c266a7cf9ae00cacc8d6e4dcaedaf9ab.ppt
- Количество слайдов: 51
 Post-Market Surveillance & Third-Party Reporting Systems A presentation to the LSRO Panel on Dietary Supplements and Adverse Event Reporting Rick Kingston Pharm. D Vice President & Senior Clinical Toxicologist PROSAR International Poison Center & Associate Professor Department of Experimental and Clinical Pharmacology College of Pharmacy, University of Minnesota
Post-Market Surveillance & Third-Party Reporting Systems A presentation to the LSRO Panel on Dietary Supplements and Adverse Event Reporting Rick Kingston Pharm. D Vice President & Senior Clinical Toxicologist PROSAR International Poison Center & Associate Professor Department of Experimental and Clinical Pharmacology College of Pharmacy, University of Minnesota
 Talking Points 1. Introduction to PROSAR International Poison Center (IPC) 2. Brief overview of Post-Market Surveillance and sources of data relied upon by Companies 3. The EPA FIFRA 6(a)(2) model 4. Product Stewardship and evolution of the PROSAR IPC model
Talking Points 1. Introduction to PROSAR International Poison Center (IPC) 2. Brief overview of Post-Market Surveillance and sources of data relied upon by Companies 3. The EPA FIFRA 6(a)(2) model 4. Product Stewardship and evolution of the PROSAR IPC model
 Talking Points (cont. ) 6. PROSAR IPC - Data Collection - Severity Assessments - Surveillance, Analysis, and Benchmarking - Responding to a serious event 7. Strengths and limitations of “spontaneous reporting” 8. Implications for the Metabolife project 9. Challenges related to Dietary Supplement Surveillance and Safety
Talking Points (cont. ) 6. PROSAR IPC - Data Collection - Severity Assessments - Surveillance, Analysis, and Benchmarking - Responding to a serious event 7. Strengths and limitations of “spontaneous reporting” 8. Implications for the Metabolife project 9. Challenges related to Dietary Supplement Surveillance and Safety
 Organizational Profile, Service Offerings, and Focus
Organizational Profile, Service Offerings, and Focus
 PROSAR • Health & Safety Information Services – Healthcare practice – leverage technology extensively • Case Manager • Case Explorer • PROSAR International Poison Center (IPC) serving clients with specialized post-market surveillance needs since 1997 – >100, 000 cases per year – private center serving >200 clients
PROSAR • Health & Safety Information Services – Healthcare practice – leverage technology extensively • Case Manager • Case Explorer • PROSAR International Poison Center (IPC) serving clients with specialized post-market surveillance needs since 1997 – >100, 000 cases per year – private center serving >200 clients
 PROSAR IPC Services • PROSAR International Poison Center (Health & Safety Call Center focused on consumer product medical support and surveillance) • International Animal Poison Center (Industry focused surveillance center for incidents involving animal health) • Pet Poison Hotline (Public) • Toxicology Consulting – Pre & Post Market Product Safety Assessments – Risk Assessments
PROSAR IPC Services • PROSAR International Poison Center (Health & Safety Call Center focused on consumer product medical support and surveillance) • International Animal Poison Center (Industry focused surveillance center for incidents involving animal health) • Pet Poison Hotline (Public) • Toxicology Consulting – Pre & Post Market Product Safety Assessments – Risk Assessments
 PROSAR International Poison Center • Markets served: – Consumer Products: Personal Care, Food, Household – Industrial Chemical – Drug/Pharmaceutical – Ag-Chem-Pesticide-Herbicide – Animal Health
PROSAR International Poison Center • Markets served: – Consumer Products: Personal Care, Food, Household – Industrial Chemical – Drug/Pharmaceutical – Ag-Chem-Pesticide-Herbicide – Animal Health
 International Animal Poison Center Service • Director, Lynn Hovda, R. Ph. , D. V. M. , M. S. , CSPI, DACVIM • Animal care provided by DVM’s, CVT’s, and Toxicologists • Serving corporate clients and the general public • EPA and FDA reporting
International Animal Poison Center Service • Director, Lynn Hovda, R. Ph. , D. V. M. , M. S. , CSPI, DACVIM • Animal care provided by DVM’s, CVT’s, and Toxicologists • Serving corporate clients and the general public • EPA and FDA reporting
 Our Staff. . . • Highly credentialed, healthcare professionals with over 150 years experience in clinical, academic, and research environments – Specific expertise in Toxicology, Pharmacology, Infectious Disease Epidemiology, and Industrial Hygiene – Physicians Board Certified in Internal Medicine, Emergency Medicine, Occupational Medicine, Veterinary Medicine, and Medical Toxicology – Academic affiliation with University of Minnesota College of Pharmacy
Our Staff. . . • Highly credentialed, healthcare professionals with over 150 years experience in clinical, academic, and research environments – Specific expertise in Toxicology, Pharmacology, Infectious Disease Epidemiology, and Industrial Hygiene – Physicians Board Certified in Internal Medicine, Emergency Medicine, Occupational Medicine, Veterinary Medicine, and Medical Toxicology – Academic affiliation with University of Minnesota College of Pharmacy
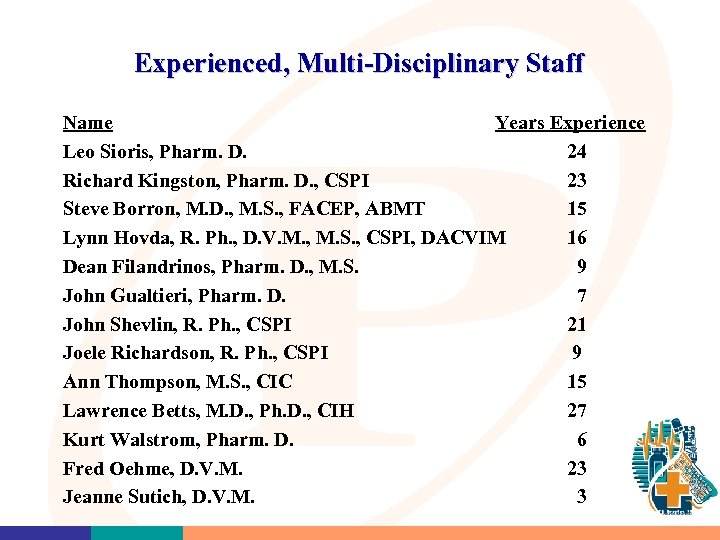 Experienced, Multi-Disciplinary Staff Name Years Experience Leo Sioris, Pharm. D. 24 Richard Kingston, Pharm. D. , CSPI 23 Steve Borron, M. D. , M. S. , FACEP, ABMT 15 Lynn Hovda, R. Ph. , D. V. M. , M. S. , CSPI, DACVIM 16 Dean Filandrinos, Pharm. D. , M. S. 9 John Gualtieri, Pharm. D. 7 John Shevlin, R. Ph. , CSPI 21 Joele Richardson, R. Ph. , CSPI 9 Ann Thompson, M. S. , CIC 15 Lawrence Betts, M. D. , Ph. D. , CIH 27 Kurt Walstrom, Pharm. D. 6 Fred Oehme, D. V. M. 23 Jeanne Sutich, D. V. M. 3
Experienced, Multi-Disciplinary Staff Name Years Experience Leo Sioris, Pharm. D. 24 Richard Kingston, Pharm. D. , CSPI 23 Steve Borron, M. D. , M. S. , FACEP, ABMT 15 Lynn Hovda, R. Ph. , D. V. M. , M. S. , CSPI, DACVIM 16 Dean Filandrinos, Pharm. D. , M. S. 9 John Gualtieri, Pharm. D. 7 John Shevlin, R. Ph. , CSPI 21 Joele Richardson, R. Ph. , CSPI 9 Ann Thompson, M. S. , CIC 15 Lawrence Betts, M. D. , Ph. D. , CIH 27 Kurt Walstrom, Pharm. D. 6 Fred Oehme, D. V. M. 23 Jeanne Sutich, D. V. M. 3
 Partial Client List Household Products: Amway Church & Dwight Dial Elmer’s Iron Out Incorporated WD-40 Company Lime-O-Sol Reckitt Benckiser SC Johnson Personal Care Products: Amway Bath & Body Works Church & Dwight Dial Playtex SC Johnson Unilever White Rain Commercial/Industrial: Avecia Castrol Chemtrec Dow Corning Dupont Ecolab Fuji USA Huntsman Corporation ICI Americas Johnson Wax Professional PRC - De. Soto Rohm & Haas Spartan Chemical Sysco Valspar Animal Health: Bayer Animal Health Fort Dodge Animal Health Intervet Lambert Kay Merial Pharmacia Animal Health Sargeants Wellmark Agricultural/Pesticide: Aventis Bayer Advanced Bayer Crop Protection Bell Laboratories Inc. Cargill Dow LLC Dow Agrosciences FMC MGK Scott’s (Ortho) Syngenta Food Products: Best Foods Dole Food Company Inc. HJ Heinz Company L. P. International Multifoods Kellogg Company Land-O-Lakes Starbucks Unilever Pharmaceutical Bioglan Health. Point Lec. Tec Corp. Medicis Zila Pharmaceutical
Partial Client List Household Products: Amway Church & Dwight Dial Elmer’s Iron Out Incorporated WD-40 Company Lime-O-Sol Reckitt Benckiser SC Johnson Personal Care Products: Amway Bath & Body Works Church & Dwight Dial Playtex SC Johnson Unilever White Rain Commercial/Industrial: Avecia Castrol Chemtrec Dow Corning Dupont Ecolab Fuji USA Huntsman Corporation ICI Americas Johnson Wax Professional PRC - De. Soto Rohm & Haas Spartan Chemical Sysco Valspar Animal Health: Bayer Animal Health Fort Dodge Animal Health Intervet Lambert Kay Merial Pharmacia Animal Health Sargeants Wellmark Agricultural/Pesticide: Aventis Bayer Advanced Bayer Crop Protection Bell Laboratories Inc. Cargill Dow LLC Dow Agrosciences FMC MGK Scott’s (Ortho) Syngenta Food Products: Best Foods Dole Food Company Inc. HJ Heinz Company L. P. International Multifoods Kellogg Company Land-O-Lakes Starbucks Unilever Pharmaceutical Bioglan Health. Point Lec. Tec Corp. Medicis Zila Pharmaceutical
 Consumer Product Post-Market/In. Market Surveillance • What is it? ? The processes whereby manufactures, regulators, health professionals, the public at large, and others monitor the performance and experience related to a given products lifecycle in the open market.
Consumer Product Post-Market/In. Market Surveillance • What is it? ? The processes whereby manufactures, regulators, health professionals, the public at large, and others monitor the performance and experience related to a given products lifecycle in the open market.
 Post-Market/In-Market Surveillance • Why is it necessary? ? “Your market population will likely be much larger than any of your pre-market test populations”
Post-Market/In-Market Surveillance • Why is it necessary? ? “Your market population will likely be much larger than any of your pre-market test populations”
 Post-Market/In-Market Surveillance • “The absence of reliable evidence of risk should not be mistaken for reliable evidence of the absence of risk”
Post-Market/In-Market Surveillance • “The absence of reliable evidence of risk should not be mistaken for reliable evidence of the absence of risk”
 Regulations and Safety The question to ask: Are there systems or processes in place to identify safety issues through methods other than serendipitous discovery? and How do you acknowledge the varying levels of quality and integrity of typical “spontaneous reports” received regarding any consumer product
Regulations and Safety The question to ask: Are there systems or processes in place to identify safety issues through methods other than serendipitous discovery? and How do you acknowledge the varying levels of quality and integrity of typical “spontaneous reports” received regarding any consumer product
 Post-Market/In-Market Surveillance What should it accomplish? ? • Helps identify intended and unintended use patterns that may potentially contribute to Adverse Events • Allows assessment of how the product performs by itself or in the presence of other products or substances • Helps insure that “unique” populations are not adversely affected • Should also help define a relative “Safety profile”
Post-Market/In-Market Surveillance What should it accomplish? ? • Helps identify intended and unintended use patterns that may potentially contribute to Adverse Events • Allows assessment of how the product performs by itself or in the presence of other products or substances • Helps insure that “unique” populations are not adversely affected • Should also help define a relative “Safety profile”
 Adverse Event Reporting Systems Utilized by Industry: CPSC Monitoring Systems National Electronic Injury Surveillance System (NEISS): • Surveys 100 ER’s selected as a nationwide probability sample of all 5, 300+ U. S. Hospitals with ER’s • Designed to provide the Commission with evidence of need for: - a product recall - a public awareness campaign - a product safety standard
Adverse Event Reporting Systems Utilized by Industry: CPSC Monitoring Systems National Electronic Injury Surveillance System (NEISS): • Surveys 100 ER’s selected as a nationwide probability sample of all 5, 300+ U. S. Hospitals with ER’s • Designed to provide the Commission with evidence of need for: - a product recall - a public awareness campaign - a product safety standard
 Adverse Event Reporting Systems Utilized by Industry: CPSC Internal Monitoring Systems Consumer Communications: Individual Reports Involving Death or Injury • Results in CPSC investigation into injury allegations • May include on-site, face-to-face interviews and analysis of the product involved as well as medical records related to the injury Manufacturer Communications (CPSC): Individual Reports Involving Death or Injury
Adverse Event Reporting Systems Utilized by Industry: CPSC Internal Monitoring Systems Consumer Communications: Individual Reports Involving Death or Injury • Results in CPSC investigation into injury allegations • May include on-site, face-to-face interviews and analysis of the product involved as well as medical records related to the injury Manufacturer Communications (CPSC): Individual Reports Involving Death or Injury
 Adverse Event Reporting Systems Utilized by Industry: : FDA Monitoring Systems • FDA Med. Watch - Depends on either the patient or a Healthcare Practitioner to identify an effect, identify a suspected cause, question a relationship and report it to a “governmental” body - reporting is typically a “one-way” street (not designed to engage the caller and provide “clinical advice”)
Adverse Event Reporting Systems Utilized by Industry: : FDA Monitoring Systems • FDA Med. Watch - Depends on either the patient or a Healthcare Practitioner to identify an effect, identify a suspected cause, question a relationship and report it to a “governmental” body - reporting is typically a “one-way” street (not designed to engage the caller and provide “clinical advice”)
 Other External Surveillance Databases, Systems or Services Medical Literature (human & animal) • More in-depth reports with medical details on the injury • Often lacking on “circumstance” info*** (focused on what was done after the exposure) • Often serves as basis or support for “pro-active” regulation (animal or human data)
Other External Surveillance Databases, Systems or Services Medical Literature (human & animal) • More in-depth reports with medical details on the injury • Often lacking on “circumstance” info*** (focused on what was done after the exposure) • Often serves as basis or support for “pro-active” regulation (animal or human data)
 Other External Surveillance Databases, Systems or Services • Media Clipping Service • Surveys/reports of death or injury in the lay press that involves consumer products • Product Liability Claims
Other External Surveillance Databases, Systems or Services • Media Clipping Service • Surveys/reports of death or injury in the lay press that involves consumer products • Product Liability Claims
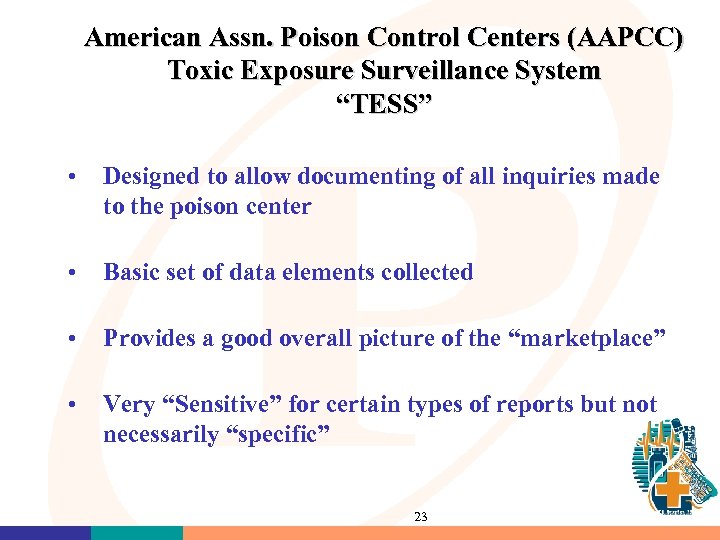 American Assn. Poison Control Centers (AAPCC) Toxic Exposure Surveillance System “TESS” • Designed to allow documenting of all inquiries made to the poison center • Basic set of data elements collected • Provides a good overall picture of the “marketplace” • Very “Sensitive” for certain types of reports but not necessarily “specific” 23
American Assn. Poison Control Centers (AAPCC) Toxic Exposure Surveillance System “TESS” • Designed to allow documenting of all inquiries made to the poison center • Basic set of data elements collected • Provides a good overall picture of the “marketplace” • Very “Sensitive” for certain types of reports but not necessarily “specific” 23
 Toxic Exposure Surveillance System “TESS” • Telephone based reporting: Phone Calls Exposures Injury/Poisonings “Hospitalization” Injury/Poisonings 24
Toxic Exposure Surveillance System “TESS” • Telephone based reporting: Phone Calls Exposures Injury/Poisonings “Hospitalization” Injury/Poisonings 24
 Minnesota Poison Prevention Findings Y 2 K • For all pediatric cases, 97. 6% result in non-significant outcomes (<=minor effects) and 91. 1% of cases result in no reported adverse effects. These outcomes are achieved with fewer than 5 patients receiving specific therapies other than decontamination. • Of all pediatric cases, 666(3. 0%) received specific decontamination therapy other than simple dilution/irrigation such as syrup of ipecac or activated charcoal. (The lack of need for specific interventions may be the result of previously cited Minnesota data reporting that more than 55% of exposures from all routes including all reasons are “taste/touch” or mucous membrane types of exposures. These types of trivial exposures represent 71% of all “ingestions”. )
Minnesota Poison Prevention Findings Y 2 K • For all pediatric cases, 97. 6% result in non-significant outcomes (<=minor effects) and 91. 1% of cases result in no reported adverse effects. These outcomes are achieved with fewer than 5 patients receiving specific therapies other than decontamination. • Of all pediatric cases, 666(3. 0%) received specific decontamination therapy other than simple dilution/irrigation such as syrup of ipecac or activated charcoal. (The lack of need for specific interventions may be the result of previously cited Minnesota data reporting that more than 55% of exposures from all routes including all reasons are “taste/touch” or mucous membrane types of exposures. These types of trivial exposures represent 71% of all “ingestions”. )
 Adverse Event Reporting Systems Utilized by Industry: EPA Monitoring Systems • Environmental Protection Agency: FIFRA 6(a)(2) • Process maximally engages the Manufacturer in the process - Encouraged and supported Product Stewardship Efforts
Adverse Event Reporting Systems Utilized by Industry: EPA Monitoring Systems • Environmental Protection Agency: FIFRA 6(a)(2) • Process maximally engages the Manufacturer in the process - Encouraged and supported Product Stewardship Efforts
 AER Systems: EPA Monitoring Systems • 6(a)(2) & Product Stewardship Identify, Manage, Evaluate: Adverse incidents reportedly associated with products • Previous 6(a)(2) Requirements: Only serious or unexpected effects judged related to a product reported to EPA
AER Systems: EPA Monitoring Systems • 6(a)(2) & Product Stewardship Identify, Manage, Evaluate: Adverse incidents reportedly associated with products • Previous 6(a)(2) Requirements: Only serious or unexpected effects judged related to a product reported to EPA
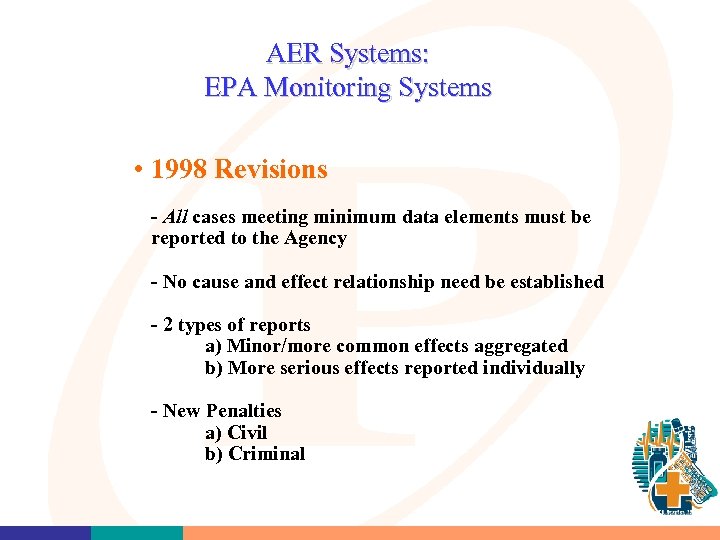 AER Systems: EPA Monitoring Systems • 1998 Revisions - All cases meeting minimum data elements must be reported to the Agency - No cause and effect relationship need be established - 2 types of reports a) Minor/more common effects aggregated b) More serious effects reported individually - New Penalties a) Civil b) Criminal
AER Systems: EPA Monitoring Systems • 1998 Revisions - All cases meeting minimum data elements must be reported to the Agency - No cause and effect relationship need be established - 2 types of reports a) Minor/more common effects aggregated b) More serious effects reported individually - New Penalties a) Civil b) Criminal
 Adverse Event Reporting Systems Utilized by Industry: EPA Monitoring Systems • Industry’s Response to the 6(a)(2) Challenge - “Industry’s Voluntary Report Forms” (see: www. fifra 6 a 2. com) General Incident Data Addendum’s for specific events Report format for single reportable incidents Guidance document form completion
Adverse Event Reporting Systems Utilized by Industry: EPA Monitoring Systems • Industry’s Response to the 6(a)(2) Challenge - “Industry’s Voluntary Report Forms” (see: www. fifra 6 a 2. com) General Incident Data Addendum’s for specific events Report format for single reportable incidents Guidance document form completion
 Adverse Event Reporting Systems Utilized by Industry: EPA Monitoring Systems • FOCUS AREAS 1) General Incident Data Addendum’s for specific events Report format for single reportable incidents Guidance document form completion 2) Aggregate Reporting Form Guidance Document form completion
Adverse Event Reporting Systems Utilized by Industry: EPA Monitoring Systems • FOCUS AREAS 1) General Incident Data Addendum’s for specific events Report format for single reportable incidents Guidance document form completion 2) Aggregate Reporting Form Guidance Document form completion
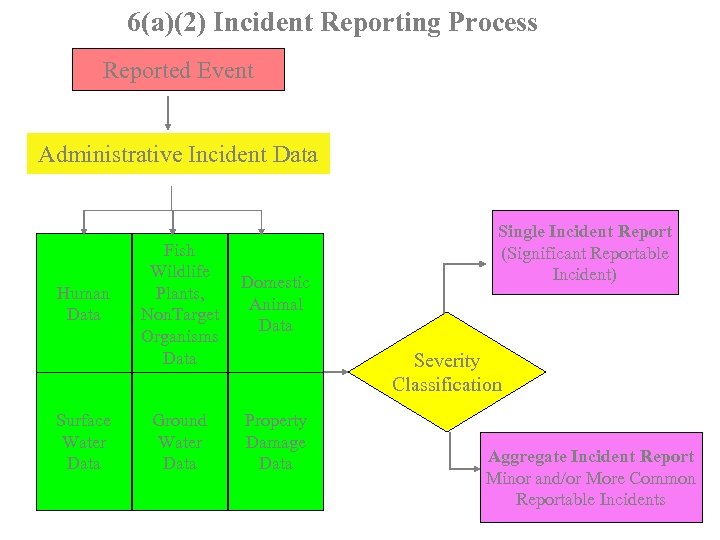 6(a)(2) Incident Reporting Process Reported Event Administrative Incident Data Human Data Surface Water Data Fish Wildlife Plants, Non. Target Organisms Data Domestic Animal Data Ground Water Data Property Damage Data Single Incident Report (Significant Reportable Incident) Severity Classification Aggregate Incident Report Minor and/or More Common Reportable Incidents
6(a)(2) Incident Reporting Process Reported Event Administrative Incident Data Human Data Surface Water Data Fish Wildlife Plants, Non. Target Organisms Data Domestic Animal Data Ground Water Data Property Damage Data Single Incident Report (Significant Reportable Incident) Severity Classification Aggregate Incident Report Minor and/or More Common Reportable Incidents
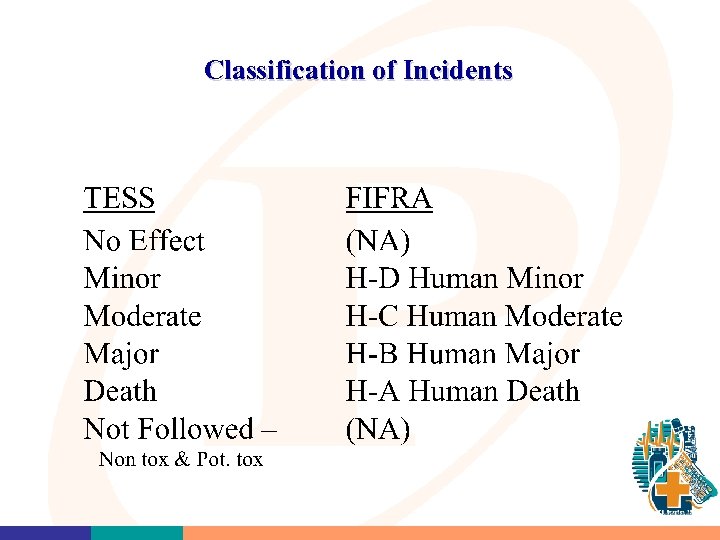
 Classification of Incidents Involving Humans • Aggregate Incident Report (Collect 3 months, report in 2) Minor and/or More Common Reportable Incidents H-D • Single Incidents Reports: Significant Reportable Incident H-B, H-C (Collect 1 month report following month) H-A (Human Death: Report within 15 days of notification)
Classification of Incidents Involving Humans • Aggregate Incident Report (Collect 3 months, report in 2) Minor and/or More Common Reportable Incidents H-D • Single Incidents Reports: Significant Reportable Incident H-B, H-C (Collect 1 month report following month) H-A (Human Death: Report within 15 days of notification)
 Classification of Incidents
Classification of Incidents
 Challenges in Incident Classification • Additional Considerations “Duration” vs. “Intensity of clinical effects”
Challenges in Incident Classification • Additional Considerations “Duration” vs. “Intensity of clinical effects”
 Evolution of the PROSAR Model • Strongly influenced by national Product Stewardship efforts by Industry - Responsible Care - Responsible Distribution - Product. Care • Also influenced by evolution of “standard of care” as the model proliferated (consumer expectations)
Evolution of the PROSAR Model • Strongly influenced by national Product Stewardship efforts by Industry - Responsible Care - Responsible Distribution - Product. Care • Also influenced by evolution of “standard of care” as the model proliferated (consumer expectations)
 Product Stewardship • CSPA – Product. Care Vision To promote the production and distribution of safe and effective formulated products that provide desirable benefits for household, commercial, institutional and industrial customers and consumers, and their families, pets and their environment.
Product Stewardship • CSPA – Product. Care Vision To promote the production and distribution of safe and effective formulated products that provide desirable benefits for household, commercial, institutional and industrial customers and consumers, and their families, pets and their environment.
 Product Stewardship (eg. CSPA Product Care) • In-Market Support, Incident Evaluation and Follow-up – Principle 5 • We will support dissemination of safety related product information regarding routine use of our products that is accurate, complete and in context to the inquiry or concern • When product-related incidents occur, we will have systems in place to minimize adverse effects, assist our consumers/customers and provide needed information
Product Stewardship (eg. CSPA Product Care) • In-Market Support, Incident Evaluation and Follow-up – Principle 5 • We will support dissemination of safety related product information regarding routine use of our products that is accurate, complete and in context to the inquiry or concern • When product-related incidents occur, we will have systems in place to minimize adverse effects, assist our consumers/customers and provide needed information
 Product Stewardship (eg. CSPA Product Care) • In-Market Support, Incident Evaluation and Follow-up – Principle 5 (cont. ) • We will strive to influence product and label design as well as develop educational messages on safe and responsible product use based on information regarding unintended events and other types of exposures involving our products
Product Stewardship (eg. CSPA Product Care) • In-Market Support, Incident Evaluation and Follow-up – Principle 5 (cont. ) • We will strive to influence product and label design as well as develop educational messages on safe and responsible product use based on information regarding unintended events and other types of exposures involving our products
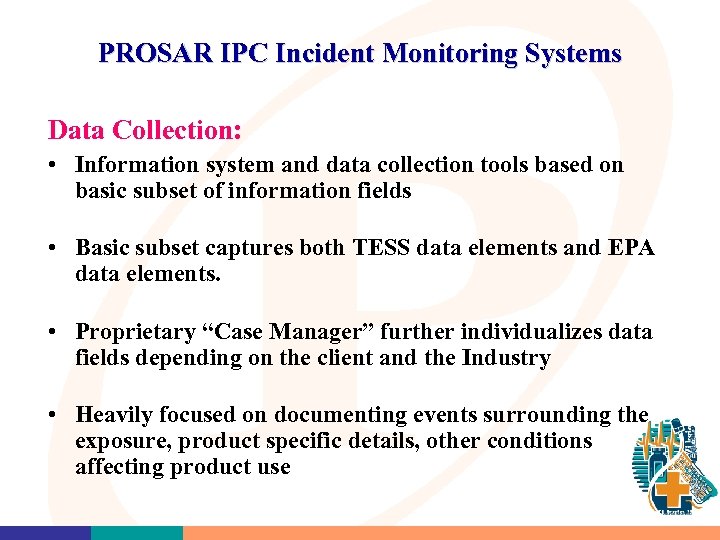 PROSAR IPC Incident Monitoring Systems Data Collection: • Information system and data collection tools based on basic subset of information fields • Basic subset captures both TESS data elements and EPA data elements. • Proprietary “Case Manager” further individualizes data fields depending on the client and the Industry • Heavily focused on documenting events surrounding the exposure, product specific details, other conditions affecting product use
PROSAR IPC Incident Monitoring Systems Data Collection: • Information system and data collection tools based on basic subset of information fields • Basic subset captures both TESS data elements and EPA data elements. • Proprietary “Case Manager” further individualizes data fields depending on the client and the Industry • Heavily focused on documenting events surrounding the exposure, product specific details, other conditions affecting product use
 PROSAR IPC Incident Monitoring Systems Data Collection: • Product Specific Details Multiple methods of product verification Evaluating the role of labeling Marketing
PROSAR IPC Incident Monitoring Systems Data Collection: • Product Specific Details Multiple methods of product verification Evaluating the role of labeling Marketing
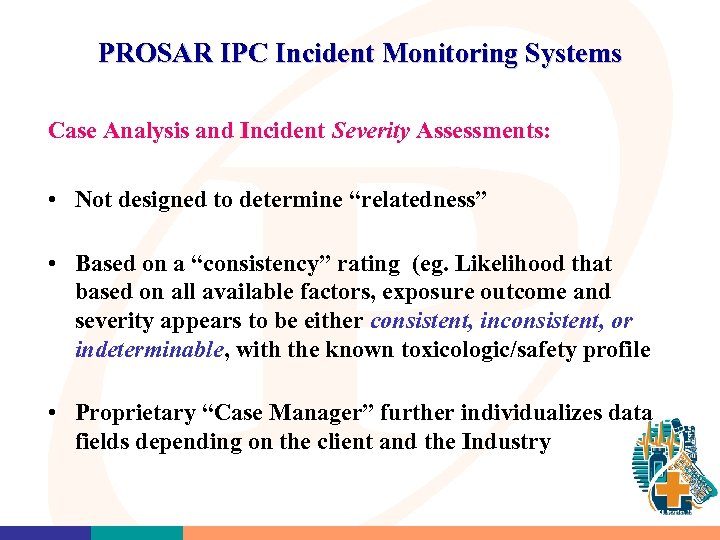 PROSAR IPC Incident Monitoring Systems Case Analysis and Incident Severity Assessments: • Not designed to determine “relatedness” • Based on a “consistency” rating (eg. Likelihood that based on all available factors, exposure outcome and severity appears to be either consistent, inconsistent, or indeterminable, with the known toxicologic/safety profile • Proprietary “Case Manager” further individualizes data fields depending on the client and the Industry
PROSAR IPC Incident Monitoring Systems Case Analysis and Incident Severity Assessments: • Not designed to determine “relatedness” • Based on a “consistency” rating (eg. Likelihood that based on all available factors, exposure outcome and severity appears to be either consistent, inconsistent, or indeterminable, with the known toxicologic/safety profile • Proprietary “Case Manager” further individualizes data fields depending on the client and the Industry
 PROSAR IPC Incident Monitoring Systems Surveillance, Analysis, and Benchmarking: • Benchmarking is provided through an automated data analysis system: PSI (PROSAR Safety Index)
PROSAR IPC Incident Monitoring Systems Surveillance, Analysis, and Benchmarking: • Benchmarking is provided through an automated data analysis system: PSI (PROSAR Safety Index)
 PROSAR Safety Index • Designed to help identify when to “take a closer look” • Analytical tool to aid in identifying products that fall outside of their normal safety profile • Based on the total number of “significant outcomes” compared against all symptomatic incidents involving a given product or class of products for a given time period • Initially calculated at the following levels: – Individual product – Company – PROSAR (also allows normalizing with product sales data)
PROSAR Safety Index • Designed to help identify when to “take a closer look” • Analytical tool to aid in identifying products that fall outside of their normal safety profile • Based on the total number of “significant outcomes” compared against all symptomatic incidents involving a given product or class of products for a given time period • Initially calculated at the following levels: – Individual product – Company – PROSAR (also allows normalizing with product sales data)
 Future Levels of Incident Evaluation • Next level of Incident Assessment: “Registry” approach to follow-up Product Surveillance The SAS Initiative (Safety Assurance Surveillance)
Future Levels of Incident Evaluation • Next level of Incident Assessment: “Registry” approach to follow-up Product Surveillance The SAS Initiative (Safety Assurance Surveillance)
 Responding to a “serious” event • Action is typically dictated by: - type of incident - supporting documentation - existing regulatory reporting requirements - ability to investigate, verify, and add definition
Responding to a “serious” event • Action is typically dictated by: - type of incident - supporting documentation - existing regulatory reporting requirements - ability to investigate, verify, and add definition
 Telephone Based Incident Data: Strengths/Limitations of Spontaneous Reported Data
Telephone Based Incident Data: Strengths/Limitations of Spontaneous Reported Data
 Strengths • Monitoring for Sentinel Events: – Critical Mass increases likelihood of discovery • Hypothesis Generation: – Background noise vs. emerging issue – Aids in Design of Research? • Generation of Safety Profile: – Sufficient “Penetrance”? – Insure representative sample relative intensity
Strengths • Monitoring for Sentinel Events: – Critical Mass increases likelihood of discovery • Hypothesis Generation: – Background noise vs. emerging issue – Aids in Design of Research? • Generation of Safety Profile: – Sufficient “Penetrance”? – Insure representative sample relative intensity
 Limitations • Outcome Severity Classifications – Some symptoms not amenable to clear-cut classification – Indirect vs. Direct Effects – Physiologic vs. Psychologic S/S • Assessment of Causation/Relatedness – Data does not lead to determination of “cause and effect” relationship – At best may suggest an Epidemiological Association • Under-reporting/Over-reporting – reporting of “real” vs. “perceived” threats – Media and promotion – Possible “link” not recognized
Limitations • Outcome Severity Classifications – Some symptoms not amenable to clear-cut classification – Indirect vs. Direct Effects – Physiologic vs. Psychologic S/S • Assessment of Causation/Relatedness – Data does not lead to determination of “cause and effect” relationship – At best may suggest an Epidemiological Association • Under-reporting/Over-reporting – reporting of “real” vs. “perceived” threats – Media and promotion – Possible “link” not recognized
 Limitations continued • Accuracy and Report Quality – Voluntary vs. Mandatory – Spontaneous Reports Rigorous Prospective Clinical Research – Objective vs. Subjective Data – Translation of layman’s symptom descriptions into complex medical terminology (i. e. paresthesias, numbness, tingling, peripheral neuropathy) – Second hand reporting – Hidden agenda’s
Limitations continued • Accuracy and Report Quality – Voluntary vs. Mandatory – Spontaneous Reports Rigorous Prospective Clinical Research – Objective vs. Subjective Data – Translation of layman’s symptom descriptions into complex medical terminology (i. e. paresthesias, numbness, tingling, peripheral neuropathy) – Second hand reporting – Hidden agenda’s
 Implications for the LSRO Project I. Metabolife Analysis -- Outstanding Issues: Report quality and integrity Ability to verify individual incidents Ability to verify exact product(s) involved Laboratory confirmation Hidden Agenda’s Collection of data in a systematic format Normalizing for product sales(eg. DEET) Independent verification of information Ability to follow-up at this late date
Implications for the LSRO Project I. Metabolife Analysis -- Outstanding Issues: Report quality and integrity Ability to verify individual incidents Ability to verify exact product(s) involved Laboratory confirmation Hidden Agenda’s Collection of data in a systematic format Normalizing for product sales(eg. DEET) Independent verification of information Ability to follow-up at this late date
 Implications for the LSRO Dietary Supplement AER Project II. Advising on a appropriate model of Post-Market Surveillance for Dietary Supplements: • Must differentiate between “ingredients” vs. “products” • For AER/Surveillance…. . No one system will suffice: All likely reporting avenues that patients, consumers, and healthcare professionals will use to request or report information must be cultivated for maximum input • Mandatory vs. Voluntary Reporting for Manufacturers
Implications for the LSRO Dietary Supplement AER Project II. Advising on a appropriate model of Post-Market Surveillance for Dietary Supplements: • Must differentiate between “ingredients” vs. “products” • For AER/Surveillance…. . No one system will suffice: All likely reporting avenues that patients, consumers, and healthcare professionals will use to request or report information must be cultivated for maximum input • Mandatory vs. Voluntary Reporting for Manufacturers


