dd15fa57f21fc21dc1ce20dbb6ff6230.ppt
- Количество слайдов: 44

PORTABLE MONITORING FOR THE DIAGNOSIS OF OBSTRUCTIVE SLEEP APNEA ADNAN ABBASI, MBBS, MPH

• Conflict of interest • None • Disclosure • None

OBJECTIVES • Understand the need of Portable Monitoring (PM) and Home Sleep Testing (HST) in evaluation of obstructive sleep apnea (OSA) in current practice model • Review technology for PM for diagnosis of OSA • Review algorithmic approach to diagnosis and management of OSA using PM and auto-PAP • Identify patients in whom HST can be effective

EPIDEMIOLOGY OF SLEEP-DISORDERED BREATHING • Prevalence of OSA (defined as an apnea-hypopnea index >5 events per hour) 33. 9% in men and 17. 4% in women • OSA syndrome (OSA + excessive daytime sleepiness) is 14. 3% in men and 5% in women • Increasing rates of obesity Peppard PE. Am J Epidemiol. 2013 May 1; 177(9): 1006 -14 Young T. N Engl J Med. 1993 Apr 29; 328(17): 1230 -5 Al Lawati NM. Prog Cardiovasc Dis. 2009 Jan-Feb; 51(4): 285 -93

CONSEQUENCES OF UNTREATED SLEEP APNEA • Excessive daytime sleepiness • Decreased workplace productivity • Car crashes • Systemic hypertension • Cardiovascular disease • Increased mortality Mulgrew AT. Sleep Med. 2007 Dec; 9(1): 42 -53 Marin JM. Lancet 2007; 365: 1046 -1053 Peppard PE. NEJM 2000; 342: 1378 -1384 Barbe F. Am Jr Respir Crit Care Med. 1998; 158: 18 -22

DIAGNOSIS OF SLEEP APNEA • Historically, in-laboratory attended polysomnography (PSG) has been considered as the preferred method • • High cost Long wait times
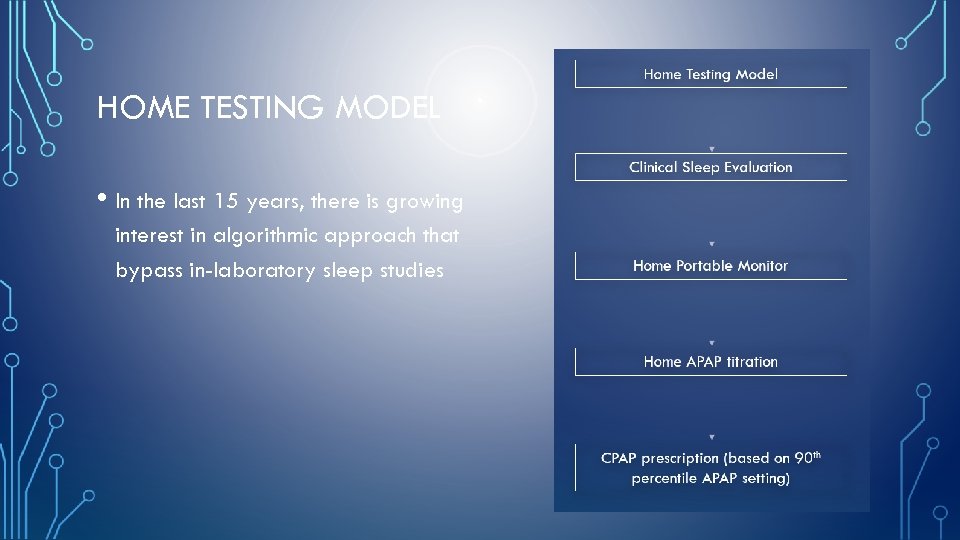
HOME TESTING MODEL • In the last 15 years, there is growing interest in algorithmic approach that bypass in-laboratory sleep studies

TERMINOLOGY FOR TECHNOLOGY USED TO DIAGNOSE SLEEP APNEA • Level 1: In-laboratory attended – EEG, chin EMG, ECG, measure of airflow, respiratory effort, oxygen saturation • Level 2: • Level 3: Same as level 1, but performed outside the sleep laboratory minimum of 4 parameters including oxygen saturation, measurement of heart rate or ECG, and at least two channels assessing respiration (e. g. , respiratory movement and airflow) • Level 4: one or two channels – nocturnal oximetry or nasal airflow alone Ferber R. Sleep. 1994; 17(4): 378 -392

REVISED CLASSIFICATION SYSTEM FOR PM- SCOPER • S: Sleep • C: Cardiac measure • O: Oximetry • P: Position • E: Effort • R: Respiration Collop NA. J Clin Sleep Med 2011; 7(5): 531 -548

IN-LABORATORY PSG VS. HST • Most PM devices lack ability to differentiate awake from sleep state • Total recording time is used as denominator for calculating AHI (Respiratory Event Index) • PM underestimates severity of OSA • Several PM are able to estimate total sleep time • • • Limited EEG 1 or 2 leads Lack of head movement Actigraphy J Clin Sleep Med 2007; 3(7): 737 -747

J Clin Sleep Med 2007; 3(7): 737 -747

INDICATIONS FOR PORTABLE MONITORING • PM for diagnosis of OSA should be performed only in conjunction with a comprehensive sleep evaluation. • Clinical sleep evaluations using PM must be supervised by a Board Certified (or Board eligible) Sleep Specialist. • In the absence of a comprehensive sleep evaluation, there is no indication for the use of PM. J Clin Sleep Med 2007; 3(7): 737 -747

INDICATIONS FOR PORTABLE MONITORING • PM may be used as an alternative to PSG for diagnosis of OSA in patients with a high pre-test probability of moderate to severe OSA. J Clin Sleep Med 2007; 3(7): 737 -747

PM IS NOT APPROPRIATE FOR THE DIAGNOSIS OF OSA • Patients with significant comorbid medical such as moderate to severe pulmonary disease, neuromuscular disease, or congestive heart failure. • Patients suspected of having other sleep disorders, including central sleep apnea, periodic limb movement disorder (PLMD), insomnia, parasomnias, circadian rhythm disorders, or narcolepsy. • For general screening of asymptomatic populations. J Clin Sleep Med 2007; 3(7): 737 -747

PM MAY BE INDICATED • PM may be indicated for the diagnosis of OSA in patients for whom in-laboratory PSG is not possible by virtue of immobility, safety, or critical illness. • PM may be indicated to monitor the response of non-CPAP treatments for OSA, including oral appliances, upper airway surgery, and weight loss. J Clin Sleep Med 2007; 3(7): 737 -747
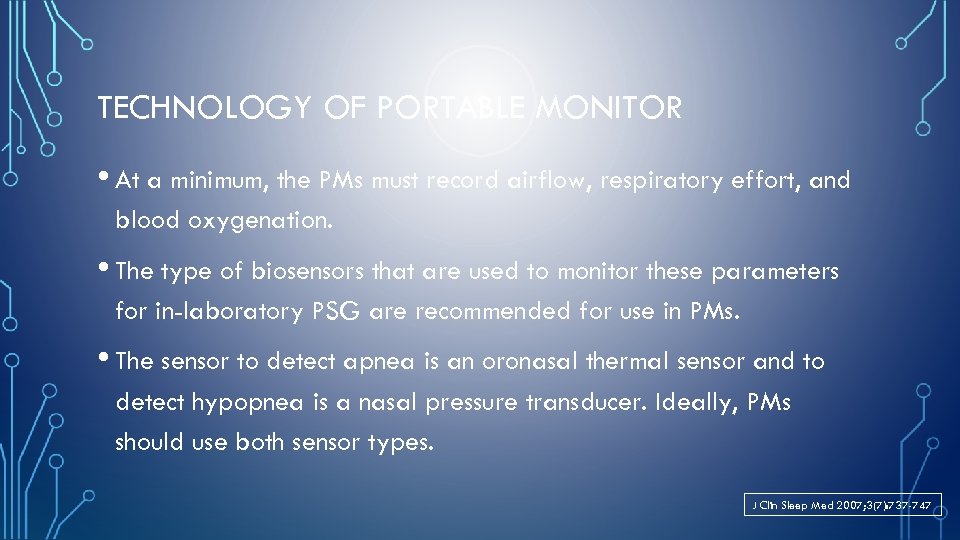
TECHNOLOGY OF PORTABLE MONITOR • At a minimum, the PMs must record airflow, respiratory effort, and blood oxygenation. • The type of biosensors that are used to monitor these parameters for in-laboratory PSG are recommended for use in PMs. • The sensor to detect apnea is an oronasal thermal sensor and to detect hypopnea is a nasal pressure transducer. Ideally, PMs should use both sensor types. J Clin Sleep Med 2007; 3(7): 737 -747
TECHNOLOGY OF PORTABLE MONITOR • Ideally the sensor for identification of respiratory effort is either calibrated or uncalibrated inductance plethesmography. • The sensor for detection of blood oxygen is pulse oximetry with appropriate signal averaging time and accommodation for motion artifact. J Clin Sleep Med 2007; 3(7): 737 -747

METHODOLOGY FOR PORTABLE MONITORING • Under the auspices of an AASM accredited comprehensive Sleep Medicine Program with policies and procedures for sensor application, scoring, and interpretation of PM. • An experienced sleep technician, sleep technologist, or appropriately trained healthcare practitioner must perform the application of PM or directly educate the patient in the correct application of sensors. J Clin Sleep Med 2007; 3(7): 737 -747

METHODOLOGY FOR PORTABLE MONITORING • PM devices must allow for the display of raw data for manual scoring or editing of automated scoring by a trained and qualified sleep technician/technologist. • Evaluation of PM data must include review of the raw data by a Board Certified (or Board eligible) Sleep Specialist. • Scoring criteria should be consistent with the current published AASM standards for scoring of apneas and hypopneas. J Clin Sleep Med 2007; 3(7): 737 -747

METHODOLOGY FOR PORTABLE MONITORING • Due to the known rate of false negative PM tests, in-laboratory PSG should be performed in cases where PM is technically inadequate or fails to establish the diagnosis of OSA in patients with a high pre-test probability. • A follow-up visit with a physician or other appropriately trained and supervised health provider should be performed on all patients undergoing PM to discuss the results of the test. J Clin Sleep Med 2007; 3(7): 737 -747
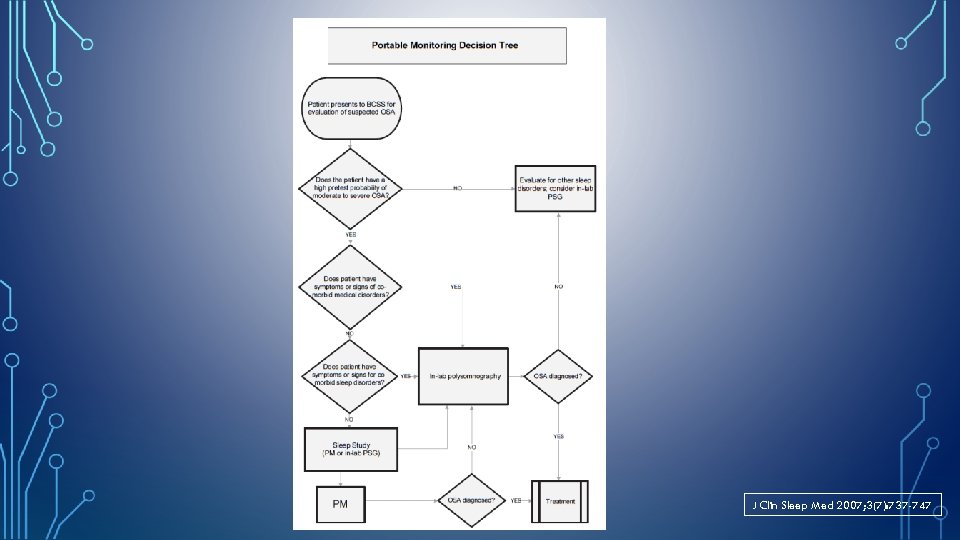
J Clin Sleep Med 2007; 3(7): 737 -747

CMS DECISION MEMO FOR SLEEP TESTING FOR OSA • In 2009 CMS approved coverage for PAP for patients diagnosed with sleep disordered breathing using PM • Devices covered for diagnosis of OSA: • • Type I sleep testing device -if performed attended in a sleep lab facility Type II or a Type III sleep testing device if performed unattended in or out of a sleep lab facility or attended in a sleep lab facility https: //www. cms. gov/medicare-coverage-database/overview-and-quicksearch. aspx? Friendly. Error=Invalid. NCAID Document ID: CAG-00405 N

CMS DECISION MEMO FOR SLEEP TESTING FOR OSA • A Type IV sleep testing device measuring three or more channels, one of which is airflow if performed unattended in or out of a sleep lab facility or attended in a sleep lab facility. • A sleep testing device measuring three or more channels that include actigraphy, oximetry, and peripheral arterial tone is covered if performed unattended in or out of a sleep lab facility or attended in a sleep lab facility. https: //www. cms. gov/medicare-coverage-database/overview-and-quicksearch. aspx? Friendly. Error=Invalid. NCAID Document ID: CAG-00405 N

PRE-TEST CLINICAL ASSESSMENT OF OSA • History and physical examination • Assessment of excessive daytime sleepiness- Epworth Sleepiness Scale • Review of overnight oximetry • Sleep Questionnaires • • • STOP-BANG Questionnaire Berlin Questionnaire Wisconsin Questionnaire

STOP-BANG QUESTIONNAIRE • • S: Snoring T: Tired O: Observed P: Pressure (Hypertension) B: Body Mass Index > 35 Kg/M 2 Low Risk : Yes to 0 - 2 questions Intermediate Risk : Yes to 3 - 4 questions High Risk : Yes to 5 - 8 questions or Yes to 2 or more of 4 STOP questions + male gender or Yes to 2 or more of 4 STOP questions + BMI > 35 kg/m 2 or Yes to 2 or more of 4 STOP questions + neck circumference > 17 inches in M or 16 inches in F A: Age > 50 years N: Neck circumference >17” in M >16” in F G: Gender= Male http: //www. stopbang. ca/osa/screening. php
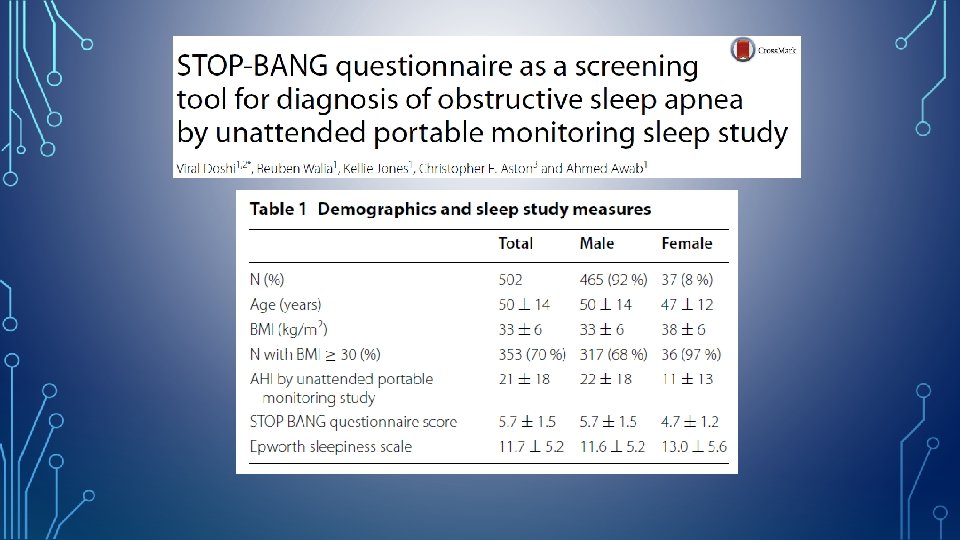
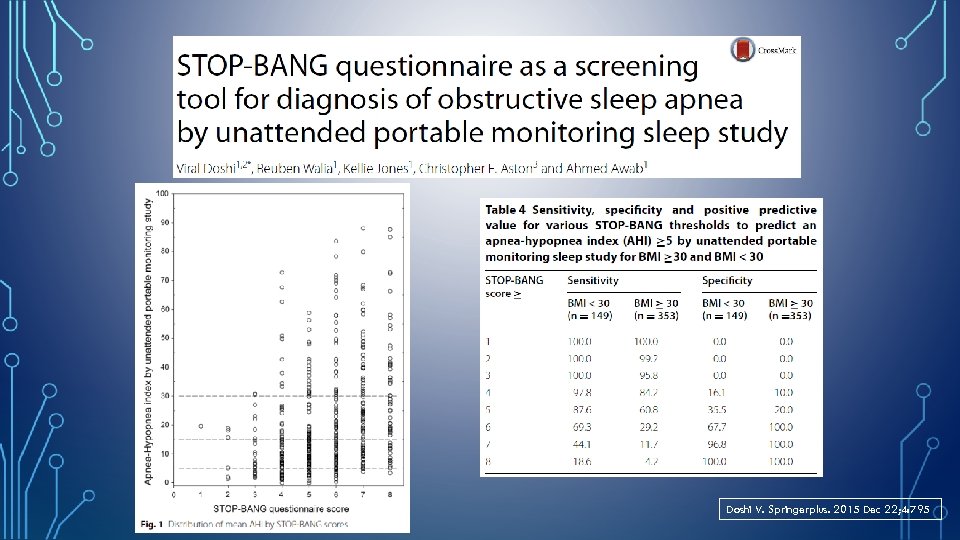
Doshi V. Springerplus. 2015 Dec 22; 4: 795
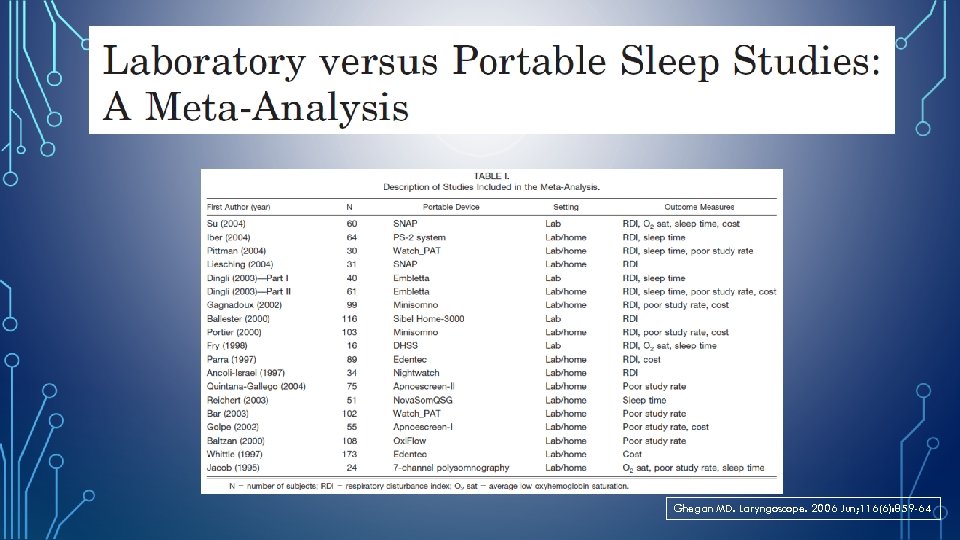
Ghegan MD. Laryngoscope. 2006 Jun; 116(6): 859 -64
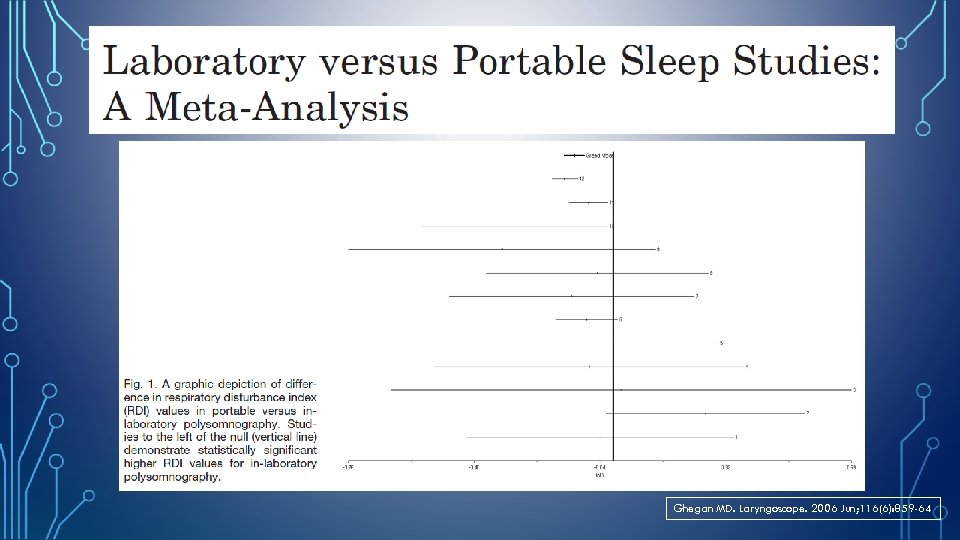
Ghegan MD. Laryngoscope. 2006 Jun; 116(6): 859 -64
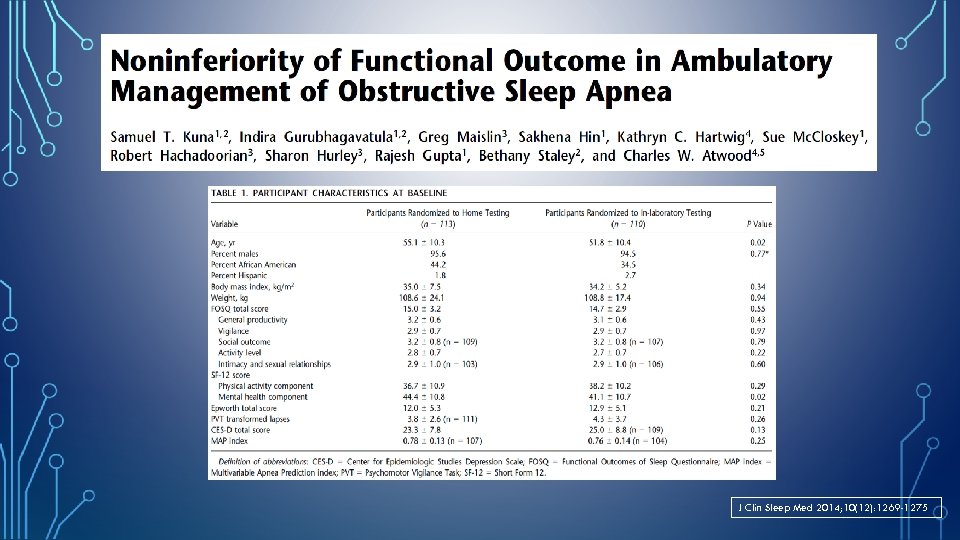
J Clin Sleep Med 2014; 10(12): 1269 -1275
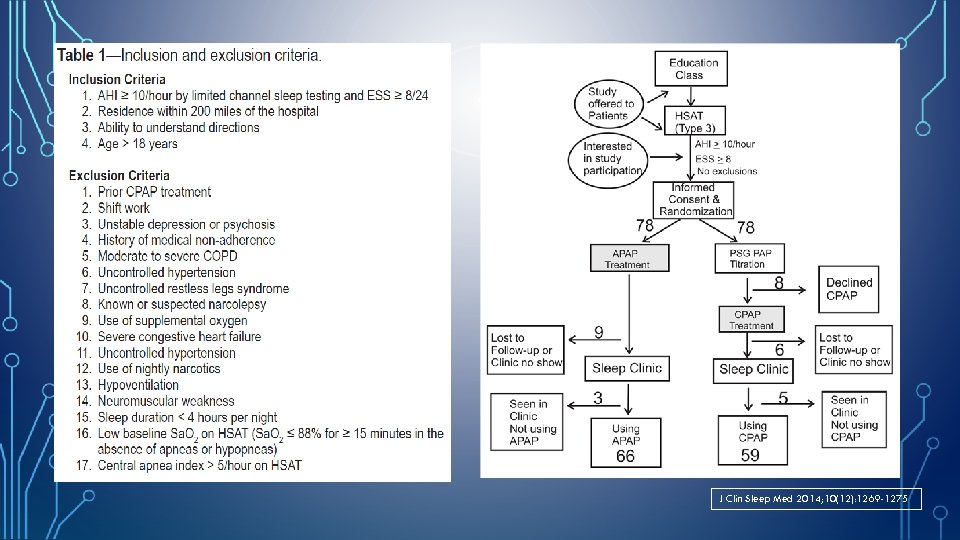
J Clin Sleep Med 2014; 10(12): 1269 -1275
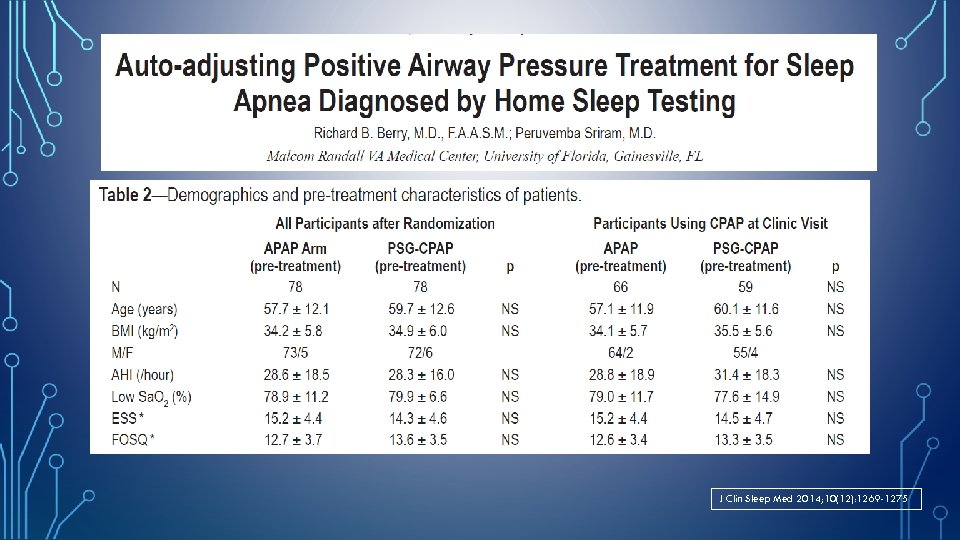
J Clin Sleep Med 2014; 10(12): 1269 -1275
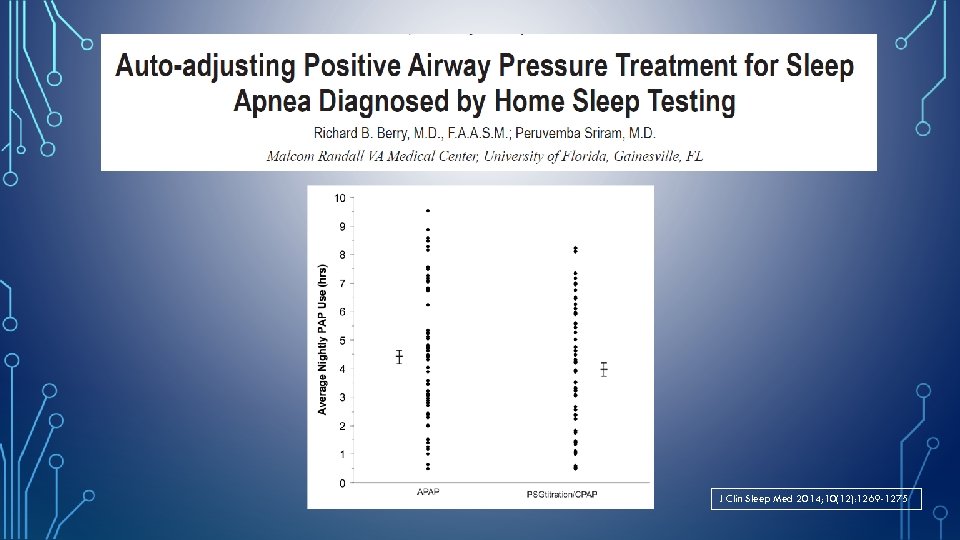
J Clin Sleep Med 2014; 10(12): 1269 -1275
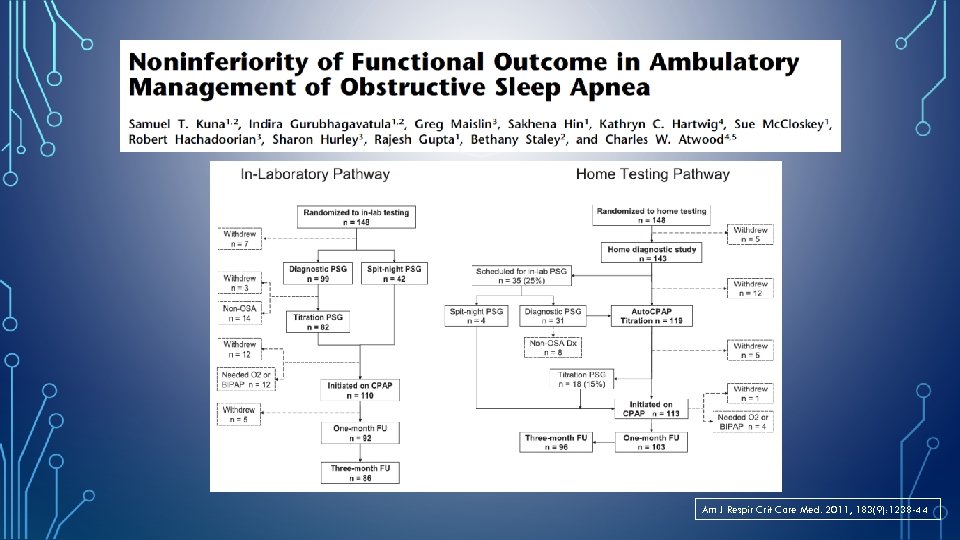
Am J Respir Crit Care Med. 2011, 183(9): 1238 -44
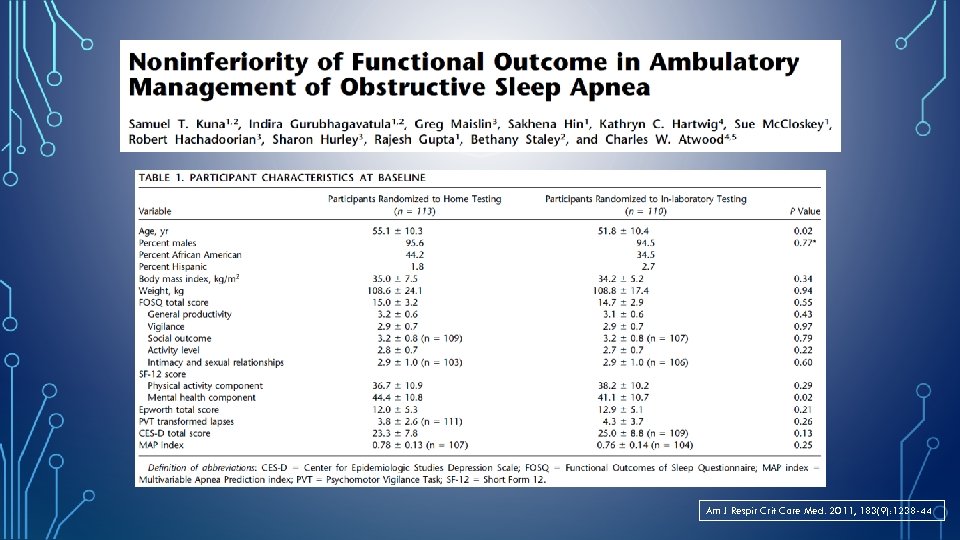
Am J Respir Crit Care Med. 2011, 183(9): 1238 -44

Am J Respir Crit Care Med. 2011, 183(9): 1238 -44
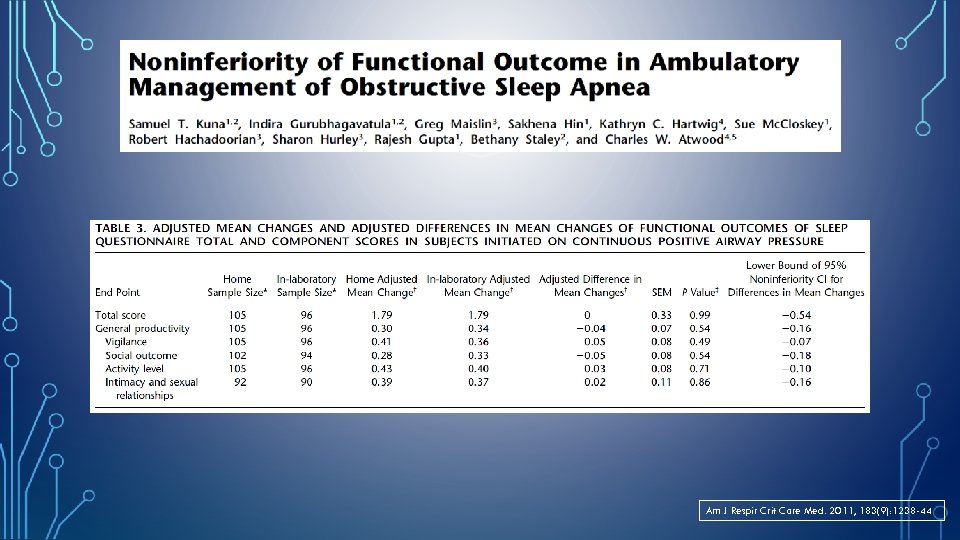
Am J Respir Crit Care Med. 2011, 183(9): 1238 -44
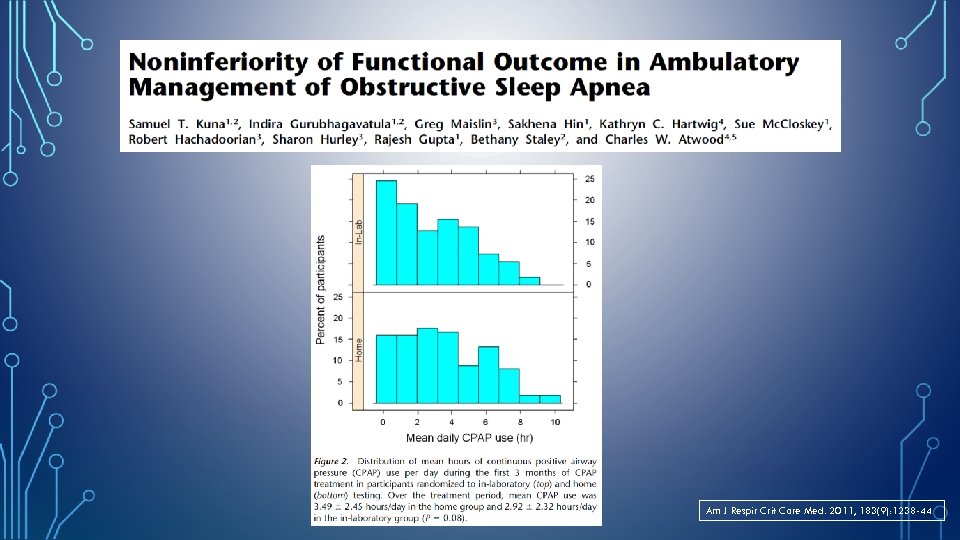
Am J Respir Crit Care Med. 2011, 183(9): 1238 -44
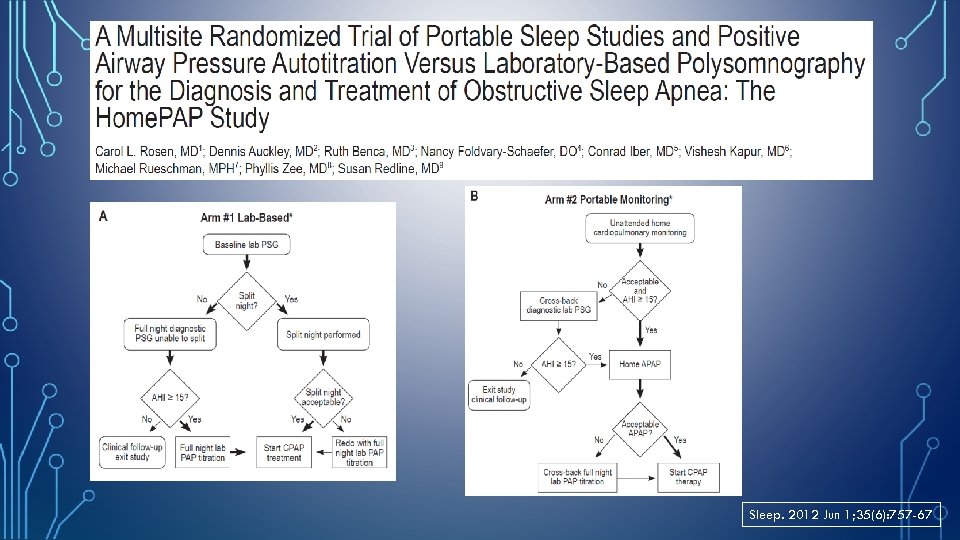
Sleep. 2012 Jun 1; 35(6): 757 -67
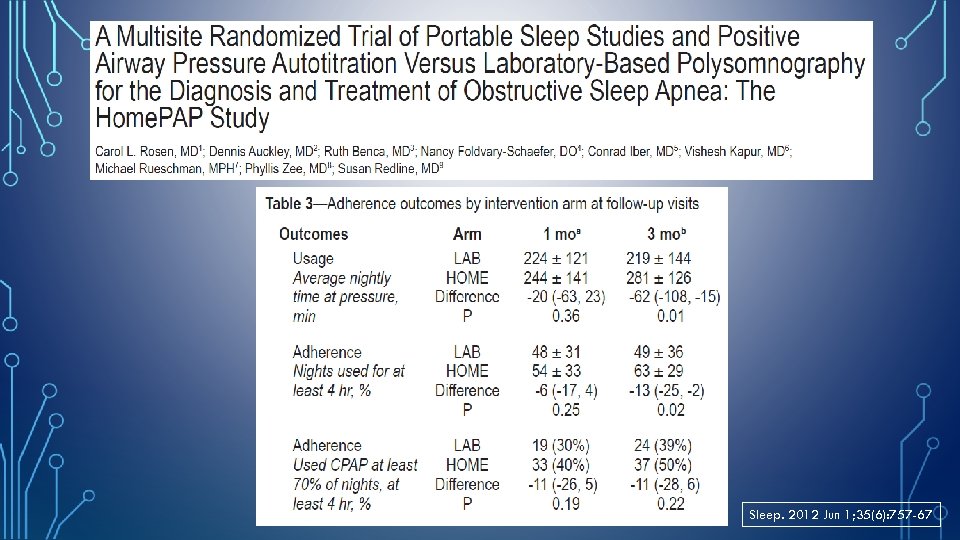
Sleep. 2012 Jun 1; 35(6): 757 -67

Sleep. 2012 Jun 1; 35(6): 757 -67

TAKE HOME MESSAGES… • PM has become an important tool in the armamentarium used to manage sleep disordered breathing. • In out-patient setting, PM/HST algorithms are non-inferior to laboratory-based diagnosis of OSA and titration of PAP therapy. • For appropriately selected patients- with moderate to high clinical suspicion of obstructive sleep apnea and without significant co-morbid conditions, HST can be used effectively to diagnose and auto-PAP to treat OSA. • Patients with normal AHI on HST but high pre-test clinical suspicion for OSA should undergo in-laboratory PSG.
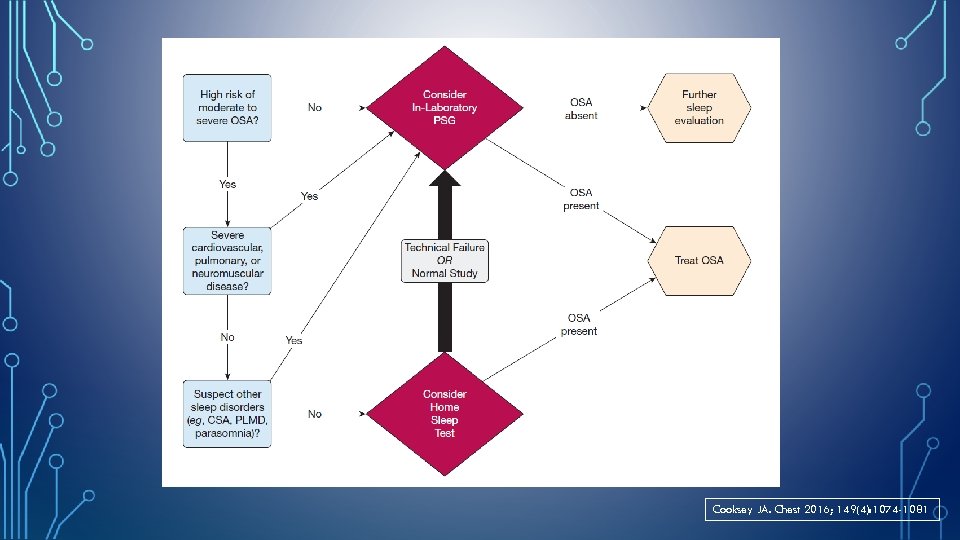
Cooksey JA. Chest 2016; 149(4): 1074 -1081
dd15fa57f21fc21dc1ce20dbb6ff6230.ppt