-column plumbing, requiring a “perfect” connection Impractical with columns smaller than ≈75 µm • Clogged tips and columns • Difficult to distinguish point of plug - is it the column or the tip? Air bubbles in line • Out-gassing, leaks, electrolysis, etc.
 Pico. Frit™ Packed Tip Performance 75 µm ID, C 18 Frit Tip: 8 - 15 µm Pack the LC column directly into the tip! “Zero” post column volume Emmett & Caprioli, J. Am. Soc. Mass. Spec. 1994, 5, 605 -613
Pico. Frit™ Packed Tip Performance 75 µm ID, C 18 Frit Tip: 8 - 15 µm Pack the LC column directly into the tip! “Zero” post column volume Emmett & Caprioli, J. Am. Soc. Mass. Spec. 1994, 5, 605 -613
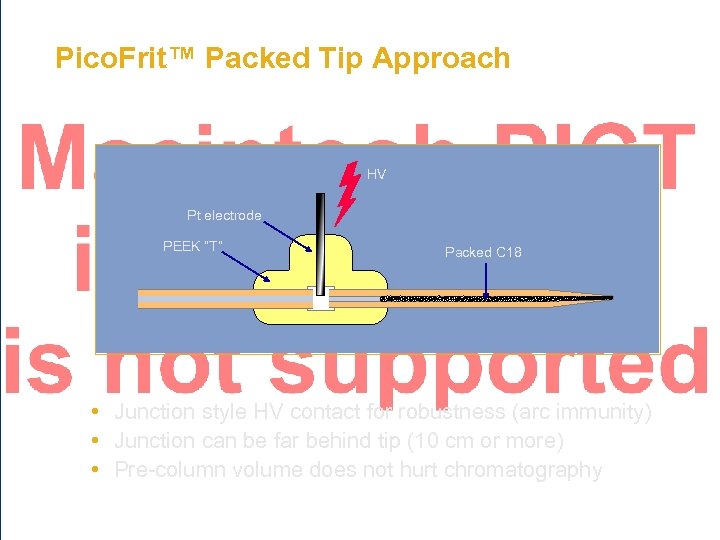 Pico. Frit™ Packed Tip Approach HV Pt electrode PEEK “T” Packed C 18 • Junction style HV contact for robustness (arc immunity) • Junction can be far behind tip (10 cm or more) • Pre-column volume does not hurt chromatography
Pico. Frit™ Packed Tip Approach HV Pt electrode PEEK “T” Packed C 18 • Junction style HV contact for robustness (arc immunity) • Junction can be far behind tip (10 cm or more) • Pre-column volume does not hurt chromatography
 Pico. Frit™ Approach Analytical Advantages • Tip size optimal for column flow rate – Typically 8 -15 µm for 75 µm ID column • HV contact on inlet side of column – Minimal contribution to band broadening w/sample stacking – Eliminates air bubbles (high pressure side of column) – Robust and easy to use • Economical – Concurrent fabrication of tip and column
Pico. Frit™ Approach Analytical Advantages • Tip size optimal for column flow rate – Typically 8 -15 µm for 75 µm ID column • HV contact on inlet side of column – Minimal contribution to band broadening w/sample stacking – Eliminates air bubbles (high pressure side of column) – Robust and easy to use • Economical – Concurrent fabrication of tip and column
 Packed Tip Appraoch Analytical Advantages • Optimal sensitivity and resolution – Spray directly from column – Virtually zero post-column volume • Virtually eliminates tip clogging – Robust lifetime – 500+ injections/column with sample trapping • Easy to use – Fewer connections to make
Packed Tip Appraoch Analytical Advantages • Optimal sensitivity and resolution – Spray directly from column – Virtually zero post-column volume • Virtually eliminates tip clogging – Robust lifetime – 500+ injections/column with sample trapping • Easy to use – Fewer connections to make
 Pico. Frit™ Columns Performance Benchmark Data courtesy James P. Murphy III, Ph. D.
Pico. Frit™ Columns Performance Benchmark Data courtesy James P. Murphy III, Ph. D.
 Pico. Frit™ Columns Performance Benchmark
Pico. Frit™ Columns Performance Benchmark
 Keys to Success
Keys to Success
 Minimize Particle Contamination
Minimize Particle Contamination
 Minimize Particle Contamination Contaminated Column Head Clean Column Head Mobile Phase Stocks • Change Stocks Regularly (weekly or better) • Use bottled water, preferrably distilled in glass • Avoid “ultrpure” meg-ohm water from in-house systems – These can contain high levels of carbon particulates Poor quality water is the primary cause of clogged columns!
Minimize Particle Contamination Contaminated Column Head Clean Column Head Mobile Phase Stocks • Change Stocks Regularly (weekly or better) • Use bottled water, preferrably distilled in glass • Avoid “ultrpure” meg-ohm water from in-house systems – These can contain high levels of carbon particulates Poor quality water is the primary cause of clogged columns!
 Minimize Particle Contamination Fittings and Unions • Use PEEK or FEP adapter sleeves • Don’t over tighten fittings • Avoid graphitized ferrules (common in GC) • Discard contaminated fittings OUCH!
Minimize Particle Contamination Fittings and Unions • Use PEEK or FEP adapter sleeves • Don’t over tighten fittings • Avoid graphitized ferrules (common in GC) • Discard contaminated fittings OUCH!
 Minimize Particle Contamination • • • Injection valves Avoid “scribing” surface of rotor with fused-silica Inspect surfaces often Pump components Inspect/replace seals, fittings, check valves and filters Watch out!
Minimize Particle Contamination • • • Injection valves Avoid “scribing” surface of rotor with fused-silica Inspect surfaces often Pump components Inspect/replace seals, fittings, check valves and filters Watch out!
 Measuring Column Flow Rate • Let a droplet collect at tip for 5 -10 minutes (ESI is off) • Collect the droplet by capillary action • Measure the volume and calculate flow rate
Measuring Column Flow Rate • Let a droplet collect at tip for 5 -10 minutes (ESI is off) • Collect the droplet by capillary action • Measure the volume and calculate flow rate
 Source Tuning: Go For the Best Spray 850 V Stream and Plume 50% ACN, 0. 1% Formic Acid 500 n. L/min, 15 µm Picofrit™ tip, LCQ™ Deca XP Inlet
Source Tuning: Go For the Best Spray 850 V Stream and Plume 50% ACN, 0. 1% Formic Acid 500 n. L/min, 15 µm Picofrit™ tip, LCQ™ Deca XP Inlet
 Source Tuning: Go For the Best Spray 1150 V Stream and Plume 850 V Stream and Plume 50% ACN, 0. 1% Formic Acid 500 n. L/min, 15 µm Picofrit™ tip, LCQ™ Deca XP Inlet
Source Tuning: Go For the Best Spray 1150 V Stream and Plume 850 V Stream and Plume 50% ACN, 0. 1% Formic Acid 500 n. L/min, 15 µm Picofrit™ tip, LCQ™ Deca XP Inlet
 Source Tuning: Go For the Best Spray 1450 V Good Plume 850 V Stream and Plume 50% ACN, 0. 1% Formic Acid 500 n. L/min, 15 µm Picofrit™ tip, LCQ™ Deca XP Inlet
Source Tuning: Go For the Best Spray 1450 V Good Plume 850 V Stream and Plume 50% ACN, 0. 1% Formic Acid 500 n. L/min, 15 µm Picofrit™ tip, LCQ™ Deca XP Inlet
 Source Tuning: Go For the Best Spray 1850 V Optimal Plume 850 V Stream and Plume 50% ACN, 0. 1% Formic Acid 500 n. L/min, 15 µm Picofrit™ tip, LCQ™ Deca XP Inlet
Source Tuning: Go For the Best Spray 1850 V Optimal Plume 850 V Stream and Plume 50% ACN, 0. 1% Formic Acid 500 n. L/min, 15 µm Picofrit™ tip, LCQ™ Deca XP Inlet
 Source Tuning: Go For the Best Spray 2050 V Split Plume 850 V Stream and Plume 50% ACN, 0. 1% Formic Acid 500 n. L/min, 15 µm Picofrit™ tip, LCQ™ Deca XP Inlet
Source Tuning: Go For the Best Spray 2050 V Split Plume 850 V Stream and Plume 50% ACN, 0. 1% Formic Acid 500 n. L/min, 15 µm Picofrit™ tip, LCQ™ Deca XP Inlet
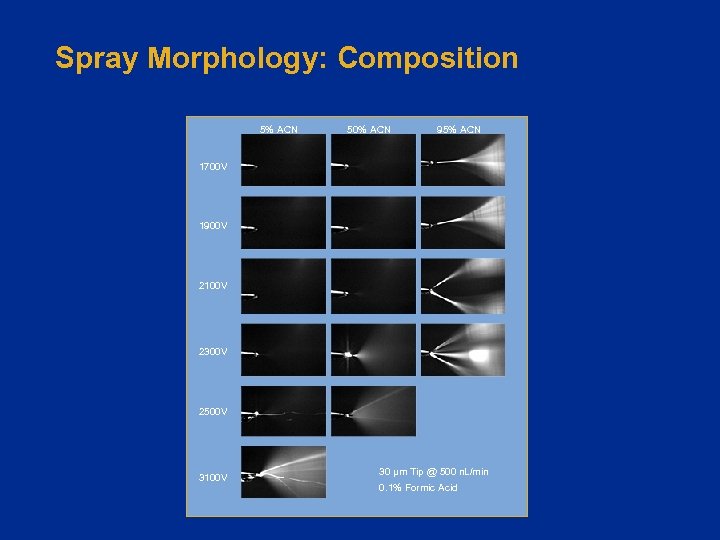 Spray Morphology: Composition 5% ACN 50% ACN 95% ACN 1700 V 1900 V 2100 V 2300 V 2500 V 3100 V 30 µm Tip @ 500 n. L/min 0. 1% Formic Acid
Spray Morphology: Composition 5% ACN 50% ACN 95% ACN 1700 V 1900 V 2100 V 2300 V 2500 V 3100 V 30 µm Tip @ 500 n. L/min 0. 1% Formic Acid
 Source Tuning: Challenges Spray characteristics are sensitive to: • • Emitter size, shape, distance Flow rate Voltage Mobile phase composition – Optimal results require a changing voltage! Bottom line: Tune your spray under “eluting conditions”
Source Tuning: Challenges Spray characteristics are sensitive to: • • Emitter size, shape, distance Flow rate Voltage Mobile phase composition – Optimal results require a changing voltage! Bottom line: Tune your spray under “eluting conditions”
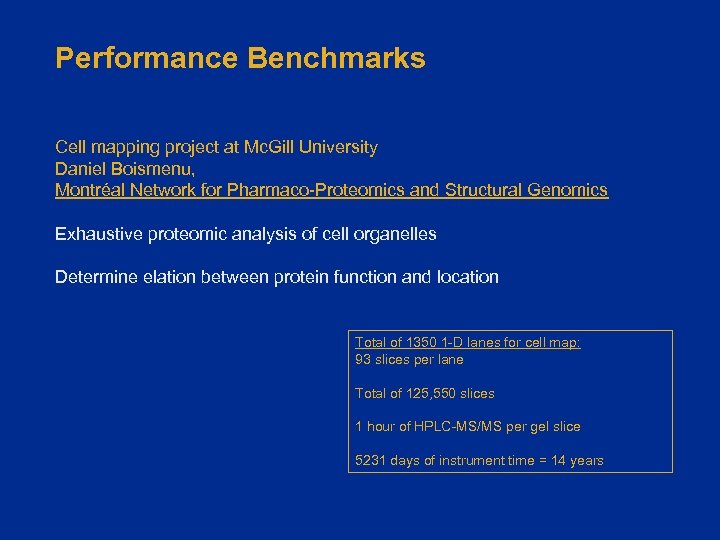 Performance Benchmarks Cell mapping project at Mc. Gill University Daniel Boismenu, Montréal Network for Pharmaco-Proteomics and Structural Genomics Exhaustive proteomic analysis of cell organelles Determine elation between protein function and location Total of 1350 1 -D lanes for cell map: 93 slices per lane Total of 125, 550 slices 1 hour of HPLC-MS/MS per gel slice 5231 days of instrument time = 14 years
Performance Benchmarks Cell mapping project at Mc. Gill University Daniel Boismenu, Montréal Network for Pharmaco-Proteomics and Structural Genomics Exhaustive proteomic analysis of cell organelles Determine elation between protein function and location Total of 1350 1 -D lanes for cell map: 93 slices per lane Total of 125, 550 slices 1 hour of HPLC-MS/MS per gel slice 5231 days of instrument time = 14 years
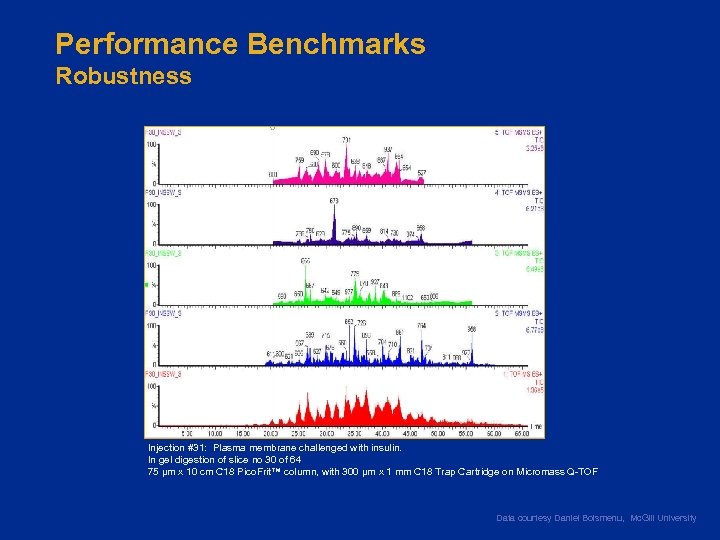 Performance Benchmarks Robustness Injection #31: Plasma membrane challenged with insulin. In gel digestion of slice no 30 of 64 75 µm x 10 cm C 18 Pico. Frit™ column, with 300 µm x 1 mm C 18 Trap Cartridge on Micromass Q-TOF Data courtesy Daniel Boismenu, Mc. Gill University
Performance Benchmarks Robustness Injection #31: Plasma membrane challenged with insulin. In gel digestion of slice no 30 of 64 75 µm x 10 cm C 18 Pico. Frit™ column, with 300 µm x 1 mm C 18 Trap Cartridge on Micromass Q-TOF Data courtesy Daniel Boismenu, Mc. Gill University
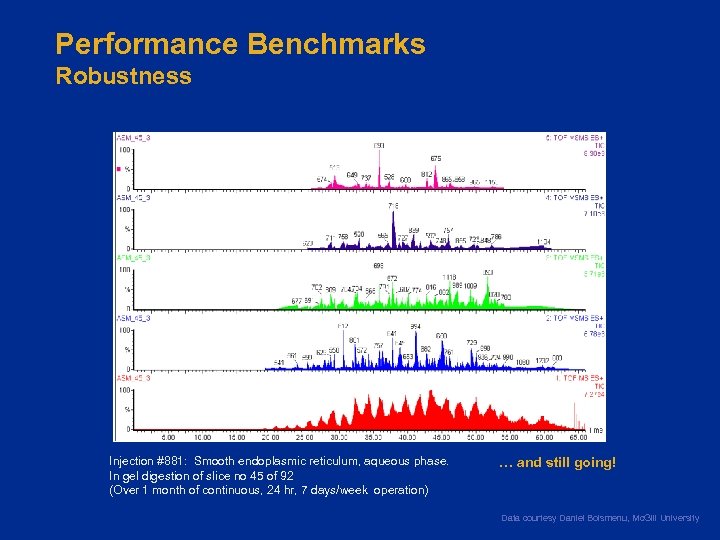 Performance Benchmarks Robustness Injection #881: Smooth endoplasmic reticulum, aqueous phase. In gel digestion of slice no 45 of 92 (Over 1 month of continuous, 24 hr, 7 days/week operation) … and still going! Data courtesy Daniel Boismenu, Mc. Gill University
Performance Benchmarks Robustness Injection #881: Smooth endoplasmic reticulum, aqueous phase. In gel digestion of slice no 45 of 92 (Over 1 month of continuous, 24 hr, 7 days/week operation) … and still going! Data courtesy Daniel Boismenu, Mc. Gill University
 Keys to Success with Nanobore LC-MS • Clean mobile phase – Minimize particulate contamination – Use multiple high quality in-line filters • Know your flow rate – Monitor through column flow periodically • Use the right injection scheme for your samples • Throughput vs. sensitivity • Minimize (or eliminate) post-column plumbing – Use special care with post-column connections – Use a tip-column (Pico. Frit™) format • Optimize electrospray conditions – Stabilize spray with voltage – Maximize S/N with emitter position – Match tip size to flow rate
Keys to Success with Nanobore LC-MS • Clean mobile phase – Minimize particulate contamination – Use multiple high quality in-line filters • Know your flow rate – Monitor through column flow periodically • Use the right injection scheme for your samples • Throughput vs. sensitivity • Minimize (or eliminate) post-column plumbing – Use special care with post-column connections – Use a tip-column (Pico. Frit™) format • Optimize electrospray conditions – Stabilize spray with voltage – Maximize S/N with emitter position – Match tip size to flow rate



