faa3fd20c95c909540e66c9d4f33ad0f.ppt
- Количество слайдов: 15
 POST MARKET CLINICAL FOLLOW UP (MEDDEV 2. 12 -2 May 2004) Dr. med. Christian Schübel TÜV SÜD Product Service Gmb. H
POST MARKET CLINICAL FOLLOW UP (MEDDEV 2. 12 -2 May 2004) Dr. med. Christian Schübel TÜV SÜD Product Service Gmb. H
 2007/47/EG – Änderungen Klin. Bewertung Historie: CETF – Report (2000) Qualität der klinischen Daten zu schlecht Zu wenige klinische Prüfungen Im Vorgriff auf die Revision der MDD publiziert: • MEDDEV 2. 7. 1. – Clinical Evaluation • MEDDEV 2. 12 -2 – Post Market Clinical Follow Up TÜV SÜD Product Service Gmb. H Abteilung: 3/19/2018 2
2007/47/EG – Änderungen Klin. Bewertung Historie: CETF – Report (2000) Qualität der klinischen Daten zu schlecht Zu wenige klinische Prüfungen Im Vorgriff auf die Revision der MDD publiziert: • MEDDEV 2. 7. 1. – Clinical Evaluation • MEDDEV 2. 12 -2 – Post Market Clinical Follow Up TÜV SÜD Product Service Gmb. H Abteilung: 3/19/2018 2
 PMCF – MEDDEV 2. 12 -2 Post market surveillance may include: § active supervision by customer surveys § inquiries of users and patient § literature reviews § post market clinical follow up TÜV SÜD Product Service Gmb. H
PMCF – MEDDEV 2. 12 -2 Post market surveillance may include: § active supervision by customer surveys § inquiries of users and patient § literature reviews § post market clinical follow up TÜV SÜD Product Service Gmb. H
 2007/47/EG – Änderungen Klin. Bewertung Annex II: 5. 2. The manufacturer must authorize the notified body to carry out all necessary inspections and supply it with all relevant information, in particular § [. . . ] § the data stipulated in the part of the quality system relating to design, such as the results of analyses, calculation test, the solutions adopted in accordance with Annex I section(2), preclinical and clinical evaluation, post-market clinical followup and results of the post-market clinical follow up, if applicable § [. . . ] TÜV SÜD Product Service Gmb. H
2007/47/EG – Änderungen Klin. Bewertung Annex II: 5. 2. The manufacturer must authorize the notified body to carry out all necessary inspections and supply it with all relevant information, in particular § [. . . ] § the data stipulated in the part of the quality system relating to design, such as the results of analyses, calculation test, the solutions adopted in accordance with Annex I section(2), preclinical and clinical evaluation, post-market clinical followup and results of the post-market clinical follow up, if applicable § [. . . ] TÜV SÜD Product Service Gmb. H
 Klinische Daten – Anhang X • Die klinische Bewertung und ihre Dokumentation müssen aktiv anhand der aus der Überwachung nach dem Inverkehrbringen erhaltenen Daten auf dem neuesten Stand gehalten werden • Wird eine klinische Überwachung PMCF – Post Market Clinical Follow UP nach dem Inverkehrbringen […] nicht für erforderlich gehalten, muss dies ordnungsgemäß begründet und dokumentiert werden. MEDDEV 2. 12 -2 TÜV SÜD Product Service Gmb. H Abteilung: 3/19/2018 5
Klinische Daten – Anhang X • Die klinische Bewertung und ihre Dokumentation müssen aktiv anhand der aus der Überwachung nach dem Inverkehrbringen erhaltenen Daten auf dem neuesten Stand gehalten werden • Wird eine klinische Überwachung PMCF – Post Market Clinical Follow UP nach dem Inverkehrbringen […] nicht für erforderlich gehalten, muss dies ordnungsgemäß begründet und dokumentiert werden. MEDDEV 2. 12 -2 TÜV SÜD Product Service Gmb. H Abteilung: 3/19/2018 5
 PMCF – MEDDEV 2. 12 -2 PMCF should always considered for devices where: § In the case the assessment of a product is performed through the concept of equivalence (“literature route” !) § identification of possible emerging risks is critical § the evaluation of long term safety and performance is critical (implants !) TÜV SÜD Product Service Gmb. H
PMCF – MEDDEV 2. 12 -2 PMCF should always considered for devices where: § In the case the assessment of a product is performed through the concept of equivalence (“literature route” !) § identification of possible emerging risks is critical § the evaluation of long term safety and performance is critical (implants !) TÜV SÜD Product Service Gmb. H
 PMCF – MEDDEV 2. 12 -2 Criteria to identify emerging risk: § innovation § severity of the disease § sensitive target population § risky anatomical site § well known risks from literature or marketed devices § obvious discrepancy between the pre-market follow up timescale and the expected life of the product TÜV SÜD Product Service Gmb. H
PMCF – MEDDEV 2. 12 -2 Criteria to identify emerging risk: § innovation § severity of the disease § sensitive target population § risky anatomical site § well known risks from literature or marketed devices § obvious discrepancy between the pre-market follow up timescale and the expected life of the product TÜV SÜD Product Service Gmb. H
 PMCF – MEDDEV 2. 12 -2 All PMCF should be planned: § form of extended follow-up of patients enrolled in the pre-market trials and/or § a prospective study of a representative subset of patients after the device is placed on the market TÜV SÜD Product Service Gmb. H
PMCF – MEDDEV 2. 12 -2 All PMCF should be planned: § form of extended follow-up of patients enrolled in the pre-market trials and/or § a prospective study of a representative subset of patients after the device is placed on the market TÜV SÜD Product Service Gmb. H
 PMCF – MEDDEV 2. 12 -2 This plan will need to take into account: § Results of the clinical investigation including Adverse events identified § Average life expectancy of the device § The claims made by the manufacturer for the device § Performances for which equivalence is claimed § New information becoming available TÜV SÜD Product Service Gmb. H
PMCF – MEDDEV 2. 12 -2 This plan will need to take into account: § Results of the clinical investigation including Adverse events identified § Average life expectancy of the device § The claims made by the manufacturer for the device § Performances for which equivalence is claimed § New information becoming available TÜV SÜD Product Service Gmb. H
 PMCF – MEDDEV 2. 12 -2 No PMCF: § Products for which the medium/long term clinical performance and safety is already known from previous use of the device or § from fully transferable experience with equivalent devices (Products quoted as "equivalent" devices where reference product is subjected to PMCF) TÜV SÜD Product Service Gmb. H
PMCF – MEDDEV 2. 12 -2 No PMCF: § Products for which the medium/long term clinical performance and safety is already known from previous use of the device or § from fully transferable experience with equivalent devices (Products quoted as "equivalent" devices where reference product is subjected to PMCF) TÜV SÜD Product Service Gmb. H
 PMCF – MEDDEV 2. 12 -2 Conclusion § PMCF – Plan § Follow up of a pre-market trial § Post-market prospective trial (cave: national requirements!) § The notified body should review the appropriateness of the plan and the procedures § The follow-up should take into account the average life expectancy § In the case the assessment of a product is performed through the concept of equivalence, PMCF should always be considered. TÜV SÜD Product Service Gmb. H
PMCF – MEDDEV 2. 12 -2 Conclusion § PMCF – Plan § Follow up of a pre-market trial § Post-market prospective trial (cave: national requirements!) § The notified body should review the appropriateness of the plan and the procedures § The follow-up should take into account the average life expectancy § In the case the assessment of a product is performed through the concept of equivalence, PMCF should always be considered. TÜV SÜD Product Service Gmb. H
 Post Market Clinical Follow Up Möglichkeiten eines PMCF: • Fortführung einer klinischen Prüfung (z. B. Herzklappen) • Planung einer neuen klinischen Prüfung, klinischen Studie (Achtung bei zusätzlichen invasiven oder belastenden Untersuchungen - MPG § 23) • Durchführung einer sogenannten Registry TÜV SÜD Product Service Gmb. H Abteilung: 3/19/2018 12
Post Market Clinical Follow Up Möglichkeiten eines PMCF: • Fortführung einer klinischen Prüfung (z. B. Herzklappen) • Planung einer neuen klinischen Prüfung, klinischen Studie (Achtung bei zusätzlichen invasiven oder belastenden Untersuchungen - MPG § 23) • Durchführung einer sogenannten Registry TÜV SÜD Product Service Gmb. H Abteilung: 3/19/2018 12
 PMCF – MEDDEV 2. 12 -2 Ø Requirements for documentation of Post Market Clinical Follow Up (PMCF) activities: NOTE: Results of PMCF and link to risk management are not addressed in MEDDEV 2. 12 -2 TÜV SÜD Product Service Gmb. H
PMCF – MEDDEV 2. 12 -2 Ø Requirements for documentation of Post Market Clinical Follow Up (PMCF) activities: NOTE: Results of PMCF and link to risk management are not addressed in MEDDEV 2. 12 -2 TÜV SÜD Product Service Gmb. H
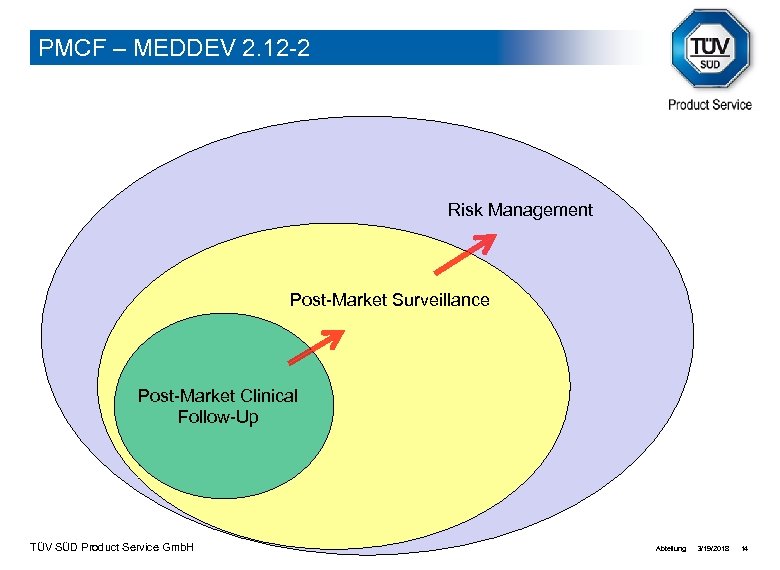 PMCF – MEDDEV 2. 12 -2 Risk Management Post-Market Surveillance Post-Market Clinical Follow-Up TÜV SÜD Product Service Gmb. H Abteilung: 3/19/2018 14
PMCF – MEDDEV 2. 12 -2 Risk Management Post-Market Surveillance Post-Market Clinical Follow-Up TÜV SÜD Product Service Gmb. H Abteilung: 3/19/2018 14
 PMCF – MEDDEV 2. 12 -2 Content of an integrated Risk Management File: NOTE: Timeframes for Risk Management File update (addressed in RM SOP / RM Plan) should be in compliance with PMCF plan and design of clinical follow up studies TÜV SÜD Product Service Gmb. H
PMCF – MEDDEV 2. 12 -2 Content of an integrated Risk Management File: NOTE: Timeframes for Risk Management File update (addressed in RM SOP / RM Plan) should be in compliance with PMCF plan and design of clinical follow up studies TÜV SÜD Product Service Gmb. H


