bc5fdd847c7b0acf23b74ebc3e3cd131.ppt
- Количество слайдов: 27

Practical aspects of standardization for a global control manufacturer Example: Enterovirus So. GAT XX Warsaw, Poland June 12, 2007 Ralf Schönbrunner, Acro. Metrix

Acro. Metrix’ Control Development • Acro. Metrix Quality System – ISO 13485: 2003 certified – FDA 21 CFR Part 820 Quality System Regulations (QSR) – c. GMP – Calibrators and Controls values assigned following ISO 17511: 2003 • In vitro diagnostic medical devices —Measurement of quantities in biological samples — Metrological traceability of values assigned to calibrators and control materials

ISO 17511: 2003 Five Metrological Levels • Upper end of the metrological traceability chain – Level 1: Traceable to SI – Levels 2 – 5: Not traceable to SI • See ISO 17511: 2003 Introduction p. vii
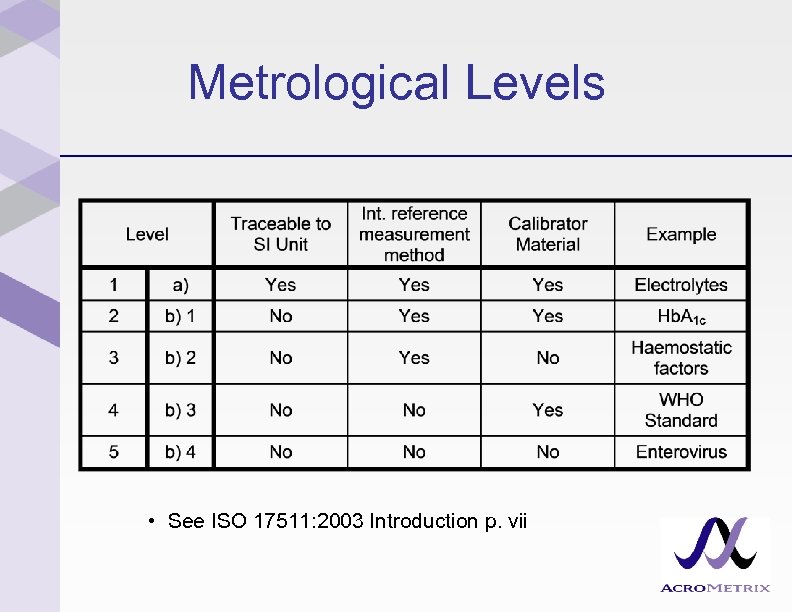
Metrological Levels • See ISO 17511: 2003 Introduction p. vii
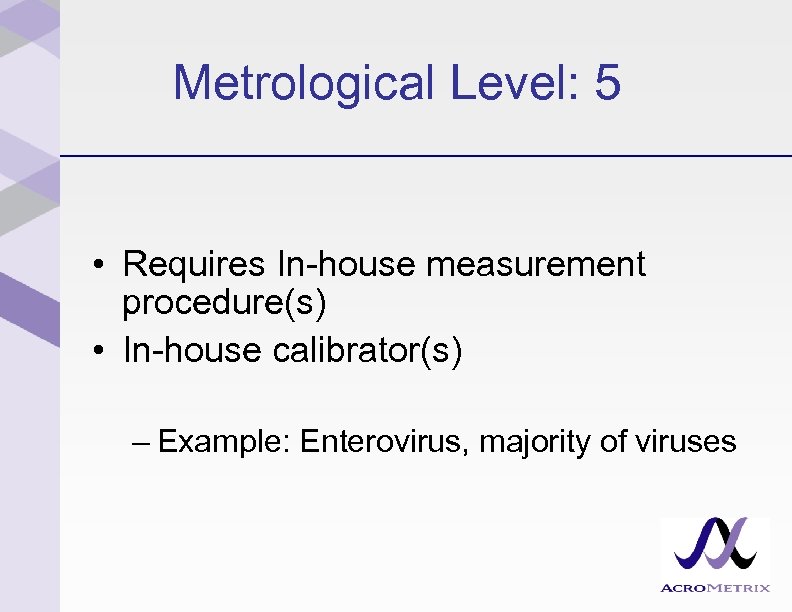
Metrological Level: 5 • Requires In-house measurement procedure(s) • In-house calibrator(s) – Example: Enterovirus, majority of viruses
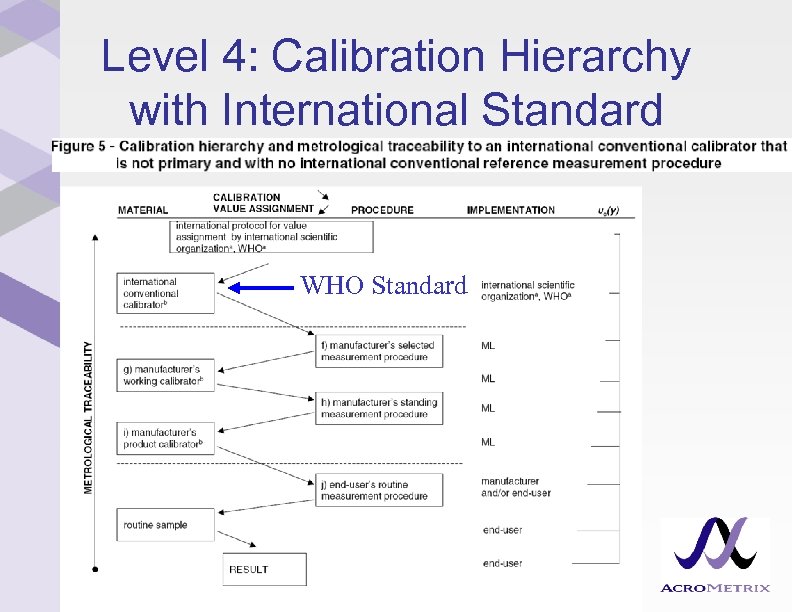
Level 4: Calibration Hierarchy with International Standard WHO Standard
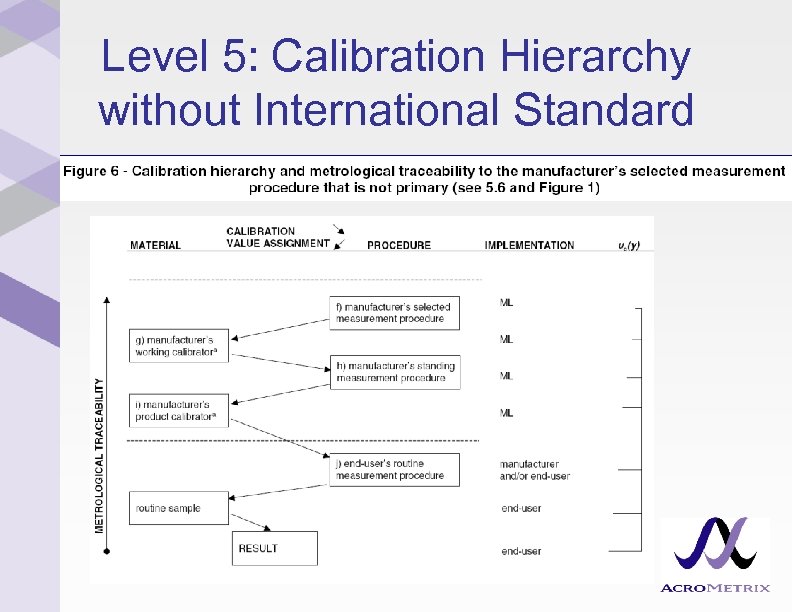
Level 5: Calibration Hierarchy without International Standard

Enteroviruses Metrological Level 5 • Group: Group IV (+)ss. RNA • Family: Picornaviridae • Genus: Enterovirus – No WHO Standard available
Genus enterovirus • • • Human enterovirus A, B, C, D Bovine enterovirus Porcine enterovirus A, B Simian enterovirus Human rhinovirus A, B • almost 100 different serotypes – Source: Fields Virology, 5 th edition, 2007 – Coxsackie viruses – Echovirus – Poliovirus

First EV Quantitation • Zhang, HY, Yousef GE, Bowles NE, Archard LC, Mann GF, Mowbray JF • J Med Virol. 1988 Dec; 26(4): 375 -86. • Detection of enterovirus RNA in experimentally infected mice by molecular hybridisation: specificity of subgenomic probes in quantitative slot blot and in situ hybridisation. • Department of Experimental Pathology, St. Mary's Hospital Medical School, London, England • In situ-hybridization reportedly detected 10 to 100 enteroviral genomes

First EV PCR Quantitation • T A Martino, M J Sole, L Z Penn, C C Liew, and P Liu • J Clin Microbiol. 1993 October; 31(10): 2634– 2640. • Quantitation of enteroviral RNA by competitive polymerase chain reaction. • Center for Cardiovascular Research, Toronto Hospital, Ontario, Canada. • • • Used competitive internal control standard Concentration measured at 260 nm Based on gels and radioactive [α-32 P]d. CTP quantitation Quantitation possible from 15 – 6000 TCID 50 Calibration Curve: [Standard] (pg) against CPM Std / CPM Virus
Cepheid Xpert EV assay • FDA approval March 16, 2007 • Reverse transcription polymerase chain reaction (RT-PCR) • Qualitative detection of enterovirus (EV) RNA • Cerebrospinal fluid (CSF) specimens • Individuals with signs and symptoms of meningitis
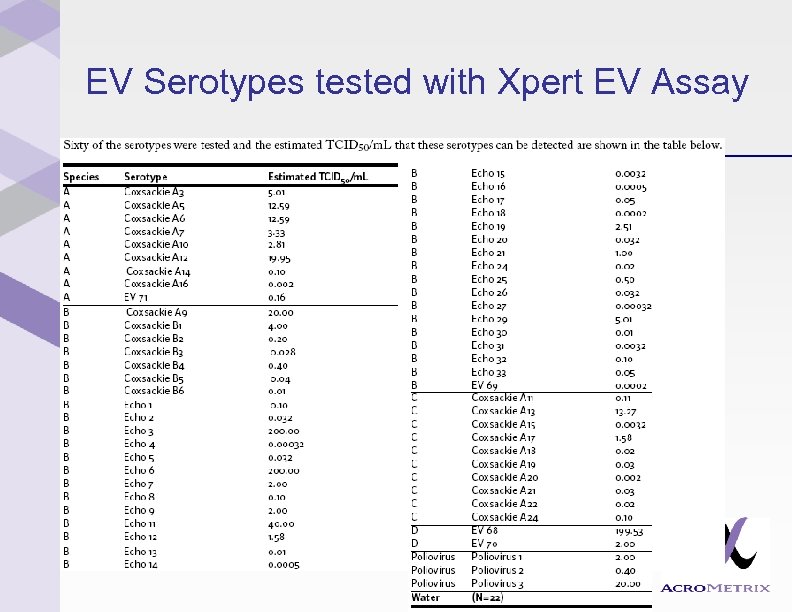
EV Serotypes tested with Xpert EV Assay
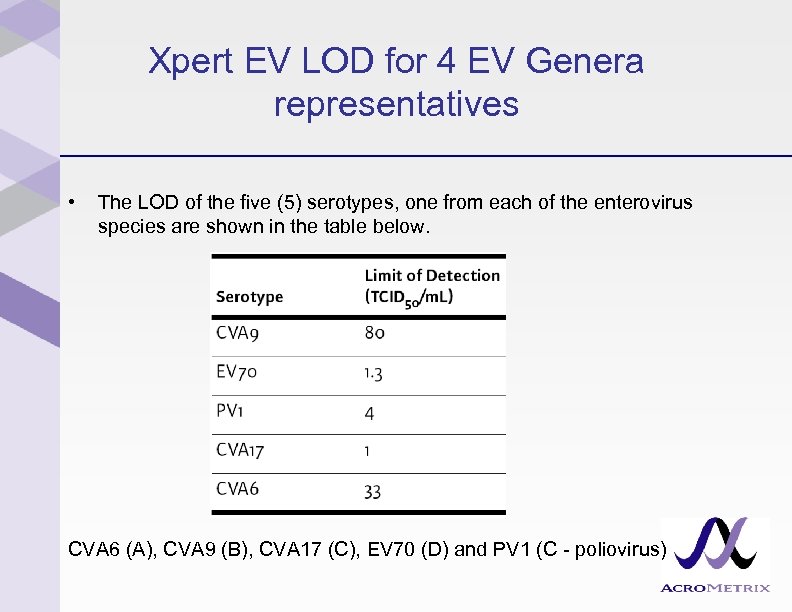
Xpert EV LOD for 4 EV Genera representatives • The LOD of the five (5) serotypes, one from each of the enterovirus species are shown in the table below. CVA 6 (A), CVA 9 (B), CVA 17 (C), EV 70 (D) and PV 1 (C - poliovirus)
Challenges for Development of a Low PC useful for Xpert EV • • No international EV standard Which enterovirus should be used? Which unit should be used? What is correct level for control? – (about 3 - 5 x LOD)
EV in-House working calibrator Step 1 in ISO 17511 to generate EV Low PC • Which EV strain should be used? – Meningitis causing – see assay intended use – Defined LOD – CVA 6 or CVA 9 • Which unit should be used? – – – Copies Genome equivalents IU TCID 50 pg RNA • What is best level for in-house working calibrator? – Significantly higher than Low PC

Objective for in-house working calibrator • Requirements: – Proxy for non existing International Standard – Same strain as Low Positive Control – Easily available stock material • Not necessary to know: – Number of viruses in calibrator – Number of Signal Generating Units (SGU)
EV strain for in-House working calibrator • • Human Enterovirus Species B Type: Coxsackie A 9 (CVA 9) Strain: P. B. (Bozek) Geographic Origin: New York Illness yielding prototype virus: Meningitis Accession Number: D 00627 Heat inactivated, 56ºC, 30 min – K. E. van Vliet et al. J. Clin. Microbiol. (2001) 39, p. 3390
in-House working calibrator unit • Copies – No. Avoid confusion with other people’s copies • SGU – No. Dependent on assay sensitivity • Genome equivalents – Ideally yes, but no general accepted reference measurement procedure available • IU – Requires international protocol for value assignment by international scientific organization • TCID 50 – Heat inactivated: TCID 50 = ∞ • pg RNA – No accepted int. reference method • Generated independent in-house unit – according ISO 17511: 2003 clause 4. 1. 1 d
Enterovirus Unit (EVU) • Basis to deliver consistent control • Not based on SI unit • Arbitrarily defined unit – similar to WHO IU • No claim about number of viruses • Traceable or commutable to other units – Qiagen copies – Cepheid TCID 50 – Future WHO Standard?
In-house working calibrator level • 500 times higher than Low Pos Control • Low Pos Control about 3 - 5 x LOD of Xpert EV – Target level of EV low PC: 100 EVU/m. L • In-house calibrator: 50 000 EVU/m. L – Named: EV APS (Acro. Metrix Primary Standard) • Product calibrator: 100 EVU/m. L – Named: EV LPC AQS (Acro. Metrix QC Standard)
EV in-house working calibrator & EV product calibrator development • Obtained high titer stock CVA 9 • Determined dilution factor to 4 x. LOD • Diluted 500 fold less to generate EV in-house working calibrator (EV APS) • Defined EV APS at 50 000 EVU/m. L • Diluted EV APS 1: 500 to generate EV product calibrator (EV LPC AQS) • Defined EV LPC AQS at 100 EVU/m. L
Opti. Qual EV Low PC • • Heat inactivated CVA 9 Level: 100 ± 30 EVU/m. L Fill volume: 300 µL Storage: -70ºC – Goal: switch to -20ºC
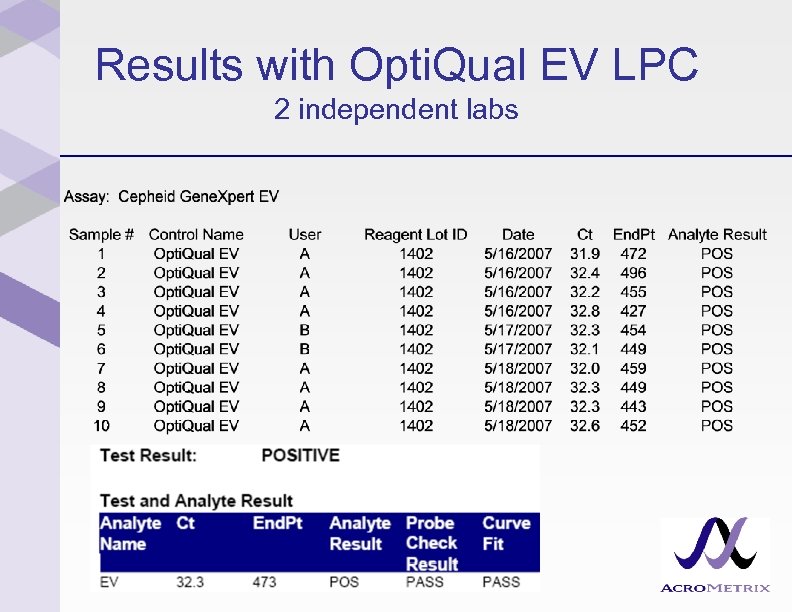
Results with Opti. Qual EV LPC 2 independent labs
Possible Downsides • Meaning of EVU? – No SI unit – Metrological based – Driven by ISO 17511 • What’s an IU? • Many different enteroviruses – CVA 9 one of many enteroviruses – Similar to HIV subtypes and HCV genotypes – Consider naming it CVA 9 unit?
Conclusions • Opti. Qual EV control very reproducible results with gene. Xpert EV assay • Level probably higher than 3 – 5 LOD – – CV very low for 3 – 5 x. LOD labs use low CV of control to asses variability evaluations ongoing some other EV assays show high CV • Adjustment to different level easily possible, due to availability of in-house calibrators

Proposal • Quickly & proactively create “WHO Standards” for “orphan pathogens”* and *: Pathogens currently without International Standard • Provide protocol for value assignment • Act before commercial assays are available • Unit: IU
bc5fdd847c7b0acf23b74ebc3e3cd131.ppt