4a01015bd403d06cf941a2c5c491d643.ppt
- Количество слайдов: 1
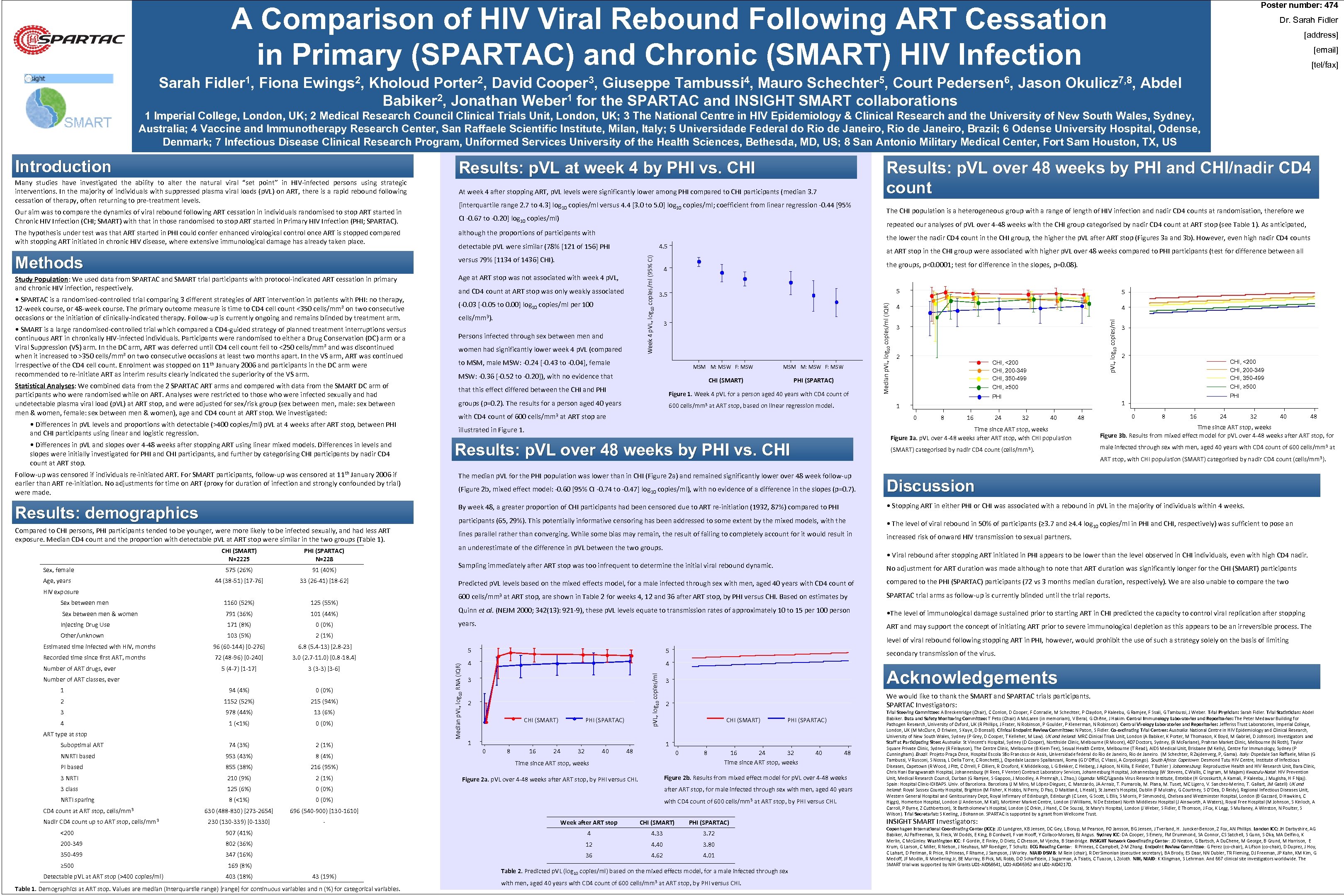
Poster number: 474 A Comparison of HIV Viral Rebound Following ART Cessation in Primary (SPARTAC) and Chronic (SMART) HIV Infection Dr. Sarah Fidler [address] [email] [tel/fax] Sarah Fidler 1, Fiona Ewings 2, Kholoud Porter 2, David Cooper 3, Giuseppe Tambussi 4, Mauro Schechter 5, Court Pedersen 6, Jason Okulicz 7, 8, Abdel Babiker 2, Jonathan Weber 1 for the SPARTAC and INSIGHT SMART collaborations 1 Imperial College, London, UK; 2 Medical Research Council Clinical Trials Unit, London, UK; 3 The National Centre in HIV Epidemiology & Clinical Research and the University of New South Wales, Sydney, Australia; 4 Vaccine and Immunotherapy Research Center, San Raffaele Scientific Institute, Milan, Italy; 5 Universidade Federal do Rio de Janeiro, Brazil; 6 Odense University Hospital, Odense, Denmark; 7 Infectious Disease Clinical Research Program, Uniformed Services University of the Health Sciences, Bethesda, MD, US; 8 San Antonio Military Medical Center, Fort Sam Houston, TX, US Introduction Results: p. VL at week 4 by PHI vs. CHI Many studies have investigated the ability to alter the natural viral “set point” in HIV-infected persons using strategic interventions. In the majority of individuals with suppressed plasma viral loads (p. VL) on ART, there is a rapid rebound following cessation of therapy, often returning to pre-treatment levels. At week 4 after stopping ART, p. VL levels were significantly lower among PHI compared to CHI participants (median 3. 7 CI -0. 67 to -0. 20] log 10 copies/ml) repeated our analyses of p. VL over 4 -48 weeks with the CHI group categorised by nadir CD 4 count at ART stop (see Table 1). As anticipated, although the proportions of participants with the lower the nadir CD 4 count in the CHI group, the higher the p. VL after ART stop (Figures 3 a and 3 b). However, even high nadir CD 4 counts detectable p. VL were similar (78% [121 of 156] PHI versus 79% [1134 of 1436] CHI). Study Population: We used data from SPARTAC and SMART trial participants with protocol-indicated ART cessation in primary and chronic HIV infection, respectively. 4. 5 Age at ART stop was not associated with week 4 p. VL, • SMART is a large randomised-controlled trial which compared a CD 4 -guided strategy of planned treatment interruptions versus continuous ART in chronically HIV-infected individuals. Participants were randomised to either a Drug Conservation (DC) arm or a Viral Suppression (VS) arm. In the DC arm, ART was deferred until CD 4 cell count fell to <250 cells/mm³ and was discontinued when it increased to >350 cells/mm³ on two consecutive occasions at least two months apart. In the VS arm, ART was continued irrespective of the CD 4 cell count. Enrolment was stopped on 11 th January 2006 and participants in the DC arm were recommended to re-initiate ART as interim results clearly indicated the superiority of the VS arm. Statistical Analyses: We combined data from the 2 SPARTAC ART arms and compared with data from the SMART DC arm of participants who were randomised while on ART. Analyses were restricted to those who were infected sexually and had undetectable plasma viral load (p. VL) at ART stop, and were adjusted for sex/risk group (sex between men, male: sex between men & women, female: sex between men & women), age and CD 4 count at ART stop. We investigated: • Differences in p. VL levels and proportions with detectable (>400 copies/ml) p. VL at 4 weeks after ART stop, between PHI and CHI participants using linear and logistic regression. • Differences in p. VL and slopes over 4 -48 weeks after stopping ART using linear mixed models. Differences in levels and slopes were initially investigated for PHI and CHI participants, and further by categorising CHI participants by nadir CD 4 count at ART stop. Week 4 p. VL, log 10 copies/ml (95% CI) Methods • SPARTAC is a randomised-controlled trial comparing 3 different strategies of ART intervention in patients with PHI: no therapy, 12 -week course, or 48 -week course. The primary outcome measure is time to CD 4 cell count <350 cells/mm³ on two consecutive occasions or the initiation of clinically-indicated therapy. Follow-up is currently ongoing and remains blinded by treatment arm. The CHI population is a heterogeneous group with a range of length of HIV infection and nadir CD 4 counts at randomisation, therefore we and CD 4 count at ART stop was only weakly associated (-0. 03 [-0. 05 to 0. 00] log 10 copies/ml per 100 cells/mm 3). Persons infected through sex between men and women had significantly lower week 4 p. VL (compared at ART stop in the CHI group were associated with higher p. VL over 48 weeks compared to PHI participants (test for difference between all the groups, p<0. 0001; test for difference in the slopes, p=0. 08). 4 5 3 to MSM, male MSW: -0. 24 [-0. 43 to -0. 04], female MSM M: MSW F: MSW: -0. 36 [-0. 52 to -0. 20]), with no evidence that MSM M: MSW F: MSW CHI (SMART) that this effect differed between the CHI and PHI (SPARTAC) Figure 1. Week 4 p. VL for a person aged 40 years with CD 4 count of groups (p=0. 2). The results for a person aged 40 years 600 cells/mm 3 at ART stop, based on linear regression model. 5 4 4 p. VL, log 10 copies/ml The hypothesis under test was that ART started in PHI could confer enhanced virological control once ART is stopped compared with stopping ART initiated in chronic HIV disease, where extensive immunological damage has already taken place. [interquartile range 2. 7 to 4. 3] log 10 copies/ml versus 4. 4 [3. 0 to 5. 0] log 10 copies/ml; coefficient from linear regression -0. 44 [95% Median p. VL, log 10 copies/ml (IQR) Our aim was to compare the dynamics of viral rebound following ART cessation in individuals randomised to stop ART started in Chronic HIV Infection (CHI; SMART) with that in those randomised to stop ART started in Primary HIV Infection (PHI; SPARTAC). Results: p. VL over 48 weeks by PHI and CHI/nadir CD 4 count 3 2 CHI, <200 CHI, 200 -349 CHI, 350 -499 CHI, ≥ 500 8 16 24 CHI, <200 CHI, 200 -349 CHI, 350 -499 PHI 0 2 CHI, ≥ 500 1 1 with CD 4 count of 600 cells/mm 3 at ART stop are 3 32 40 0 48 8 16 24 32 40 48 Time since ART stop, weeks Figure 3 a. p. VL over 4 -48 weeks after ART stop, with CHI population Results: p. VL over 48 weeks by PHI vs. CHI Time since ART stop, weeks Figure 3 b. Results from mixed effect model for p. VL over 4 -48 weeks after ART stop, for (SMART) categorised by nadir CD 4 count (cells/mm 3). illustrated in Figure 1. male infected through sex with men, aged 40 years with CD 4 count of 600 cells/mm 3 at ART stop, with CHI population (SMART) categorised by nadir CD 4 count (cells/mm 3). Follow-up was censored if individuals re-initiated ART. For SMART participants, follow-up was censored at 11 th January 2006 if earlier than ART re-initiation. No adjustments for time on ART (proxy for duration of infection and strongly confounded by trial) were made. The median p. VL for the PHI population was lower than in CHI (Figure 2 a) and remained significantly lower over 48 week follow-up (Figure 2 b, mixed effect model: -0. 60 [95% CI -0. 74 to -0. 47] log 10 copies/ml), with no evidence of a difference in the slopes (p=0. 7). Discussion Results: demographics By week 48, a greater proportion of CHI participants had been censored due to ART re-initiation (1932, 87%) compared to PHI • Stopping ART in either PHI or CHI was associated with a rebound in p. VL in the majority of individuals within 4 weeks. participants (65, 29%). This potentially informative censoring has been addressed to some extent by the mixed models, with the Compared to CHI persons, PHI participants tended to be younger, were more likely to be infected sexually, and had less ART exposure. Median CD 4 count and the proportion with detectable p. VL at ART stop were similar in the two groups (Table 1). • The level of viral rebound in 50% of participants (≥ 3. 7 and ≥ 4. 4 log 10 copies/ml in PHI and CHI, respectively) was sufficient to pose an lines parallel rather than converging. While some bias may remain, the result of failing to completely account for it would result in increased risk of onward HIV transmission to sexual partners. CHI (SMART) N=2225 PHI (SPARTAC) N=228 Sex, female 575 (26%) 91 (40%) Age, years 44 (38 -51) [17 -76] 33 (26 -41) [18 -62] HIV exposure 125 (55%) Sex between men & women 791 (36%) 101 (44%) Injecting Drug Use 171 (8%) 0 (0%) Other/unknown 103 (5%) 96 (60 -144) [0 -276] 72 (48 -96) [0 -240] 3 (3 -3) [3 -6] 600 cells/mm 3 at ART stop, are shown in Table 2 for weeks 4, 12 and 36 after ART stop, by PHI versus CHI. Based on estimates by SPARTAC trial arms as follow-up is currently blinded until the trial reports. Quinn et al. (NEJM 2000; 342(13): 921 -9), these p. VL levels equate to transmission rates of approximately 10 to 15 per 100 person • The level of immunological damage sustained prior to starting ART in CHI predicted the capacity to control viral replication after stopping years. ART and may support the concept of initiating ART prior to severe immunological depletion as this appears to be an irreversible process. The 3. 0 (2. 7 -11. 0) [0. 8 -18. 4] 5 (4 -7) [1 -17] compared to the PHI (SPARTAC) participants (72 vs 3 months median duration, respectively). We are also unable to compare the two 6. 8 (5. 4 -13) [2. 8 -23] Recorded time since first ART, months Predicted p. VL levels based on the mixed effects model, for a male infected through sex with men, aged 40 years with CD 4 count of 2 (1%) Estimated time infected with HIV, months No adjustment for ART duration was made although to note that ART duration was significantly longer for the CHI (SMART) participants Number of ART drugs, ever Number of ART classes, ever level of viral rebound following stopping ART in PHI, however, would prohibit the use of such a strategy solely on the basis of limiting 5 5 4 4 p. VL, log 10 copies/ml 1160 (52%) • Viral rebound after stopping ART initiated in PHI appears to be lower than the level observed in CHI individuals, even with high CD 4 nadir. Sampling immediately after ART stop was too infrequent to determine the initial viral rebound dynamic. Median p. VL, log 10 RNA (IQR) Sex between men an underestimate of the difference in p. VL between the two groups. 3 secondary transmission of the virus. Acknowledgements 3 1 94 (4%) 0 (0%) 2 1152 (52%) 215 (94%) 3 978 (44%) 13 (6%) 4 1 (<1%) 0 (0%) 74 (3%) 2 (1%) NNRTI based 953 (43%) 8 (4%) PI based 855 (38%) 216 (95%) 3 NRTI 210 (9%) 2 (1%) 3 class 125 (6%) 0 (0%) after ART stop, for male infected through sex with men, aged 40 years NRTI sparing 8 (<1%) 0 (0%) with CD 4 count of 600 cells/mm 3 at ART stop, by PHI versus CHI. 630 (488 -830) [273 -2654] 696 (540 -900) [130 -1610] 230 (130 -339) [0 -1330] - ART type at stop Suboptimal ART CD 4 count at ART stop, cells/mm 3 Nadir CD 4 count up to ART stop, cells/mm 3 2 CHI (SMART) PHI (SPARTAC) 1 0 8 16 24 32 40 We would like to thank the SMART and SPARTAC trials participants. SPARTAC Investigators: 2 CHI (SMART) PHI (SPARTAC) 1 48 0 8 Time since ART stop, weeks 16 24 32 Time since ART stop, weeks Week after ART stop CHI (SMART) PHI (SPARTAC) 907 (41%) 4 4. 33 3. 72 200 -349 802 (36%) 12 4. 40 3. 80 350 -499 347 (16%) 36 4. 62 4. 01 Detectable p. VL at ART stop (>400 copies/ml) 169 (8%) 403 (18%) 43 (19%) Table 1. Demographics at ART stop. Values are median (interquartile range) [range] for continuous variables and n (%) for categorical variables. 48 Figure 2 b. Results from mixed effect model for p. VL over 4 -48 weeks Figure 2 a. p. VL over 4 -48 weeks after ART stop, by PHI versus CHI. <200 ≥ 500 40 Table 2. Predicted p. VL (log 10 copies/ml) based on the mixed effects model, for a male infected through sex with men, aged 40 years with CD 4 count of 600 cells/mm 3 at ART stop, by PHI versus CHI. Trial Steering Committee: A Breckenridge (Chair), C Conlon, D Cooper, F Conradie, M Schechter, P Claydon, P Kaleebu, G Ramjee, F Ssali, G Tambussi, J Weber. Trial Physician: Sarah Fidler. Trial Statistician: Abdel Babiker. Data and Safety Monitoring Committee: T Peto (Chair) A Mc. Laren (in memoriam), V Beral, G Chêne, J Hakim. Central Immunology Laboratories and Repositories: The Peter Medawar Building for Pathogen Research, University of Oxford, UK (R Phillips, J Frater, N Robinson, P Goulder, P Klenerman, N Robinson). Central Virology Laboratories and Repositories: Jefferiss Trust Laboratories, Imperial College, London, UK (M Mc. Clure, O Erlwien, S Kaye, D Bonsall). Clinical Endpoint Review Committee: N Paton, S Fidler. Co-ordinating Trial Centres: Australia: National Centre in HIV Epidemiology and Clinical Research, University of New South Wales, Sydney (P Grey, D Cooper, T Kelleher, M Law). UK and Ireland: MRC Clinical Trials Unit, London (A Babiker, K Porter, M Thomason, K Boyd, M Gabriel, D Johnson). Investigators and Staff at Participating Sites: Australia: St Vincent’s Hospital, Sydney (D Cooper), Northside Clinic, Melbourne (R Moore), 407 Doctors, Sydney, (R Mc. Farlane), Prahran Market Clinic, Melbourne (N Roth), Taylor Square Private Clinic, Sydney (R Finlayson), The Centre Clinic, Melbourne (B Kiem Tee), Sexual Health Centre, Melbourne (T Read), AIDS Medical Unit, Brisbane (M Kelly), Centre for Immunology, Sydney (P Cunningham). Brazil: Projeto Praça Onze, Hospital Escola São Francisco de Assis, Universidade federal do Rio de Janeiro, Rio de Janeiro. (M Schechter, R Zajdenverg, P, Gama). Italy: Ospedale San Raffaele, Milan (G Tambussi, V Rusconi, S Nossa, L Della Torre, C Ronchetti, ), Ospedale Lazzaro Spallanzani, Roma (G D’Offizi, C Vlassi, A Corpolongo). South Africa: Capetown: Desmond Tutu HIV Centre, Institute of Infectious Diseases, Capetown (R Wood, J Pitt, C Orrell, F Cilliers, R Croxford, K Middelkoop, L G Bekker, C Heiberg, J Aploon, N Killa, E Fielder, T Buhler ) Johannesburg: Reproductive Health and HIV Research Unit, Bara Clinic, Chris Hani Baragwanath Hospital, Johannesburg (H Rees, F Venter) Contract Laboratory Services, Johannesburg Hospital, Johannesburg (W Stevens, C Wallis, C Ingram, M Majam) Kwazulu-Natal: HIV Prevention Unit, Medical Research Council, Durban (G Ramjee, S Gappoo, J Moodley, A Premrajh, L Zhao, ) Uganda: MRC/Uganda Virus Research Institute, Entebbe (H Grosskurth, A Kamali, P Kaleebu, J Mugisha, H F Njaj). Spain: Hospital Clinic-IDIBAPS. Univ. of Barcelona (J M Miro, M López-Dieguez, C. Manzardo, JA Arnaiz, T. Pumarola, M. Plana, M. Tuset, MC Ligero, V. Sanchez-Merino, T. Gallart, JM Gatell) UK and Ireland: Royal Sussex County Hospital, Brighton (M Fisher, K Hobbs, N Perry, D Pao, D Maitland, L Heald), St James’s Hospital, Dublin (F Mulcahy, G Courtney, S O’Dea, D Reidy), Regional Infectious Diseases Unit, Western General Hospital and Genitourinary Dept, Royal Infirmary of Edinburgh, Edinburgh (C Leen, G Scott, L Ellis, S Morris, P Simmonds), Chelsea and Westminster Hospital, London (B Gazzard, D Hawkins, C Higgs), Homerton Hospital, London (J Anderson, M Kall), Mortimer Market Centre, London (I Williams, N De Esteban) North Middlesex Hospital (J Ainsworth, A Waters), Royal Free Hospital (M Johnson, S Kinloch, A Carroll, P Byrne, Z Cuthbertson), St Bartholomew’s Hospital, London (C Orkin, J Hand, C De Souza), St Mary’s Hospital, London (J Weber, S Fidler, E Thomson, J Fox, K Legg, S Mullaney, A Winston, N Poulter, S Wilson). Trial Secretariat: S Keeling, J Bohannon. SPARTAC is supported by a grant from Wellcome Trust. INSIGHT SMART Investigators: Copenhagen International Coordinating Center (ICC): JD Lundgren, KB Jensen, DC Gey, L Borup, M Pearson, PO Jansson, BG Jensen, J Tverland, H. Juncker-Benzon, Z Fox, AN Phillips. London ICC: JH Darbyshire, AG Babiker, AJ Palfreeman, SL Fleck, W Dodds, E King, B Cordwell, F van Hooff, Y Collaco-Moraes, BJ Angus. Sydney ICC: DA Cooper, S Emery, FM Drummond, SA Connor, CS Satchell, S Gunn, S Oka, MA Delfino, K Merlin, C Mc. Ginley. Washington ICC: F Gordin, E Finley, D Dietz, C Chesson, M Vjecha, B Standridge. INSIGHT Network Coordinating Center: JD Neaton, G Bartsch, A Du. Chene, M George, B Grund, M Harrison, E Krum, G Larson, C Miller, R Nelson, J Neuhaus, MP Roediger, T Schultz. ECG Reading Center: R Prineas, C Campbell, Z-M Zhang. Endpoint Review Committee: G Perez (co-chair), A Lifson (co-chair), D Duprez, J Hoy, C Lahart, D Perlman, R Price, R Prineas, F Rhame, J Sampson, J Worley. NIAID DSMB: M Rein (chair), R Der. Simonian (executive secretary), BA Brody, ES Daar, NN Dubler, TR Fleming, DJ Freeman, JP Kahn, KM Kim, G Medoff, JF Modlin, R Moellering Jr, BE Murray, B Pick, ML Robb, DO Scharfstein, J Sugarman, A Tsiatis, C Tuazon, L Zoloth. NIH, NIAID: K Klingman, S Lehrman. And 667 clinical site investigators worldwide. The SMART trial was supported by NIH Grants U 01 -AI 068641, U 01 -AI 046362 and U 01 -AI 042170.
4a01015bd403d06cf941a2c5c491d643.ppt