81072c1c744242d88b517d12c696fc5b.ppt
- Количество слайдов: 14
 Pooled Analysis of Clinical Events From Prospective Trials of the Resolute Zotarolimus-eluting Stent: Two Year Outcomes of 5130 Patients J. Belardi, MD, FACC On Behalf of A. Yeung, M. Leon, P. Serruys, I. Meredith, S. Windecker, S. Silber, F-J. Neumann, P. Widimský, S. Saito, J. Massaro, L. Mauri and the RESOLUTE Clinical Program Investigators ACC 2012 For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
Pooled Analysis of Clinical Events From Prospective Trials of the Resolute Zotarolimus-eluting Stent: Two Year Outcomes of 5130 Patients J. Belardi, MD, FACC On Behalf of A. Yeung, M. Leon, P. Serruys, I. Meredith, S. Windecker, S. Silber, F-J. Neumann, P. Widimský, S. Saito, J. Massaro, L. Mauri and the RESOLUTE Clinical Program Investigators ACC 2012 For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
 Background • Individual trials while powered for many composite endpoints are often underpowered to show real differences for low-frequency but important adverse clinical events such as stent thrombosis (ST). • We analyzed pooled patient level data from five trials to evaluate clinical events, in particular, safety outcomes with the Resolute ZES. For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
Background • Individual trials while powered for many composite endpoints are often underpowered to show real differences for low-frequency but important adverse clinical events such as stent thrombosis (ST). • We analyzed pooled patient level data from five trials to evaluate clinical events, in particular, safety outcomes with the Resolute ZES. For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
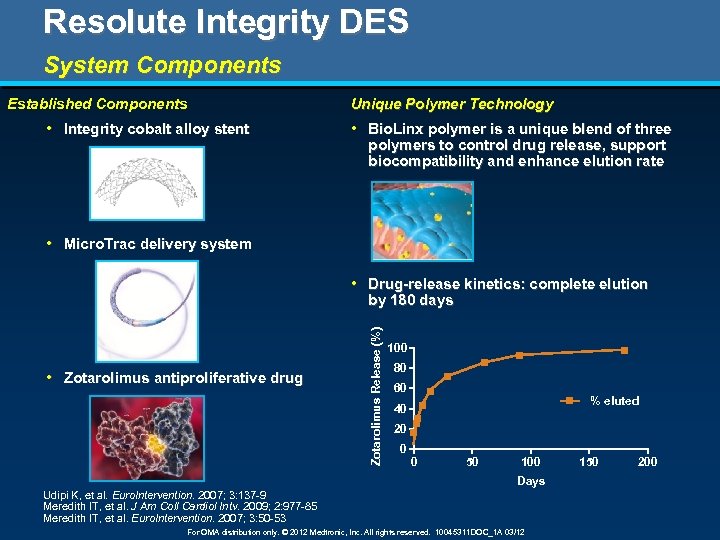 Resolute Integrity DES System Components Unique Polymer Technology Established Components • Integrity cobalt alloy stent • Bio. Linx polymer is a unique blend of three polymers to control drug release, support biocompatibility and enhance elution rate • Micro. Trac delivery system • Zotarolimus antiproliferative drug Zotarolimus Release (%) • Drug-release kinetics: complete elution by 180 days 100 80 60 % eluted 40 20 0 0 50 100 Days Udipi K, et al. Euro. Intervention. 2007; 3: 137 -9 Meredith IT, et al. J Am Coll Cardiol Intv. 2009; 2: 977 -85 Meredith IT, et al. Euro. Intervention. 2007; 3: 50 -53 For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12 150 200
Resolute Integrity DES System Components Unique Polymer Technology Established Components • Integrity cobalt alloy stent • Bio. Linx polymer is a unique blend of three polymers to control drug release, support biocompatibility and enhance elution rate • Micro. Trac delivery system • Zotarolimus antiproliferative drug Zotarolimus Release (%) • Drug-release kinetics: complete elution by 180 days 100 80 60 % eluted 40 20 0 0 50 100 Days Udipi K, et al. Euro. Intervention. 2007; 3: 137 -9 Meredith IT, et al. J Am Coll Cardiol Intv. 2009; 2: 977 -85 Meredith IT, et al. Euro. Intervention. 2007; 3: 50 -53 For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12 150 200
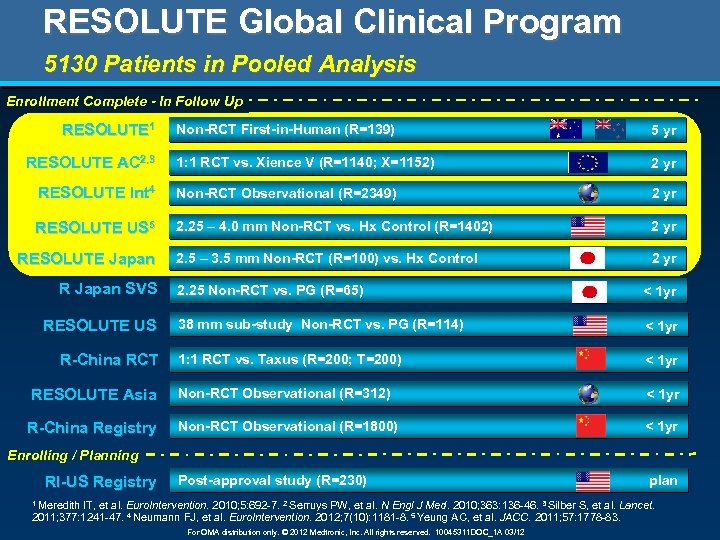 RESOLUTE Global Clinical Program 5130 Patients in Pooled Analysis Enrollment Complete - In Follow Up RESOLUTE 1 Non-RCT First-in-Human (R=139) 5 yr 1: 1 RCT vs. Xience V (R=1140; X=1152) 2 yr RESOLUTE Int 4 Non-RCT Observational (R=2349) 2 yr RESOLUTE US 5 2. 25 – 4. 0 mm Non-RCT vs. Hx Control (R=1402) 2 yr 2. 5 – 3. 5 mm Non-RCT (R=100) vs. Hx Control 2 yr RESOLUTE AC 2, 3 RESOLUTE Japan R Japan SVS 2. 25 Non-RCT vs. PG (R=65) < 1 yr 38 mm sub-study Non-RCT vs. PG (R=114) < 1 yr 1: 1 RCT vs. Taxus (R=200; T=200) < 1 yr RESOLUTE Asia Non-RCT Observational (R=312) < 1 yr R-China Registry Non-RCT Observational (R=1800) < 1 yr RESOLUTE US R-China RCT Enrolling / Planning RI-US Registry Post-approval study (R=230) 1 Meredith plan IT, et al. Euro. Intervention. 2010; 5: 692 -7. 2 Serruys PW, et al. N Engl J Med. 2010; 363: 136 -46. 3 Silber S, et al. Lancet. 2011; 377: 1241 -47. 4 Neumann FJ, et al. Euro. Intervention. 2012; 7(10): 1181 -8. 5 Yeung AC, et al. JACC. 2011; 57: 1778 -83. For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
RESOLUTE Global Clinical Program 5130 Patients in Pooled Analysis Enrollment Complete - In Follow Up RESOLUTE 1 Non-RCT First-in-Human (R=139) 5 yr 1: 1 RCT vs. Xience V (R=1140; X=1152) 2 yr RESOLUTE Int 4 Non-RCT Observational (R=2349) 2 yr RESOLUTE US 5 2. 25 – 4. 0 mm Non-RCT vs. Hx Control (R=1402) 2 yr 2. 5 – 3. 5 mm Non-RCT (R=100) vs. Hx Control 2 yr RESOLUTE AC 2, 3 RESOLUTE Japan R Japan SVS 2. 25 Non-RCT vs. PG (R=65) < 1 yr 38 mm sub-study Non-RCT vs. PG (R=114) < 1 yr 1: 1 RCT vs. Taxus (R=200; T=200) < 1 yr RESOLUTE Asia Non-RCT Observational (R=312) < 1 yr R-China Registry Non-RCT Observational (R=1800) < 1 yr RESOLUTE US R-China RCT Enrolling / Planning RI-US Registry Post-approval study (R=230) 1 Meredith plan IT, et al. Euro. Intervention. 2010; 5: 692 -7. 2 Serruys PW, et al. N Engl J Med. 2010; 363: 136 -46. 3 Silber S, et al. Lancet. 2011; 377: 1241 -47. 4 Neumann FJ, et al. Euro. Intervention. 2012; 7(10): 1181 -8. 5 Yeung AC, et al. JACC. 2011; 57: 1778 -83. For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
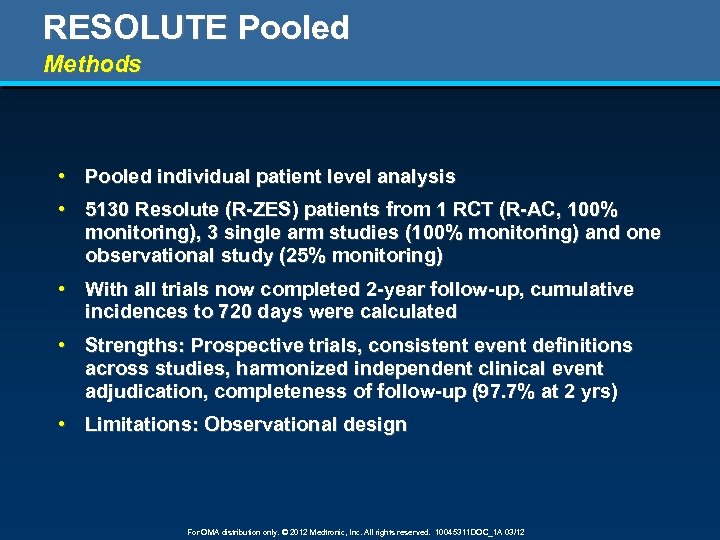 RESOLUTE Pooled Methods • Pooled individual patient level analysis • 5130 Resolute (R-ZES) patients from 1 RCT (R-AC, 100% monitoring), 3 single arm studies (100% monitoring) and one observational study (25% monitoring) • With all trials now completed 2 -year follow-up, cumulative incidences to 720 days were calculated • Strengths: Prospective trials, consistent event definitions across studies, harmonized independent clinical event adjudication, completeness of follow-up (97. 7% at 2 yrs) • Limitations: Observational design For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
RESOLUTE Pooled Methods • Pooled individual patient level analysis • 5130 Resolute (R-ZES) patients from 1 RCT (R-AC, 100% monitoring), 3 single arm studies (100% monitoring) and one observational study (25% monitoring) • With all trials now completed 2 -year follow-up, cumulative incidences to 720 days were calculated • Strengths: Prospective trials, consistent event definitions across studies, harmonized independent clinical event adjudication, completeness of follow-up (97. 7% at 2 yrs) • Limitations: Observational design For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
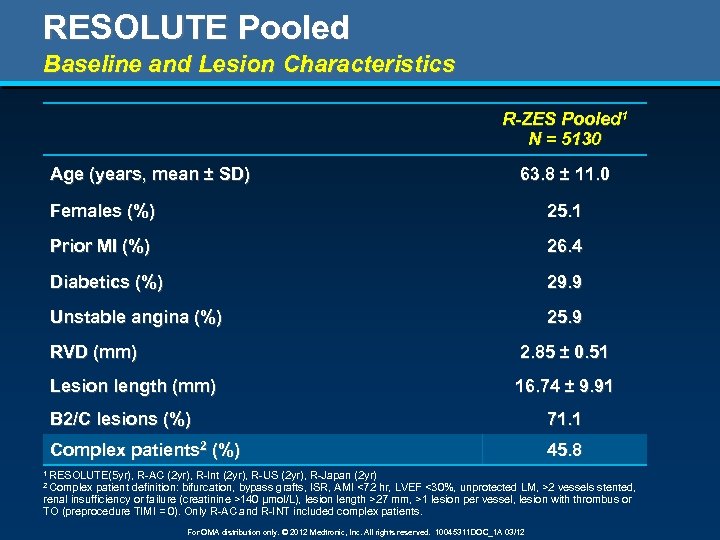 RESOLUTE Pooled Baseline and Lesion Characteristics R-ZES Pooled 1 N = 5130 Age (years, mean ± SD) 63. 8 ± 11. 0 Females (%) 25. 1 Prior MI (%) 26. 4 Diabetics (%) 29. 9 Unstable angina (%) 25. 9 RVD (mm) 2. 85 ± 0. 51 Lesion length (mm) 16. 74 ± 9. 91 B 2/C lesions (%) 71. 1 Complex patients 2 (%) 45. 8 1 RESOLUTE(5 yr), R-AC (2 yr), R-Int (2 yr), R-US (2 yr), R-Japan (2 yr) patient definition: bifurcation, bypass grafts, ISR, AMI <72 hr, LVEF <30%, unprotected LM, >2 vessels stented, renal insufficiency or failure (creatinine >140 µmol/L), lesion length >27 mm, >1 lesion per vessel, lesion with thrombus or TO (preprocedure TIMI = 0). Only R-AC and R-INT included complex patients. 2 Complex For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
RESOLUTE Pooled Baseline and Lesion Characteristics R-ZES Pooled 1 N = 5130 Age (years, mean ± SD) 63. 8 ± 11. 0 Females (%) 25. 1 Prior MI (%) 26. 4 Diabetics (%) 29. 9 Unstable angina (%) 25. 9 RVD (mm) 2. 85 ± 0. 51 Lesion length (mm) 16. 74 ± 9. 91 B 2/C lesions (%) 71. 1 Complex patients 2 (%) 45. 8 1 RESOLUTE(5 yr), R-AC (2 yr), R-Int (2 yr), R-US (2 yr), R-Japan (2 yr) patient definition: bifurcation, bypass grafts, ISR, AMI <72 hr, LVEF <30%, unprotected LM, >2 vessels stented, renal insufficiency or failure (creatinine >140 µmol/L), lesion length >27 mm, >1 lesion per vessel, lesion with thrombus or TO (preprocedure TIMI = 0). Only R-AC and R-INT included complex patients. 2 Complex For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
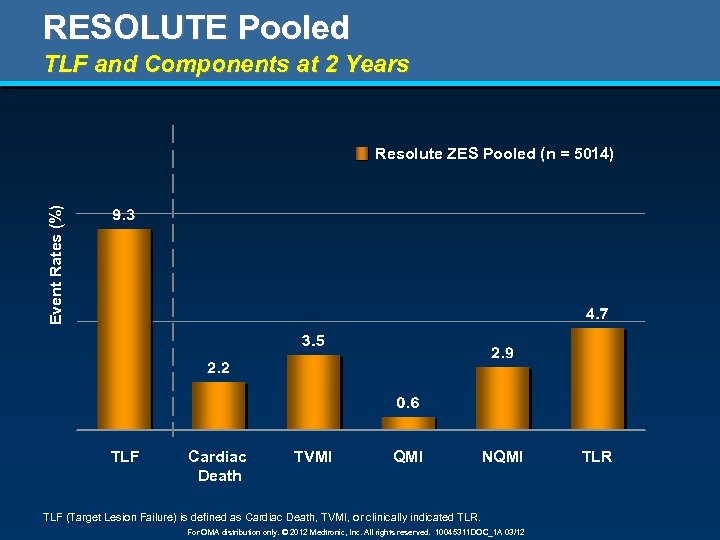 RESOLUTE Pooled TLF and Components at 2 Years Event Rates (%) Resolute ZES Pooled (n = 5014) TLF Cardiac Death TVMI QMI NQMI TLF (Target Lesion Failure) is defined as Cardiac Death, TVMI, or clinically indicated TLR. For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12 TLR
RESOLUTE Pooled TLF and Components at 2 Years Event Rates (%) Resolute ZES Pooled (n = 5014) TLF Cardiac Death TVMI QMI NQMI TLF (Target Lesion Failure) is defined as Cardiac Death, TVMI, or clinically indicated TLR. For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12 TLR
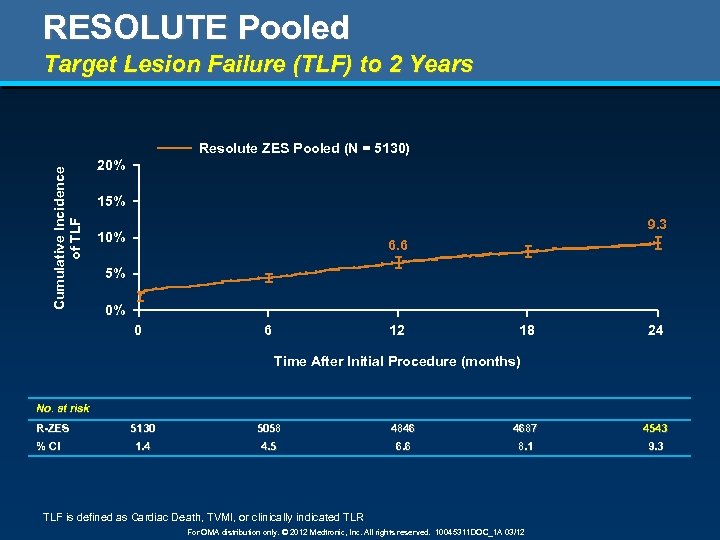 RESOLUTE Pooled Target Lesion Failure (TLF) to 2 Years Cumulative Incidence of TLF Resolute ZES Pooled (N = 5130) 20% 15% 9. 3 10% 6. 6 5% 0% 0 6 12 18 24 Time After Initial Procedure (months) No. at risk R-ZES % CI 5130 5058 4846 4687 4543 1. 4 4. 5 6. 6 8. 1 9. 3 TLF is defined as Cardiac Death, TVMI, or clinically indicated TLR For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
RESOLUTE Pooled Target Lesion Failure (TLF) to 2 Years Cumulative Incidence of TLF Resolute ZES Pooled (N = 5130) 20% 15% 9. 3 10% 6. 6 5% 0% 0 6 12 18 24 Time After Initial Procedure (months) No. at risk R-ZES % CI 5130 5058 4846 4687 4543 1. 4 4. 5 6. 6 8. 1 9. 3 TLF is defined as Cardiac Death, TVMI, or clinically indicated TLR For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
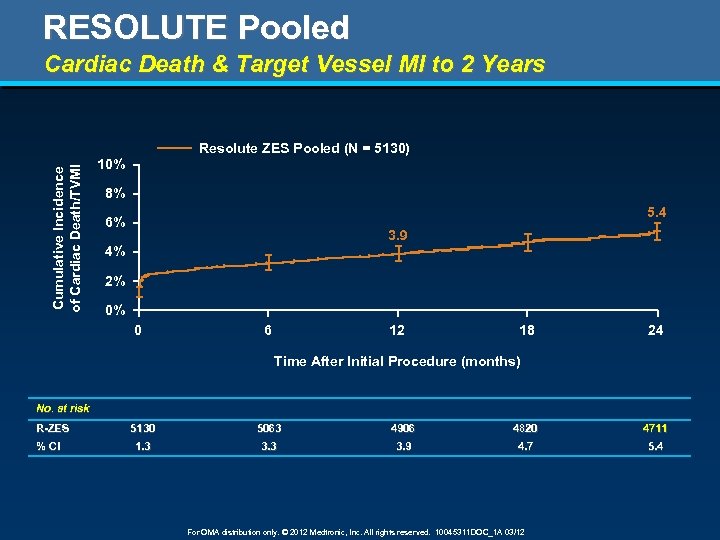 RESOLUTE Pooled Cardiac Death & Target Vessel MI to 2 Years Cumulative Incidence of Cardiac Death/TVMI Resolute ZES Pooled (N = 5130) 10% 8% 5. 4 6% 3. 9 4% 2% 0% 0 6 12 18 24 Time After Initial Procedure (months) No. at risk R-ZES % CI 5130 5063 4906 4820 4711 1. 3 3. 9 4. 7 5. 4 For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
RESOLUTE Pooled Cardiac Death & Target Vessel MI to 2 Years Cumulative Incidence of Cardiac Death/TVMI Resolute ZES Pooled (N = 5130) 10% 8% 5. 4 6% 3. 9 4% 2% 0% 0 6 12 18 24 Time After Initial Procedure (months) No. at risk R-ZES % CI 5130 5063 4906 4820 4711 1. 3 3. 9 4. 7 5. 4 For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
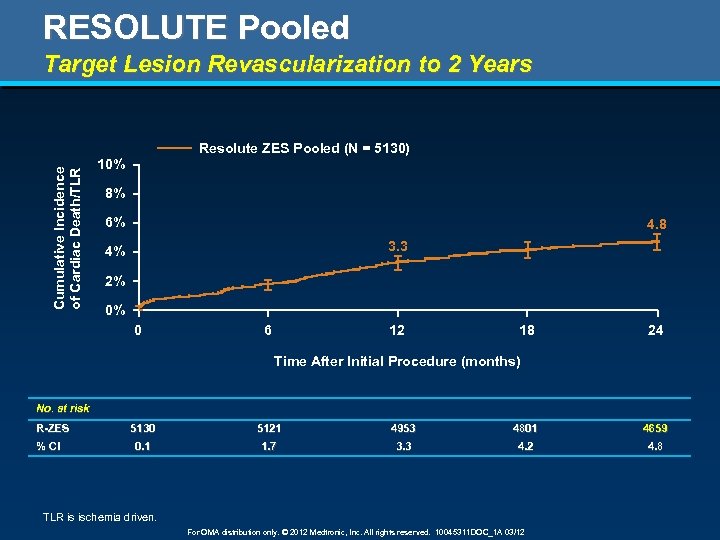 RESOLUTE Pooled Target Lesion Revascularization to 2 Years Cumulative Incidence of Cardiac Death/TLR Resolute ZES Pooled (N = 5130) 10% 8% 6% 4. 8 3. 3 4% 2% 0% 0 6 12 18 24 Time After Initial Procedure (months) No. at risk R-ZES % CI 5130 5121 4953 4801 4659 0. 1 1. 7 3. 3 4. 2 4. 8 TLR is ischemia driven. For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
RESOLUTE Pooled Target Lesion Revascularization to 2 Years Cumulative Incidence of Cardiac Death/TLR Resolute ZES Pooled (N = 5130) 10% 8% 6% 4. 8 3. 3 4% 2% 0% 0 6 12 18 24 Time After Initial Procedure (months) No. at risk R-ZES % CI 5130 5121 4953 4801 4659 0. 1 1. 7 3. 3 4. 2 4. 8 TLR is ischemia driven. For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
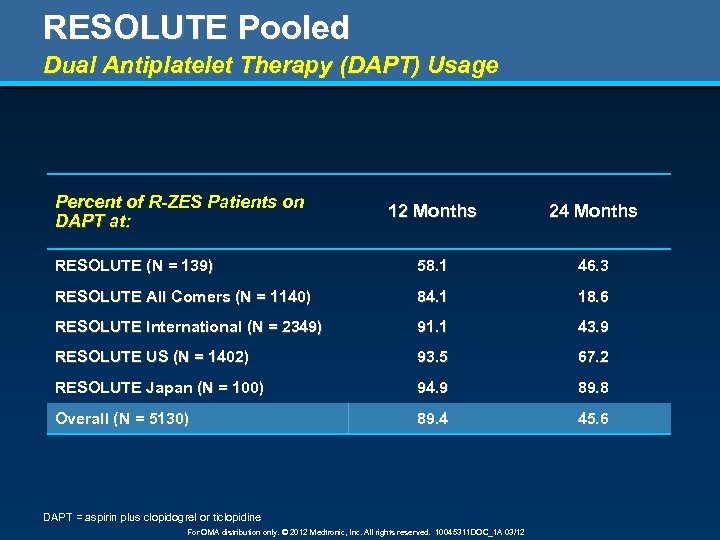 RESOLUTE Pooled Dual Antiplatelet Therapy (DAPT) Usage Percent of R-ZES Patients on DAPT at: 12 Months 24 Months RESOLUTE (N = 139) 58. 1 46. 3 RESOLUTE All Comers (N = 1140) 84. 1 18. 6 RESOLUTE International (N = 2349) 91. 1 43. 9 RESOLUTE US (N = 1402) 93. 5 67. 2 RESOLUTE Japan (N = 100) 94. 9 89. 8 Overall (N = 5130) 89. 4 45. 6 DAPT = aspirin plus clopidogrel or ticlopidine For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
RESOLUTE Pooled Dual Antiplatelet Therapy (DAPT) Usage Percent of R-ZES Patients on DAPT at: 12 Months 24 Months RESOLUTE (N = 139) 58. 1 46. 3 RESOLUTE All Comers (N = 1140) 84. 1 18. 6 RESOLUTE International (N = 2349) 91. 1 43. 9 RESOLUTE US (N = 1402) 93. 5 67. 2 RESOLUTE Japan (N = 100) 94. 9 89. 8 Overall (N = 5130) 89. 4 45. 6 DAPT = aspirin plus clopidogrel or ticlopidine For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
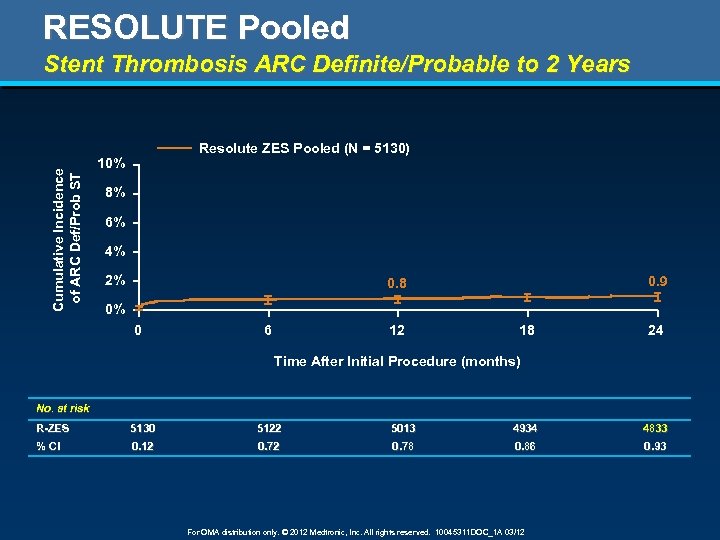 RESOLUTE Pooled Stent Thrombosis ARC Definite/Probable to 2 Years Cumulative Incidence of ARC Def/Prob ST Resolute ZES Pooled (N = 5130) 10% 8% 6% 4% 2% 0. 9 0. 8 0% 0 6 12 18 24 Time After Initial Procedure (months) No. at risk R-ZES 5130 5122 5013 4934 4833 % CI 0. 12 0. 78 0. 86 0. 93 For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
RESOLUTE Pooled Stent Thrombosis ARC Definite/Probable to 2 Years Cumulative Incidence of ARC Def/Prob ST Resolute ZES Pooled (N = 5130) 10% 8% 6% 4% 2% 0. 9 0. 8 0% 0 6 12 18 24 Time After Initial Procedure (months) No. at risk R-ZES 5130 5122 5013 4934 4833 % CI 0. 12 0. 78 0. 86 0. 93 For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
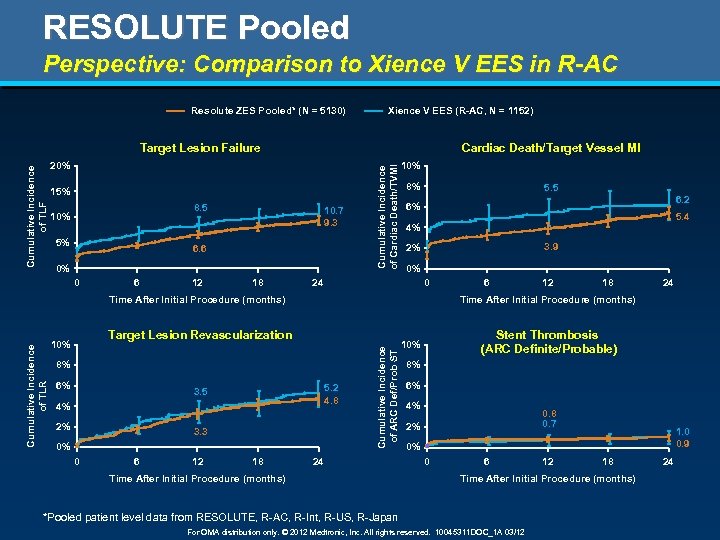 RESOLUTE Pooled Perspective: Comparison to Xience V EES in R-AC Resolute ZES Pooled* (N = 5130) Xience V EES (R-AC, N = 1152) Cardiac Death/Target Vessel MI 20% 15% 8. 5 10% 5% 10. 7 9. 3 6. 6 0% 0 6 12 18 Cumulative Incidence of Cardiac Death/TVMI Cumulative Incidence of TLF Target Lesion Failure 10% 8% 5. 5 6. 2 6% 5. 4 4% 3. 9 2% 0% 24 0 Target Lesion Revascularization 10% 8% 6% 5. 2 4. 8 3. 5 4% 2% 3. 3 0% 0 6 12 18 24 Time After Initial Procedure (months) Cumulative Incidence of ARC Def/Prob ST Cumulative Incidence of TLR Time After Initial Procedure (months) 6 24 Time After Initial Procedure (months) Stent Thrombosis (ARC Definite/Probable) 10% 8% 6% 4% 0. 8 0. 7 2% 1. 0 0. 9 0% 0 6 12 18 Time After Initial Procedure (months) *Pooled patient level data from RESOLUTE, R-AC, R-Int, R-US, R-Japan For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12 24
RESOLUTE Pooled Perspective: Comparison to Xience V EES in R-AC Resolute ZES Pooled* (N = 5130) Xience V EES (R-AC, N = 1152) Cardiac Death/Target Vessel MI 20% 15% 8. 5 10% 5% 10. 7 9. 3 6. 6 0% 0 6 12 18 Cumulative Incidence of Cardiac Death/TVMI Cumulative Incidence of TLF Target Lesion Failure 10% 8% 5. 5 6. 2 6% 5. 4 4% 3. 9 2% 0% 24 0 Target Lesion Revascularization 10% 8% 6% 5. 2 4. 8 3. 5 4% 2% 3. 3 0% 0 6 12 18 24 Time After Initial Procedure (months) Cumulative Incidence of ARC Def/Prob ST Cumulative Incidence of TLR Time After Initial Procedure (months) 6 24 Time After Initial Procedure (months) Stent Thrombosis (ARC Definite/Probable) 10% 8% 6% 4% 0. 8 0. 7 2% 1. 0 0. 9 0% 0 6 12 18 Time After Initial Procedure (months) *Pooled patient level data from RESOLUTE, R-AC, R-Int, R-US, R-Japan For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12 24
 RESOLUTE Pooled Conclusions • In this pooled analysis of 5130 patients treated with the Resolute ZES out to 2 years, adverse clinical events were very low (TLF 9. 3%, TLR 4. 7% and def/prob ST 0. 9% at 2 yrs). • Follow-up is ongoing to determine whether these results will remain generalizable to follow-up beyond 2 years and in specific higher risk subgroups. For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12
RESOLUTE Pooled Conclusions • In this pooled analysis of 5130 patients treated with the Resolute ZES out to 2 years, adverse clinical events were very low (TLF 9. 3%, TLR 4. 7% and def/prob ST 0. 9% at 2 yrs). • Follow-up is ongoing to determine whether these results will remain generalizable to follow-up beyond 2 years and in specific higher risk subgroups. For OMA distribution only. © 2012 Medtronic, Inc. All rights reserved. 10045311 DOC_1 A 03/12


