3925c396cbcced5d89eb5971fb26cee1.ppt
- Количество слайдов: 46

Post-Exposure Prophylaxis Antonio Urbina, M. D. Associate Medical Director Center for Comprehensive Care, West Village Division St. Luke’s Roosevelt Hospital

24/7 NYS PEP Line 888 -448 -4911

NYS PEP/AIDS Widget To Download Widget visit www. ceitraining. org

Overview n HIV Post-Exposure Prophylaxis (PEP) n o. PEP (occupational PEP) n n. PEP (non-occupational PEP) n Background n Guidelines n Hepatitis B and C post-exposure protocols

NON-OCCUPATIONAL PEP (n. PEP)

n. PEP n n n Buy in from institution and staffs Establish protocol and policies Have first dose available to patient immediately Educate Staff on follow up Include Mental Health and SW
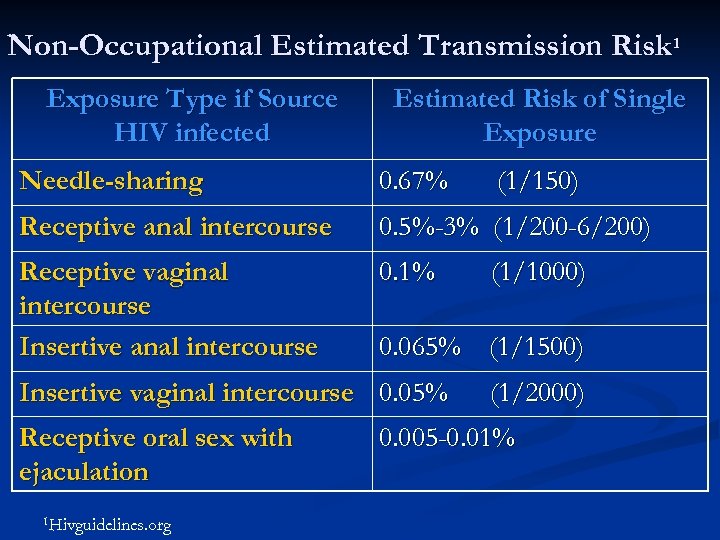
Non-Occupational Estimated Transmission Risk 1 Exposure Type if Source HIV infected Estimated Risk of Single Exposure Needle-sharing 0. 67% Receptive anal intercourse 0. 5%-3% (1/200 -6/200) Receptive vaginal intercourse Insertive anal intercourse 0. 1% 1 Hivguidelines. org (1/1000) 0. 065% (1/1500) Insertive vaginal intercourse 0. 05% Receptive oral sex with ejaculation (1/150) (1/2000) 0. 005 -0. 01%
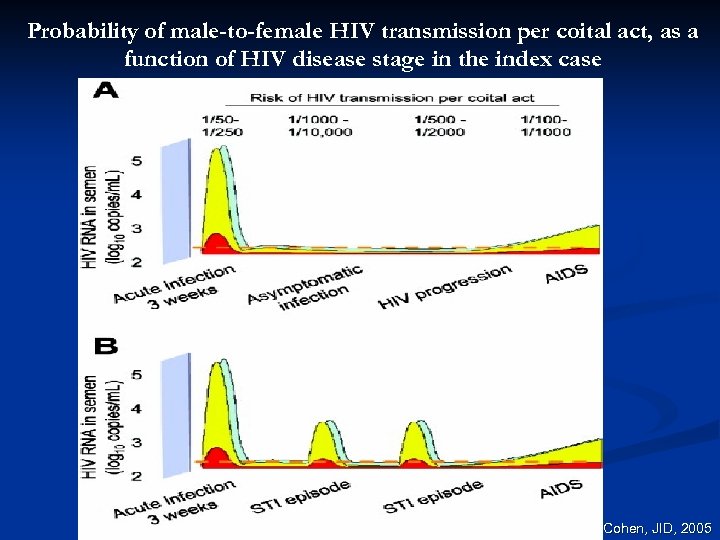
Probability of male-to-female HIV transmission per coital act, as a function of HIV disease stage in the index case Cohen, JID, 2005
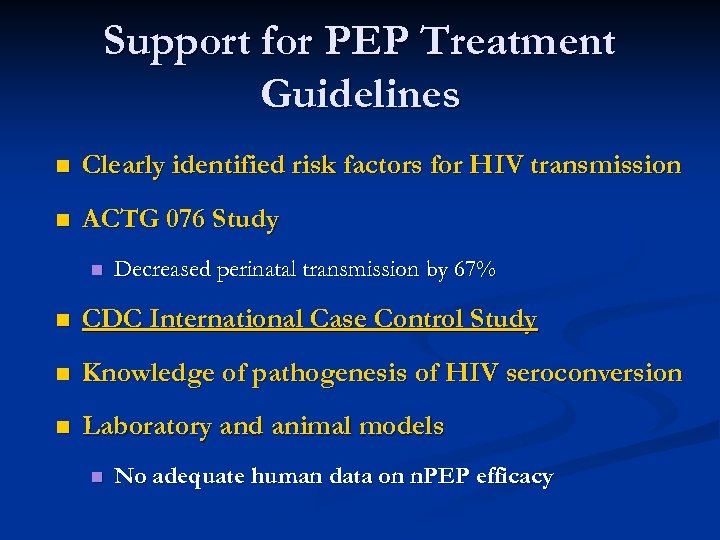
Support for PEP Treatment Guidelines n Clearly identified risk factors for HIV transmission n ACTG 076 Study n Decreased perinatal transmission by 67% n CDC International Case Control Study n Knowledge of pathogenesis of HIV seroconversion n Laboratory and animal models n No adequate human data on n. PEP efficacy
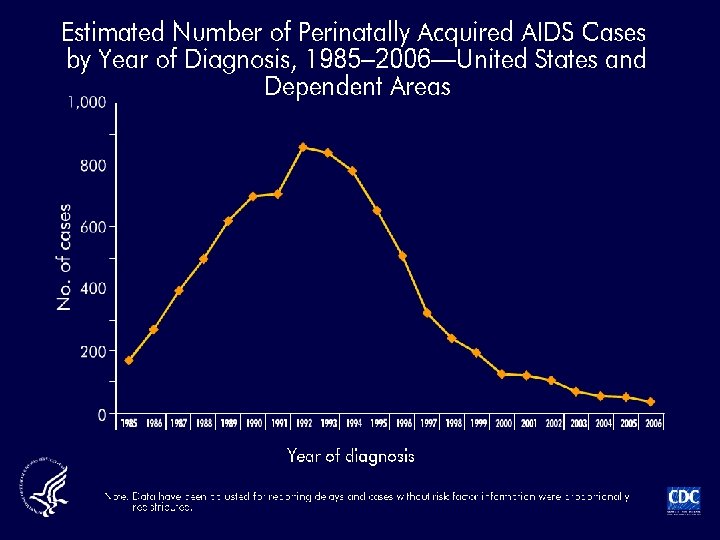
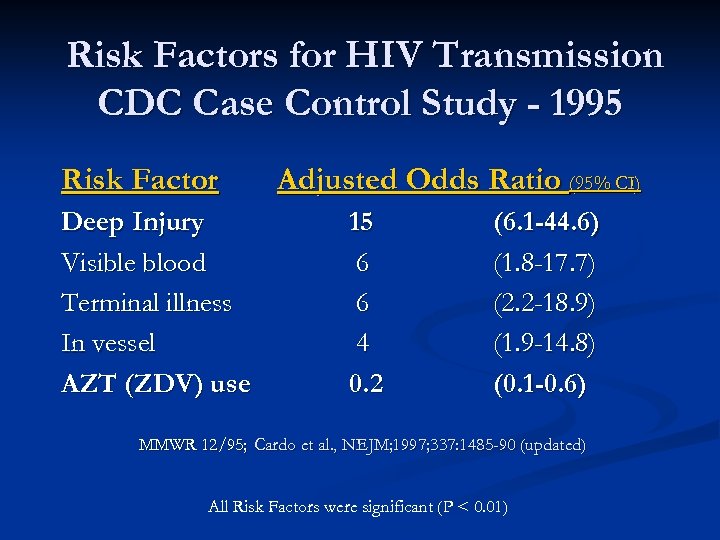
Risk Factors for HIV Transmission CDC Case Control Study - 1995 Risk Factor Deep Injury Visible blood Terminal illness In vessel AZT (ZDV) use Adjusted Odds Ratio (95% CI) 15 6 6 4 0. 2 (6. 1 -44. 6) (1. 8 -17. 7) (2. 2 -18. 9) (1. 9 -14. 8) (0. 1 -0. 6) MMWR 12/95; Cardo et al. , NEJM; 1997; 337: 1485 -90 (updated) All Risk Factors were significant (P < 0. 01)

NYS DOH Guidelines n o. PEP (occupational) n n January 2008 Updated guidelines 2009: n n January 2008 n. PEP (non-occupational) n CDC Guidelines o. PEP (occupational) n n MMWR, 54 (RR-9), 9/30/05 n. PEP (non-occupational) n MMWR, 54 (RR-2), 1/21/05 Medical Care Criteria Committee of AIDS Institute n www. cdc. gov/mmwr www. hivguidelines. org

Key Elements of Post-Exposure Prophylaxis (PEP) Programs n Medical knowledge Indications n Regimens n Follow-up n n Programmatic readiness Awareness of need n Timely availability of medical evaluation and PEP agents n Availability of follow-up n Confidentiality and documentation n

Key Elements 1. Assess Risk 2. Manage Exposure 3. Determine HIV Status of source, when possible 4. Dispense PEP if indicated 5. Educate and Counsel Exposed Patient 6. Documentation

1. Assess Risk n Percutaneous injury n Contact of mucous membrane n Contact non-intact skin
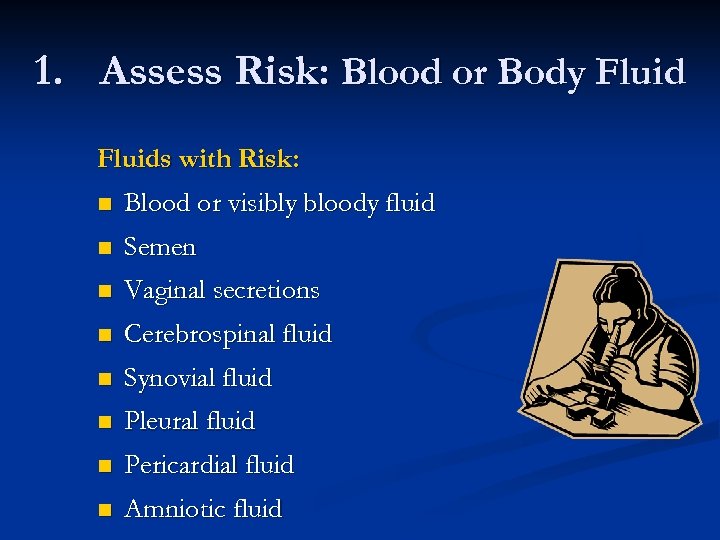
1. Assess Risk: Blood or Body Fluids with Risk: n Blood or visibly bloody fluid n Semen n Vaginal secretions n Cerebrospinal fluid n Synovial fluid n Pleural fluid n Pericardial fluid n Amniotic fluid

Community Needlestick Injuries n Consider: n n n HIV prevalence in the community or facility Surrounding prevalence of injection drug use Do not test discarded needles for HIV n False negatives n Risk

1. Assess Risk, cont. Non-risky Fluids* n Saliva, sputum or nasal secretions n Tears n Sweat n Urine n Stool n Emesis *Unless there is visible blood
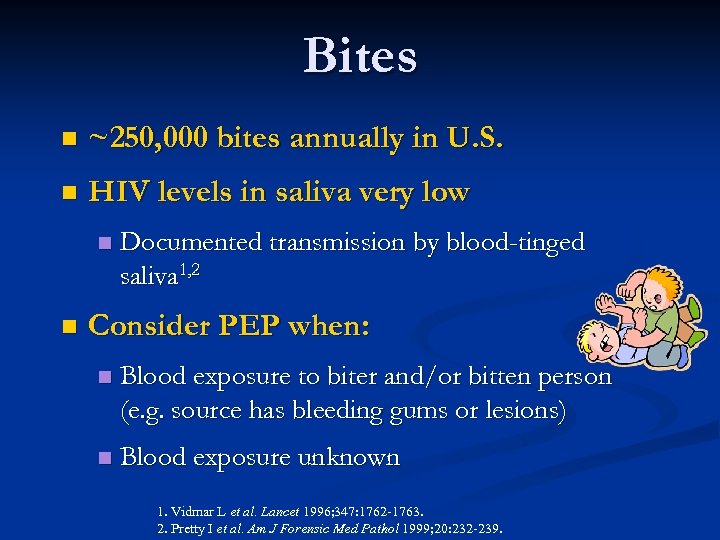
Bites n ~250, 000 bites annually in U. S. n HIV levels in saliva very low n n Documented transmission by blood-tinged saliva 1, 2 Consider PEP when: n Blood exposure to biter and/or bitten person (e. g. source has bleeding gums or lesions) n Blood exposure unknown 1. Vidmar L et al. Lancet 1996; 347: 1762 -1763. 2. Pretty I et al. Am J Forensic Med Pathol 1999; 20: 232 -239.
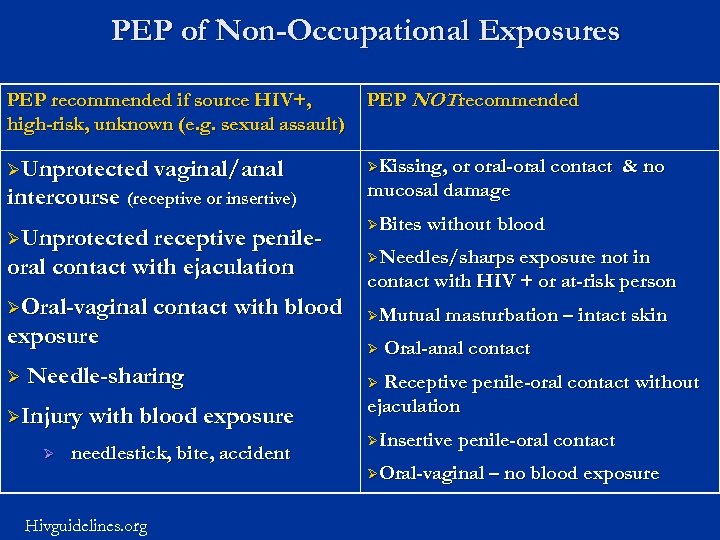
PEP of Non-Occupational Exposures PEP recommended if source HIV+, high-risk, unknown (e. g. sexual assault) PEP NOT recommended ØUnprotected vaginal/anal ØKissing, or oral-oral contact intercourse (receptive or insertive) ØUnprotected receptive penile- oral contact with ejaculation ØOral-vaginal contact with blood exposure Ø Needle-sharing ØInjury with blood exposure Ø needlestick, bite, accident Hivguidelines. org & no mucosal damage ØBites without blood ØNeedles/sharps exposure not in contact with HIV + or at-risk person ØMutual masturbation – intact skin Ø Oral-anal contact Receptive penile-oral contact without ejaculation Ø ØInsertive penile-oral contact ØOral-vaginal – no blood exposure

2. Exposure Management n Wash immediately w/ soap and water n Flush mucous membranes with water n No evidence that use of antiseptics or expressing fluid reduces potential transmission n Antiseptics not indicated; caustic agents (bleach) not recommended n “Milking the wound” may increase risk n increases local inflammation

3. Source HIV Status n Source HIV status unknown n Voluntary HIV testing Document if source unwilling or unable to consent Rapid HIV testing +/- HIV RNA viral load test recommended n n n OSHA regulations require rapid testing for occupational exposures HIV RNA if recent potential HIV exposure to source Rapid test result positive n Give result to source; confirm by HIV Western blot
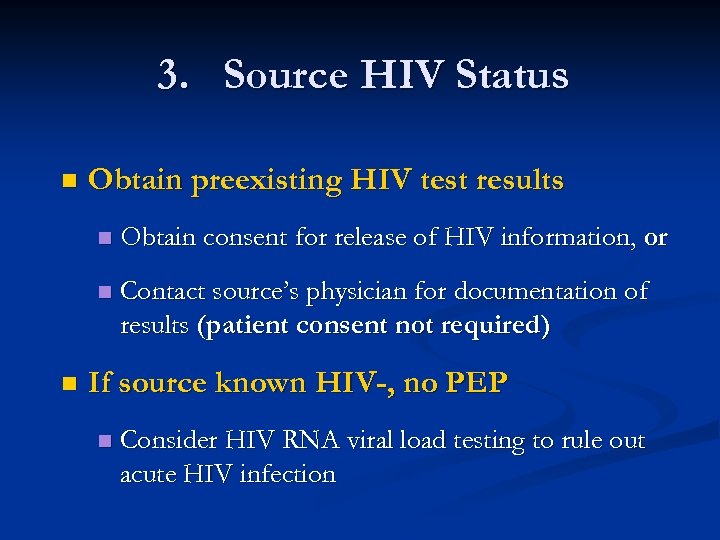
3. Source HIV Status n Obtain preexisting HIV test results n n n Obtain consent for release of HIV information, or Contact source’s physician for documentation of results (patient consent not required) If source known HIV-, no PEP n Consider HIV RNA viral load testing to rule out acute HIV infection

3. Source HIV Status n Known HIV Infection n Stage of infection (early, late or end stage) n CD 4, viral load testing, resistance testing n Current and previous antiretroviral therapies n Consider involvement of HIV Specialist n Don’t delay PEP while waiting for results

Risk Behavior n PEP recommended n n Lapse in risk-reduction practices n n Isolated exposure (sexual, needle, trauma) Interest in behavioral change Repeated high-risk behavior or presentation for repeat courses of PEP n Intensify education & prevention n Behavioral change

4. PEP Recommendations NYS DOH Guidelines n PEP ASAP, ideally within 2 hrs, no later than 36 hrs from exposure n HAART (3 antiretroviral drugs) x 4 weeks n Baseline HIV serology of exposed person within 72 hours of initiating PEP n HIV specialist follow-up within 72 hours

4. PEP Recommendations Beyond 36 Hours? “Decisions regarding initiation of n. PEP beyond 36 hours post exposure should be made by the clinician in conjunction with the patient with the realization of diminished potential for success when timing of initiation is prolonged” 1 n Consider likelihood of HIV transmission n http: //hivguidelines. org/Guide. Line. aspx? page. ID=78&guide. Line. ID=2

4. PEP Recommendations Selection of HIV PEP Regimen n PEP regimen usually empiric n If source known HIV+: n consider HIV resistance n genotype/phenotype/past antiretroviral drug history
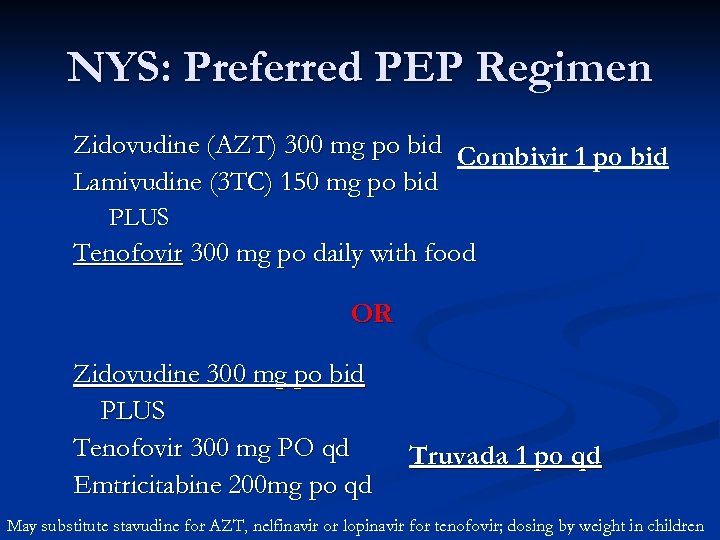
NYS: Preferred PEP Regimen Zidovudine (AZT) 300 mg po bid Combivir 1 po bid Lamivudine (3 TC) 150 mg po bid PLUS Tenofovir 300 mg po daily with food OR Zidovudine 300 mg po bid PLUS Tenofovir 300 mg PO qd Emtricitabine 200 mg po qd Truvada 1 po qd May substitute stavudine for AZT, nelfinavir or lopinavir for tenofovir; dosing by weight in children
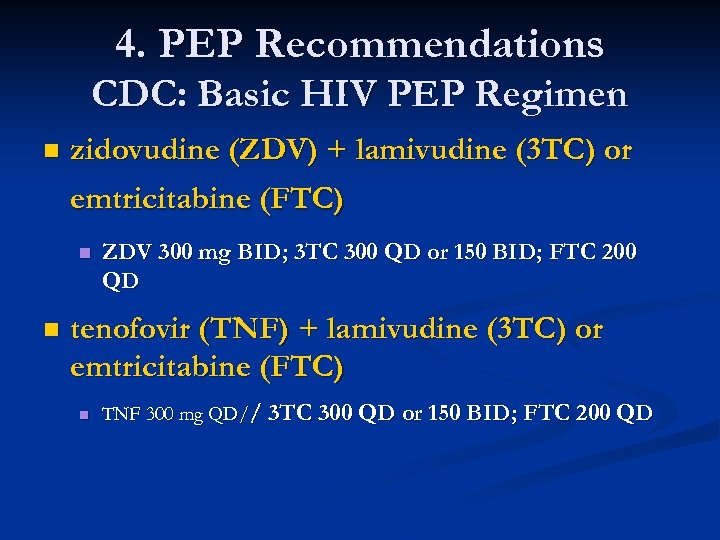
4. PEP Recommendations CDC: Basic HIV PEP Regimen n zidovudine (ZDV) + lamivudine (3 TC) or emtricitabine (FTC) n n ZDV 300 mg BID; 3 TC 300 QD or 150 BID; FTC 200 QD tenofovir (TNF) + lamivudine (3 TC) or emtricitabine (FTC) n TNF 300 mg QD// 3 TC 300 QD or 150 BID; FTC 200 QD

4. PEP Recommendations CDC: Preferred Expanded PEP Regimen n Basic regimen plus n lopinavir/ritonavir (LPV/RTV) (or efavirenz [n. PEP] n LPV/RTV: 400/100 mg = 2 tablets twice daily with food

5. Education and Counseling Explain to patient: n Potential exposure risk n Risks and benefits of PEP n To report signs and symptoms of acute (primary) HIV infection n Prevention of secondary transmissions n Acknowledge fear/anxiety commonly encountered by exposed health care workers and offer counseling services
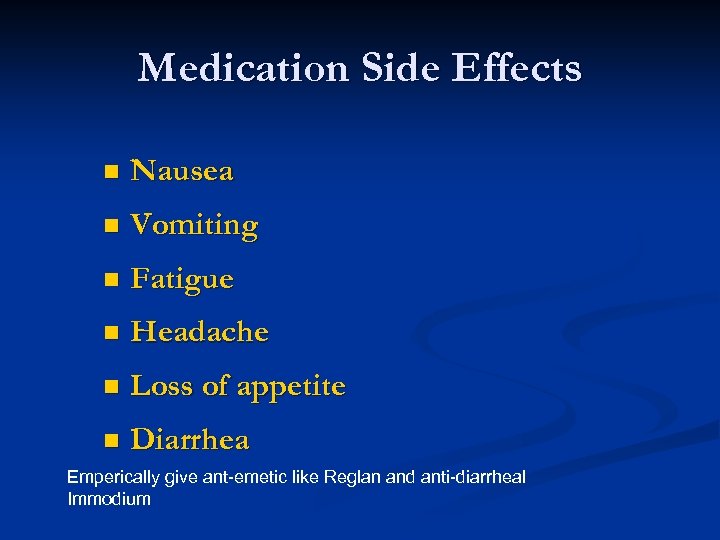
Medication Side Effects n Nausea n Vomiting n Fatigue n Headache n Loss of appetite n Diarrhea Emperically give ant-emetic like Reglan and anti-diarrheal Immodium

5. More Education and Counseling n What prescribed person needs to know: n Knowledge about efficacy of PEP is limited n ZDV best shown to prevent HIV transmission in humans n No data on combination therapy, but experts recommend multiple drugs to increase potency and overcome potential drug-resistant virus n Any or all drugs for PEP may be declined by the HCW
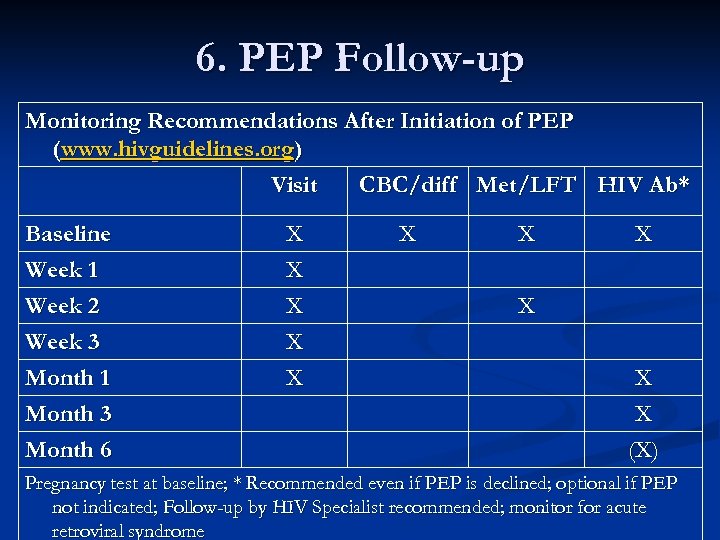
6. PEP Follow-up Monitoring Recommendations After Initiation of PEP (www. hivguidelines. org) Visit CBC/diff Met/LFT HIV Ab* Baseline Week 1 Week 2 Week 3 Month 1 Month 3 Month 6 X X X X X X X (X) Pregnancy test at baseline; * Recommended even if PEP is declined; optional if PEP not indicated; Follow-up by HIV Specialist recommended; monitor for acute retroviral syndrome

Longitudinal Care n Recommend barrier protection for 3 -6 months, while monitoring for PEP is ongoing n Avoid breastfeeding for 3 -6 months n Women preferring to breastfeed between 3 -6 months should carefully weigh risks/benefits
Failure of Post-Exposure Prophylaxis n Failure documented with zidovudine (AZT; Retrovir) monotherapy and with combination therapies n Potential explanations: n Viral resistance n Large inoculum n Delay in PEP n Lack of adherence to PEP n Infection at a time other than the known potential exposure AIDS 2001; 1593: 430, Arch Int Med 1999; 159: 2361 -3
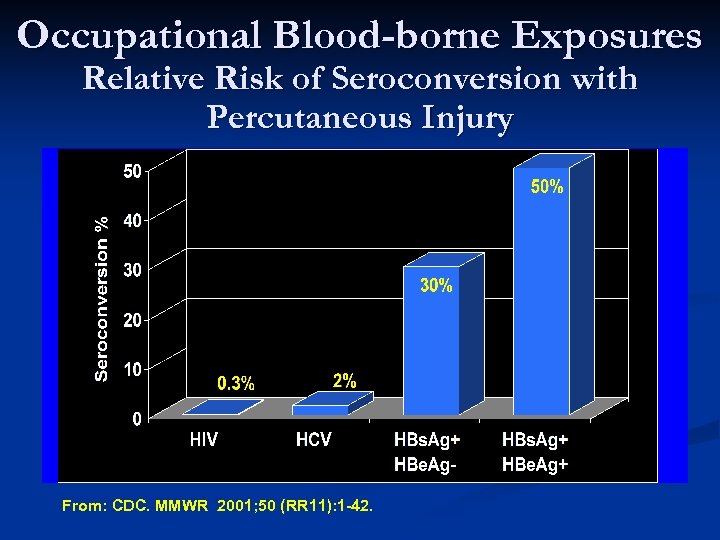
Occupational Blood-borne Exposures Relative Risk of Seroconversion with Percutaneous Injury . From: CDC. MMWR 2001; 50 (RR 11): 1 -42.

Hepatitis B n Management depends on: n Source hepatitis B surface antigen status n Whether exposed person vaccinated n Whether exposed person has immunity
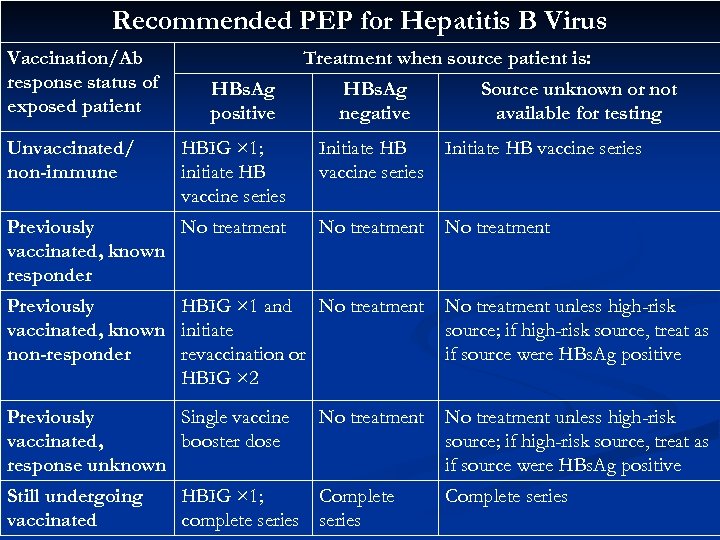
Recommended PEP for Hepatitis B Virus Vaccination/Ab response status of exposed patient Unvaccinated/ non-immune Treatment when source patient is: HBs. Ag positive HBs. Ag negative Source unknown or not available for testing HBIG × 1; initiate HB vaccine series Initiate HB vaccine series Previously No treatment vaccinated, known responder No treatment Previously HBIG × 1 and No treatment vaccinated, known initiate non-responder revaccination or HBIG × 2 No treatment unless high-risk source; if high-risk source, treat as if source were HBs. Ag positive Previously Single vaccinated, booster dose response unknown No treatment unless high-risk source; if high-risk source, treat as if source were HBs. Ag positive Still undergoing vaccinated No treatment HBIG × 1; Complete complete series Complete series

Hepatitis C n No vaccine or treatment will prevent infection n n Immune globulin not recommended; does not work Early infection effectively treated with Peg-interferon +/- ribavirin
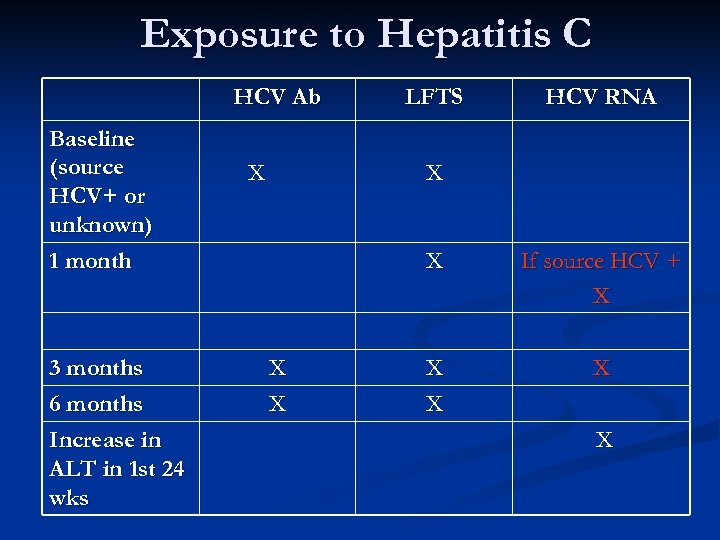
Exposure to Hepatitis C HCV Ab Baseline (source HCV+ or unknown) 1 month 3 months 6 months Increase in ALT in 1 st 24 wks X LFTS HCV RNA X X If source HCV + X X X

n. PEP Case n n JW, 21 year old WM presents to CCC, West Village on 7/15/10 for PEP. Reports going online and finding about PEP through www. pep 411. com Unprotected receptive anal sex with multiple partners the prior night Last tested HIV -, 2 weeks ago in St. Louis, MO First dose of PEP given in clinic within 32 hours of exposure

n. PEP Case n History of poly-substance abuse starting at age 16 n n Cocaine, crystal meth (now reports IV use for last 4 -6 months) SW visit n n Unemployed, moved to NYC to get away from drug scene Referred to drug treatment programs (Realization Center, Andres Hoyas at Center) n Assisted in applying for Medicaid, food stamps and Waverly Job Center
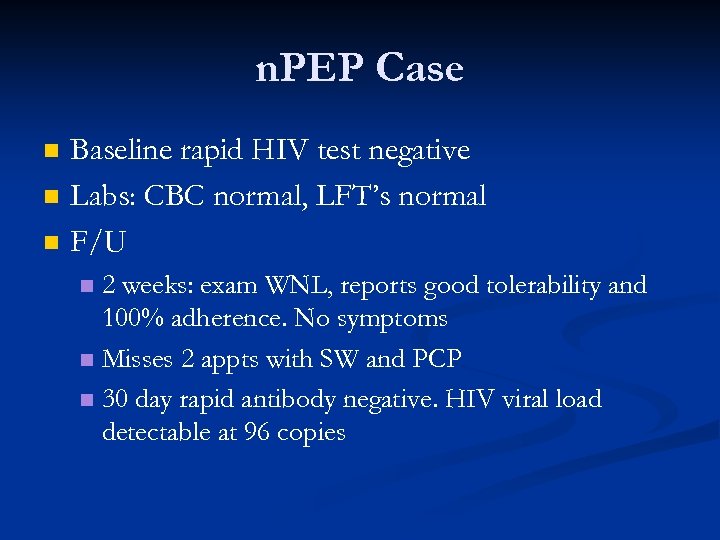
n. PEP Case n n n Baseline rapid HIV test negative Labs: CBC normal, LFT’s normal F/U 2 weeks: exam WNL, reports good tolerability and 100% adherence. No symptoms n Misses 2 appts with SW and PCP n 30 day rapid antibody negative. HIV viral load detectable at 96 copies n

n. PEP Case n n PE reveals exudative pharyngitis (L tonsil) Repeat Labs are Drawn: DNA PCR: 690 copies n HIV Elisa and WB are positive n
3925c396cbcced5d89eb5971fb26cee1.ppt