a0d0997dd013436e54d96640849333a8.ppt
- Количество слайдов: 22
 PPCW Workshop NIH 3/28/2004 Transient Transfection into Eukaryotic Cells: An Alternative to Bacterial and Insect Cell Systems for the Rapid Generation of Recombinant Proteins? Bethesda, MD, 30. 3. 2004 Sabine Geisse, Nicola di Maiuta, Thomas Cremer, Mario Henke Novartis Institutes for Biomedical Research, Basle, Switzerland 1
PPCW Workshop NIH 3/28/2004 Transient Transfection into Eukaryotic Cells: An Alternative to Bacterial and Insect Cell Systems for the Rapid Generation of Recombinant Proteins? Bethesda, MD, 30. 3. 2004 Sabine Geisse, Nicola di Maiuta, Thomas Cremer, Mario Henke Novartis Institutes for Biomedical Research, Basle, Switzerland 1
 PPCW Workshop NIH 3/28/2004 Background and Rationale • Recombinant proteins are essential research tools for assay development and screening structural biology antibody generation, selectivity assays. . . • In the post-genomic era translation of thousands of ORFs into proteins are a major challenge • Technologies and processes increasing the throughput and the success rate in protein production are needed • Novartis Research is currently building a Protein Production Center in which streamlined processes will be applied in a factory-like fashion. In parallel, an Intensive Care Approach is applied to generate proteins by non-generic means. 2
PPCW Workshop NIH 3/28/2004 Background and Rationale • Recombinant proteins are essential research tools for assay development and screening structural biology antibody generation, selectivity assays. . . • In the post-genomic era translation of thousands of ORFs into proteins are a major challenge • Technologies and processes increasing the throughput and the success rate in protein production are needed • Novartis Research is currently building a Protein Production Center in which streamlined processes will be applied in a factory-like fashion. In parallel, an Intensive Care Approach is applied to generate proteins by non-generic means. 2
 PPCW Workshop NIH 3/28/2004 Double-Track Strategy • Target output: 400 tool proteins/year – Quantity 1 - 100 mg – Purity >80 % (application-dependent) • “Protein Factory”: > 300 proteins/year – 3 standardized expression systems – Streamlined, generic, partly automated processes • “Intensive Care”: < 100 proteins/year – Difficult-to-express proteins – Recombinant cell lines, membrane proteins – Monoclonal and recombinant antibodies 3
PPCW Workshop NIH 3/28/2004 Double-Track Strategy • Target output: 400 tool proteins/year – Quantity 1 - 100 mg – Purity >80 % (application-dependent) • “Protein Factory”: > 300 proteins/year – 3 standardized expression systems – Streamlined, generic, partly automated processes • “Intensive Care”: < 100 proteins/year – Difficult-to-express proteins – Recombinant cell lines, membrane proteins – Monoclonal and recombinant antibodies 3
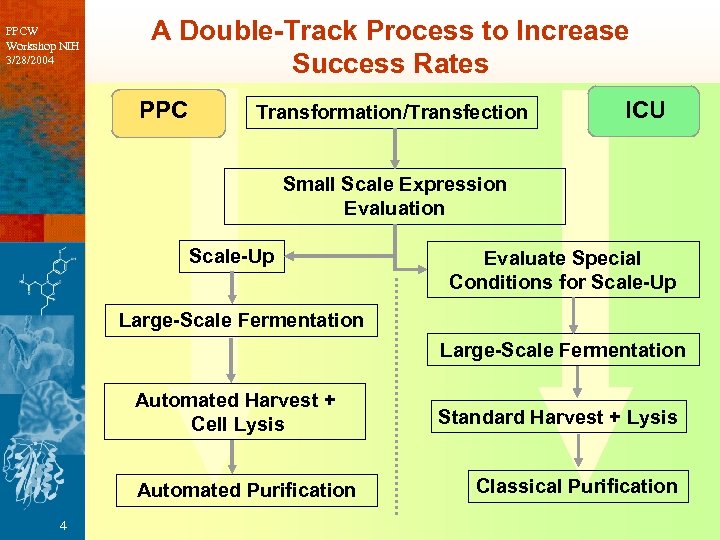 PPCW Workshop NIH 3/28/2004 A Double-Track Process to Increase Success Rates PPC Transformation/Transfection ICU Small Scale Expression Evaluation Scale-Up Evaluate Special Conditions for Scale-Up Large-Scale Fermentation Automated Harvest + Cell Lysis Automated Purification 4 Standard Harvest + Lysis Classical Purification
PPCW Workshop NIH 3/28/2004 A Double-Track Process to Increase Success Rates PPC Transformation/Transfection ICU Small Scale Expression Evaluation Scale-Up Evaluate Special Conditions for Scale-Up Large-Scale Fermentation Automated Harvest + Cell Lysis Automated Purification 4 Standard Harvest + Lysis Classical Purification
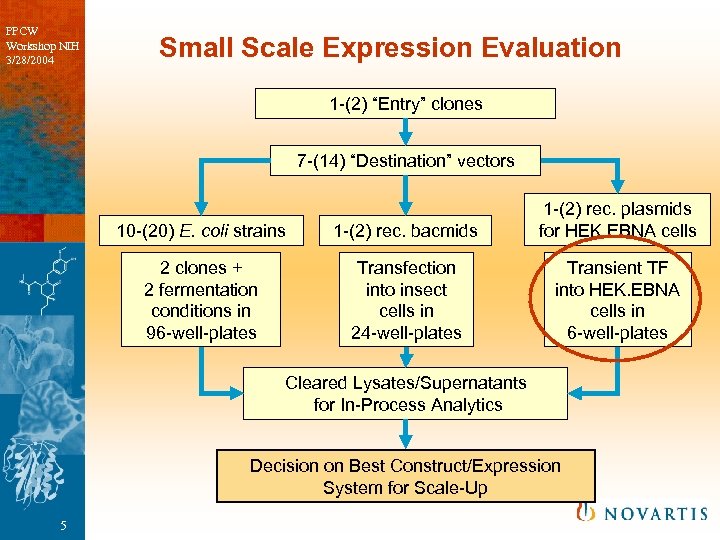 PPCW Workshop NIH 3/28/2004 Small Scale Expression Evaluation 1 -(2) “Entry” clones 7 -(14) “Destination” vectors 10 -(20) E. coli strains 1 -(2) rec. bacmids 1 -(2) rec. plasmids for HEK. EBNA cells 2 clones + 2 fermentation conditions in 96 -well-plates Transfection into insect cells in 24 -well-plates Transient TF into HEK. EBNA cells in 6 -well-plates Cleared Lysates/Supernatants for In-Process Analytics Decision on Best Construct/Expression System for Scale-Up 5
PPCW Workshop NIH 3/28/2004 Small Scale Expression Evaluation 1 -(2) “Entry” clones 7 -(14) “Destination” vectors 10 -(20) E. coli strains 1 -(2) rec. bacmids 1 -(2) rec. plasmids for HEK. EBNA cells 2 clones + 2 fermentation conditions in 96 -well-plates Transfection into insect cells in 24 -well-plates Transient TF into HEK. EBNA cells in 6 -well-plates Cleared Lysates/Supernatants for In-Process Analytics Decision on Best Construct/Expression System for Scale-Up 5
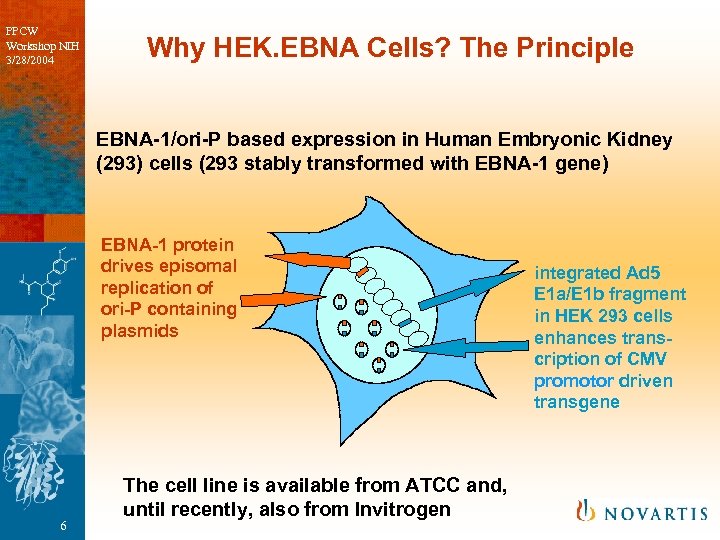 PPCW Workshop NIH 3/28/2004 Why HEK. EBNA Cells? The Principle EBNA-1/ori-P based expression in Human Embryonic Kidney (293) cells (293 stably transformed with EBNA-1 gene) EBNA-1 protein drives episomal replication of ori-P containing plasmids 6 The cell line is available from ATCC and, until recently, also from Invitrogen integrated Ad 5 E 1 a/E 1 b fragment in HEK 293 cells enhances transcription of CMV promotor driven transgene
PPCW Workshop NIH 3/28/2004 Why HEK. EBNA Cells? The Principle EBNA-1/ori-P based expression in Human Embryonic Kidney (293) cells (293 stably transformed with EBNA-1 gene) EBNA-1 protein drives episomal replication of ori-P containing plasmids 6 The cell line is available from ATCC and, until recently, also from Invitrogen integrated Ad 5 E 1 a/E 1 b fragment in HEK 293 cells enhances transcription of CMV promotor driven transgene
 PPCW Workshop NIH 3/28/2004 Why HEK. EBNA Cells? Advantages • In comparison to other eukaryotic expression systems the HEK. EBNA Expression System is rapid: from gene to protein in 4 -6 weeks • The cells can be grown adherently and in serum-free suspension culture • It can be applied to generate stable cell lines (pools/ clones) and in transient mode on small and large scale • In transient mode not only secreted and membranebound, but also intracellular proteins can successfully be expressed 7
PPCW Workshop NIH 3/28/2004 Why HEK. EBNA Cells? Advantages • In comparison to other eukaryotic expression systems the HEK. EBNA Expression System is rapid: from gene to protein in 4 -6 weeks • The cells can be grown adherently and in serum-free suspension culture • It can be applied to generate stable cell lines (pools/ clones) and in transient mode on small and large scale • In transient mode not only secreted and membranebound, but also intracellular proteins can successfully be expressed 7
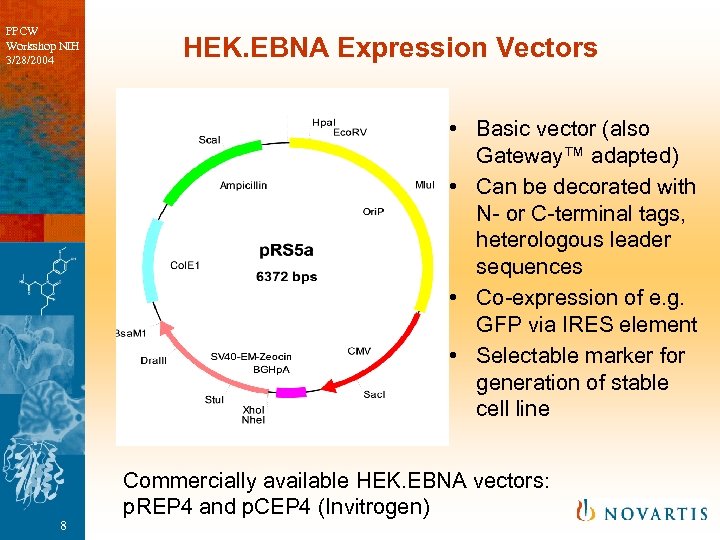 PPCW Workshop NIH 3/28/2004 HEK. EBNA Expression Vectors • Basic vector (also Gateway™ adapted) • Can be decorated with N- or C-terminal tags, heterologous leader sequences • Co-expression of e. g. GFP via IRES element • Selectable marker for generation of stable cell line 8 Commercially available HEK. EBNA vectors: p. REP 4 and p. CEP 4 (Invitrogen)
PPCW Workshop NIH 3/28/2004 HEK. EBNA Expression Vectors • Basic vector (also Gateway™ adapted) • Can be decorated with N- or C-terminal tags, heterologous leader sequences • Co-expression of e. g. GFP via IRES element • Selectable marker for generation of stable cell line 8 Commercially available HEK. EBNA vectors: p. REP 4 and p. CEP 4 (Invitrogen)
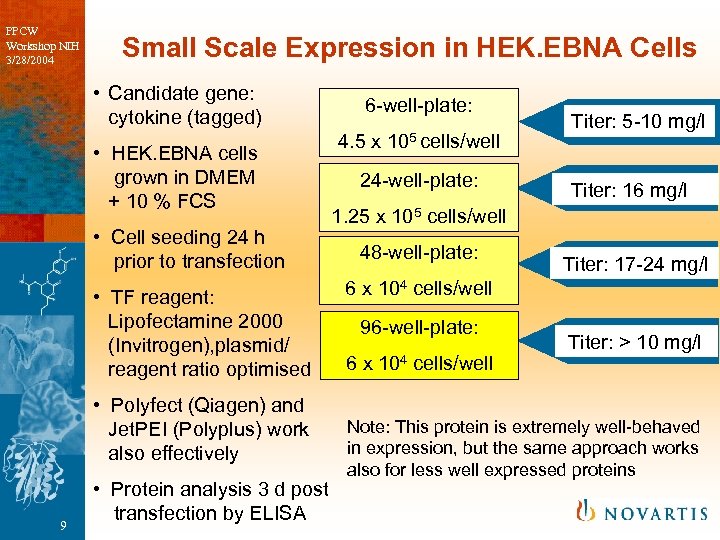 PPCW Workshop NIH 3/28/2004 Small Scale Expression in HEK. EBNA Cells • Candidate gene: cytokine (tagged) • HEK. EBNA cells grown in DMEM + 10 % FCS • Cell seeding 24 h prior to transfection • TF reagent: Lipofectamine 2000 (Invitrogen), plasmid/ reagent ratio optimised • Polyfect (Qiagen) and Jet. PEI (Polyplus) work also effectively 9 • Protein analysis 3 d post transfection by ELISA 6 -well-plate: 4. 5 x 105 cells/well 24 -well-plate: Titer: 5 -10 mg/l Titer: 16 mg/l 1. 25 x 105 cells/well 48 -well-plate: Titer: 17 -24 mg/l 6 x 104 cells/well 96 -well-plate: 6 x 104 cells/well Titer: > 10 mg/l Note: This protein is extremely well-behaved in expression, but the same approach works also for less well expressed proteins
PPCW Workshop NIH 3/28/2004 Small Scale Expression in HEK. EBNA Cells • Candidate gene: cytokine (tagged) • HEK. EBNA cells grown in DMEM + 10 % FCS • Cell seeding 24 h prior to transfection • TF reagent: Lipofectamine 2000 (Invitrogen), plasmid/ reagent ratio optimised • Polyfect (Qiagen) and Jet. PEI (Polyplus) work also effectively 9 • Protein analysis 3 d post transfection by ELISA 6 -well-plate: 4. 5 x 105 cells/well 24 -well-plate: Titer: 5 -10 mg/l Titer: 16 mg/l 1. 25 x 105 cells/well 48 -well-plate: Titer: 17 -24 mg/l 6 x 104 cells/well 96 -well-plate: 6 x 104 cells/well Titer: > 10 mg/l Note: This protein is extremely well-behaved in expression, but the same approach works also for less well expressed proteins
 PPCW Workshop NIH 3/28/2004 Summary of Small Scale Expression Trials using HEK. EBNA Cells work very efficiently using • Adherent cultures in DMEM+ 10 % FCS • Different well-plate formats • Pre-coating with Poly-D-lysine: facilitates attachment of cells and minimizes cell losses during transfection • Lipofectamine 2000™ (Invitrogen): >90 % transfection efficiency (other reagents also possible – try!) • Can be also done using serum-free suspension cultures, but less efficient 10
PPCW Workshop NIH 3/28/2004 Summary of Small Scale Expression Trials using HEK. EBNA Cells work very efficiently using • Adherent cultures in DMEM+ 10 % FCS • Different well-plate formats • Pre-coating with Poly-D-lysine: facilitates attachment of cells and minimizes cell losses during transfection • Lipofectamine 2000™ (Invitrogen): >90 % transfection efficiency (other reagents also possible – try!) • Can be also done using serum-free suspension cultures, but less efficient 10
 PPCW Workshop NIH 3/28/2004 Large Scale Transient Transfection (1) What is “large scale”? 1 – 10 liter 1. Prerequisite: Adaptation of cell culture to serum-free suspension ® Several vendors offer cell culture media either specifically developed for HEK 293 cells or for other cells (Per. C. 6, hybridoma) which can be also used for cultivation of HEK 293 or HEK. EBNA cells (see Table on Slide 14) 11
PPCW Workshop NIH 3/28/2004 Large Scale Transient Transfection (1) What is “large scale”? 1 – 10 liter 1. Prerequisite: Adaptation of cell culture to serum-free suspension ® Several vendors offer cell culture media either specifically developed for HEK 293 cells or for other cells (Per. C. 6, hybridoma) which can be also used for cultivation of HEK 293 or HEK. EBNA cells (see Table on Slide 14) 11
 PPCW Workshop NIH 3/28/2004 Large Scale Transient Transfection (2) 2. Prerequisite: a suitable transfection reagent which is cost-effective, readily available in large quantities and gives rise to high transfection efficiencies ® Commercially available reagents, such as lipoplexes are by far too expensive at this scale ® Transfection using Ca. PO 4 precipitation or cationic polymers such as Polyethylenimine, however, meet the above mentioned criteria and have been successfully used at multi-liter scale 12
PPCW Workshop NIH 3/28/2004 Large Scale Transient Transfection (2) 2. Prerequisite: a suitable transfection reagent which is cost-effective, readily available in large quantities and gives rise to high transfection efficiencies ® Commercially available reagents, such as lipoplexes are by far too expensive at this scale ® Transfection using Ca. PO 4 precipitation or cationic polymers such as Polyethylenimine, however, meet the above mentioned criteria and have been successfully used at multi-liter scale 12
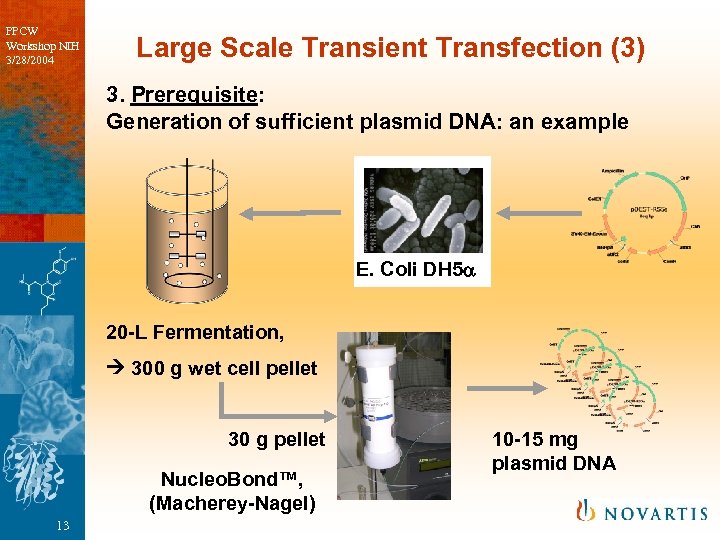 PPCW Workshop NIH 3/28/2004 Large Scale Transient Transfection (3) 3. Prerequisite: Generation of sufficient plasmid DNA: an example E. Coli DH 5 20 -L Fermentation, 300 g wet cell pellet 30 g pellet Nucleo. Bond™, (Macherey-Nagel) 13 10 -15 mg plasmid DNA
PPCW Workshop NIH 3/28/2004 Large Scale Transient Transfection (3) 3. Prerequisite: Generation of sufficient plasmid DNA: an example E. Coli DH 5 20 -L Fermentation, 300 g wet cell pellet 30 g pellet Nucleo. Bond™, (Macherey-Nagel) 13 10 -15 mg plasmid DNA
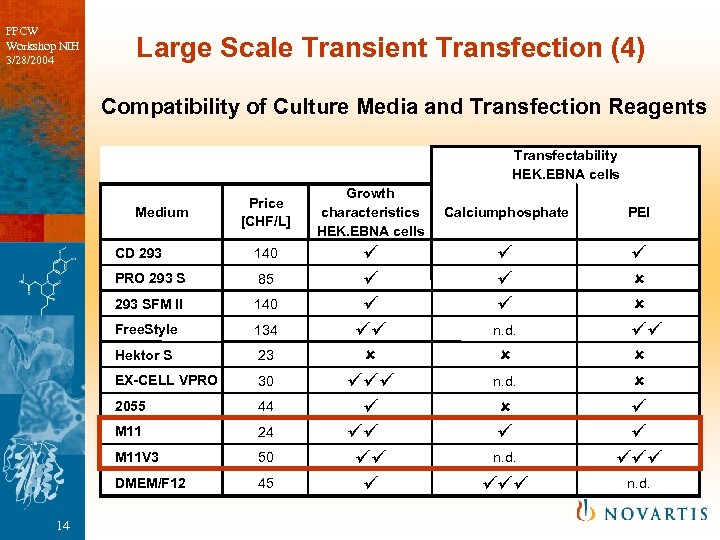 PPCW Workshop NIH 3/28/2004 Large Scale Transient Transfection (4) Compatibility of Culture Media and Transfection Reagents Transfectability HEK. EBNA cells Medium Price [CHF/L] CD 293 PRO 293 S 85 293 SFM II 140 Free. Style 134 Hektor S 23 EX-CELL VPRO 30 2055 44 M 11 24 M 11 V 3 50 DMEM/F 12 14 140 45 Growth characteristics HEK. EBNA cells û Calciumphosphate PEI n. d. û û n. d. û
PPCW Workshop NIH 3/28/2004 Large Scale Transient Transfection (4) Compatibility of Culture Media and Transfection Reagents Transfectability HEK. EBNA cells Medium Price [CHF/L] CD 293 PRO 293 S 85 293 SFM II 140 Free. Style 134 Hektor S 23 EX-CELL VPRO 30 2055 44 M 11 24 M 11 V 3 50 DMEM/F 12 14 140 45 Growth characteristics HEK. EBNA cells û Calciumphosphate PEI n. d. û û n. d. û
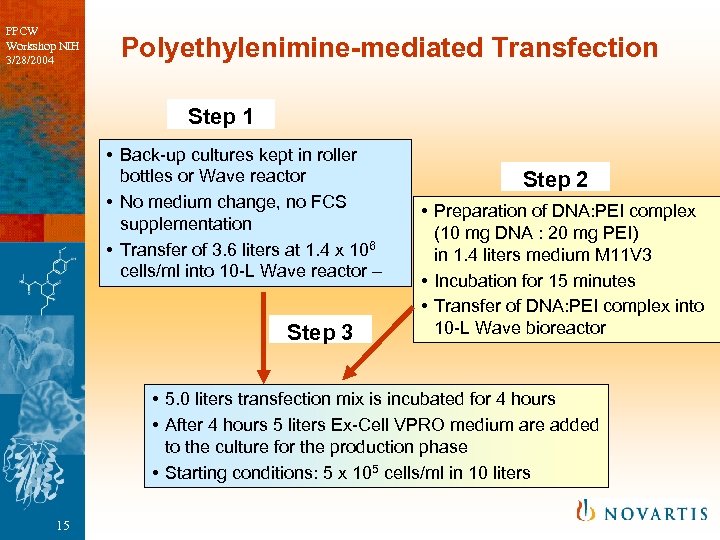 PPCW Workshop NIH 3/28/2004 Polyethylenimine-mediated Transfection Step 1 • Back-up cultures kept in roller bottles or Wave reactor • No medium change, no FCS supplementation • Transfer of 3. 6 liters at 1. 4 x 106 cells/ml into 10 -L Wave reactor – Step 3 Step 2 • Preparation of DNA: PEI complex (10 mg DNA : 20 mg PEI) in 1. 4 liters medium M 11 V 3 • Incubation for 15 minutes • Transfer of DNA: PEI complex into 10 -L Wave bioreactor • 5. 0 liters transfection mix is incubated for 4 hours • After 4 hours 5 liters Ex-Cell VPRO medium are added to the culture for the production phase • Starting conditions: 5 x 105 cells/ml in 10 liters 15
PPCW Workshop NIH 3/28/2004 Polyethylenimine-mediated Transfection Step 1 • Back-up cultures kept in roller bottles or Wave reactor • No medium change, no FCS supplementation • Transfer of 3. 6 liters at 1. 4 x 106 cells/ml into 10 -L Wave reactor – Step 3 Step 2 • Preparation of DNA: PEI complex (10 mg DNA : 20 mg PEI) in 1. 4 liters medium M 11 V 3 • Incubation for 15 minutes • Transfer of DNA: PEI complex into 10 -L Wave bioreactor • 5. 0 liters transfection mix is incubated for 4 hours • After 4 hours 5 liters Ex-Cell VPRO medium are added to the culture for the production phase • Starting conditions: 5 x 105 cells/ml in 10 liters 15
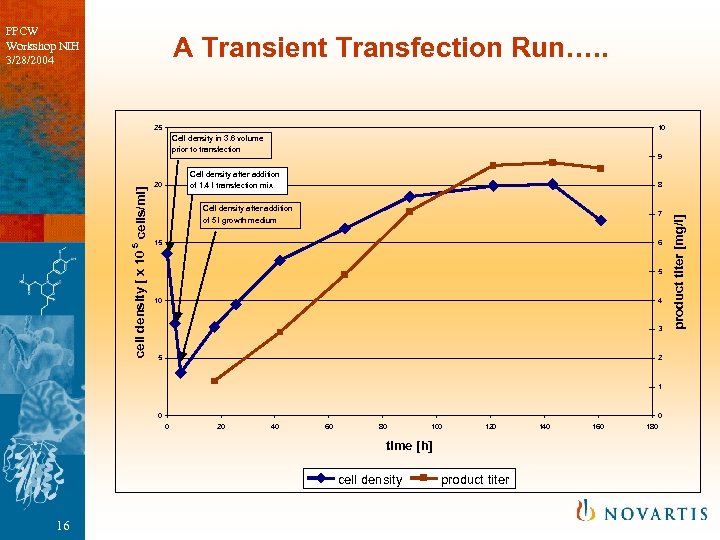 PPCW Workshop NIH 3/28/2004 A Transient Transfection Run…. . 25 10 9 Cell density after addition of 1. 4 l transfection mix 20 8 Cell density after addition of 5 l growth medium 7 15 6 5 10 4 3 5 2 1 0 0 0 20 40 60 80 100 120 time [h] cell density 16 product titer 140 160 180 product titer [mg/l] cell density [ x 10 5 cells/ml] Cell density in 3. 6 volume prior to transfection
PPCW Workshop NIH 3/28/2004 A Transient Transfection Run…. . 25 10 9 Cell density after addition of 1. 4 l transfection mix 20 8 Cell density after addition of 5 l growth medium 7 15 6 5 10 4 3 5 2 1 0 0 0 20 40 60 80 100 120 time [h] cell density 16 product titer 140 160 180 product titer [mg/l] cell density [ x 10 5 cells/ml] Cell density in 3. 6 volume prior to transfection
 PPCW Workshop NIH 3/28/2004 …. in Multiparallel Fashion The “Wave Factory” 17
PPCW Workshop NIH 3/28/2004 …. in Multiparallel Fashion The “Wave Factory” 17
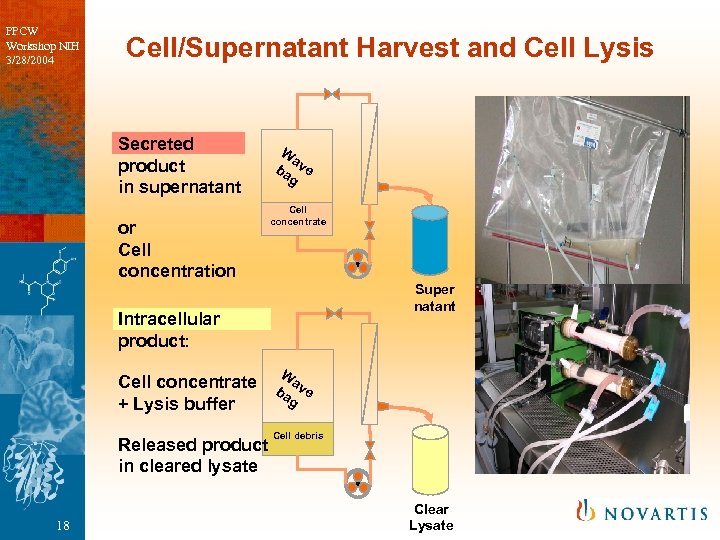 PPCW Workshop NIH 3/28/2004 Cell/Supernatant Harvest and Cell Lysis Secreted product in supernatant or Cell concentration W a ba ve g Cell concentrate Super natant Intracellular product: Cell concentrate + Lysis buffer Released product in cleared lysate 18 W a ba ve g Cell debris Clear Lysate
PPCW Workshop NIH 3/28/2004 Cell/Supernatant Harvest and Cell Lysis Secreted product in supernatant or Cell concentration W a ba ve g Cell concentrate Super natant Intracellular product: Cell concentrate + Lysis buffer Released product in cleared lysate 18 W a ba ve g Cell debris Clear Lysate
 PPCW Workshop NIH 3/28/2004 Protein Purification For Secreted Proteins: affinity chromatography on antibody or Protein A column -tag dependent. For Intracellularly Expressed Proteins: his and/or his-Strep tag Ni-chelate and/or Streptactin column To date, > 30 proteins were generated successfully using this approach. The overall success rate in expression trials was >80 %. 19
PPCW Workshop NIH 3/28/2004 Protein Purification For Secreted Proteins: affinity chromatography on antibody or Protein A column -tag dependent. For Intracellularly Expressed Proteins: his and/or his-Strep tag Ni-chelate and/or Streptactin column To date, > 30 proteins were generated successfully using this approach. The overall success rate in expression trials was >80 %. 19
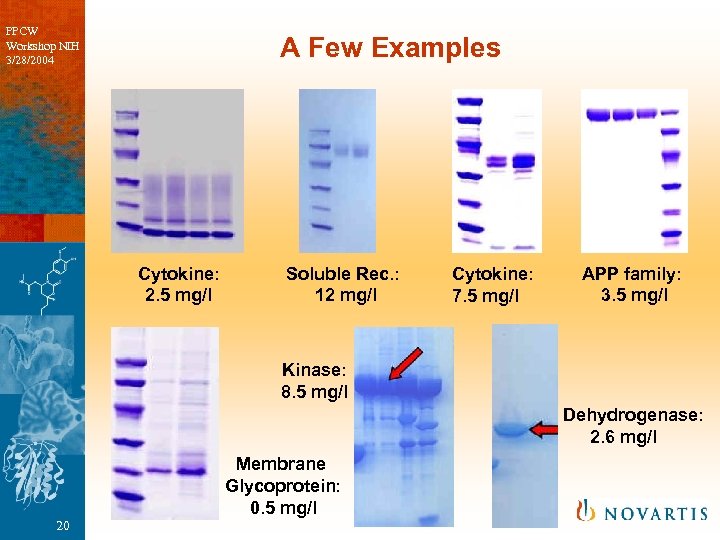 PPCW Workshop NIH 3/28/2004 A Few Examples Cytokine: 2. 5 mg/l Soluble Rec. : 12 mg/l Cytokine: 7. 5 mg/l APP family: 3. 5 mg/l Kinase: 8. 5 mg/l Dehydrogenase: 2. 6 mg/l 20 Membrane Glycoprotein: 0. 5 mg/l
PPCW Workshop NIH 3/28/2004 A Few Examples Cytokine: 2. 5 mg/l Soluble Rec. : 12 mg/l Cytokine: 7. 5 mg/l APP family: 3. 5 mg/l Kinase: 8. 5 mg/l Dehydrogenase: 2. 6 mg/l 20 Membrane Glycoprotein: 0. 5 mg/l
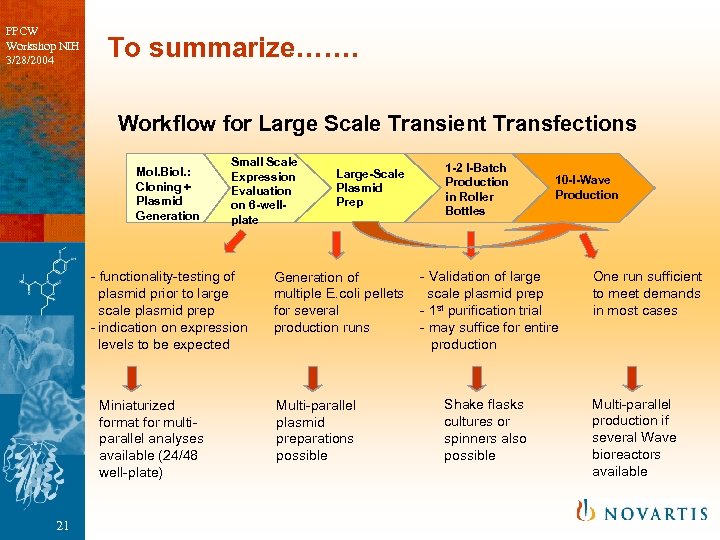 PPCW Workshop NIH 3/28/2004 To summarize……. Workflow for Large Scale Transient Transfections Mol. Biol. : Cloning + Plasmid Generation Small Scale Expression Evaluation on 6 -wellplate - functionality-testing of plasmid prior to large scale plasmid prep - indication on expression levels to be expected Miniaturized format for multiparallel analyses available (24/48 well-plate) 21 Large-Scale Plasmid Prep Generation of multiple E. coli pellets for several production runs Multi-parallel plasmid preparations possible 1 -2 l-Batch Production in Roller Bottles 10 -l-Wave Production - Validation of large scale plasmid prep - 1 st purification trial - may suffice for entire production Shake flasks cultures or spinners also possible One run sufficient to meet demands in most cases Multi-parallel production if several Wave bioreactors available
PPCW Workshop NIH 3/28/2004 To summarize……. Workflow for Large Scale Transient Transfections Mol. Biol. : Cloning + Plasmid Generation Small Scale Expression Evaluation on 6 -wellplate - functionality-testing of plasmid prior to large scale plasmid prep - indication on expression levels to be expected Miniaturized format for multiparallel analyses available (24/48 well-plate) 21 Large-Scale Plasmid Prep Generation of multiple E. coli pellets for several production runs Multi-parallel plasmid preparations possible 1 -2 l-Batch Production in Roller Bottles 10 -l-Wave Production - Validation of large scale plasmid prep - 1 st purification trial - may suffice for entire production Shake flasks cultures or spinners also possible One run sufficient to meet demands in most cases Multi-parallel production if several Wave bioreactors available
 PPCW Workshop NIH 3/28/2004 Acknowledgements: The BTP team • HP Kocher (UH, Biomolecule Production) • • S Geisse (Baculo, Mammalian) M. Buchs A Patoux B Rudin • • • S Hartmann (IPC, In process & funct. analytics) D Rinaldi T Kuiper • • • M Henke (Fermentation) S Dalcher R Uhrhahn • • 22 F Kolbinger (Mol. Bio, Mammalian) PTH BTP Program A Schildhauer • • M Mahnke (E. coli) PTH EXA Program S Deutsch E Eglin Y Pouliquen • • JM Schlaeppi (Protein Pur. ) A Berner • • • R Schmitz (Mol. Bio) N Charara K Leon • T Soellick (Bioinf, Pro. Track) • • M Zurini (Protein Pur. ) J Causevic R Enderlin D Plattner
PPCW Workshop NIH 3/28/2004 Acknowledgements: The BTP team • HP Kocher (UH, Biomolecule Production) • • S Geisse (Baculo, Mammalian) M. Buchs A Patoux B Rudin • • • S Hartmann (IPC, In process & funct. analytics) D Rinaldi T Kuiper • • • M Henke (Fermentation) S Dalcher R Uhrhahn • • 22 F Kolbinger (Mol. Bio, Mammalian) PTH BTP Program A Schildhauer • • M Mahnke (E. coli) PTH EXA Program S Deutsch E Eglin Y Pouliquen • • JM Schlaeppi (Protein Pur. ) A Berner • • • R Schmitz (Mol. Bio) N Charara K Leon • T Soellick (Bioinf, Pro. Track) • • M Zurini (Protein Pur. ) J Causevic R Enderlin D Plattner


