Polymers.ppt
- Количество слайдов: 32

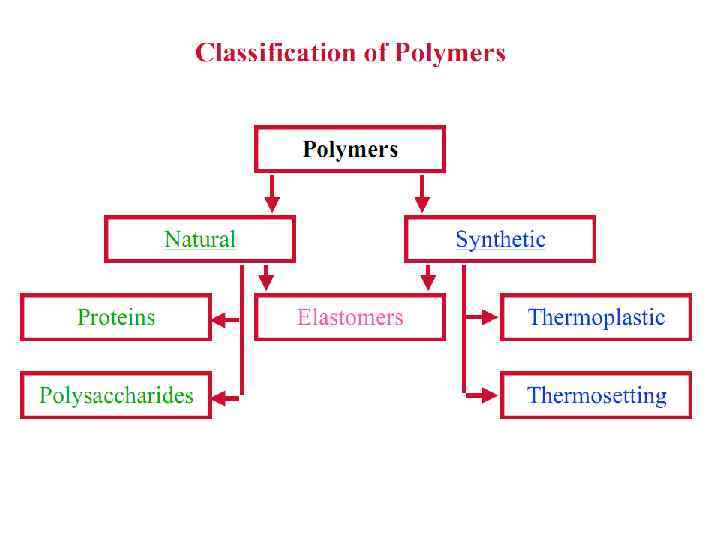
Polymers
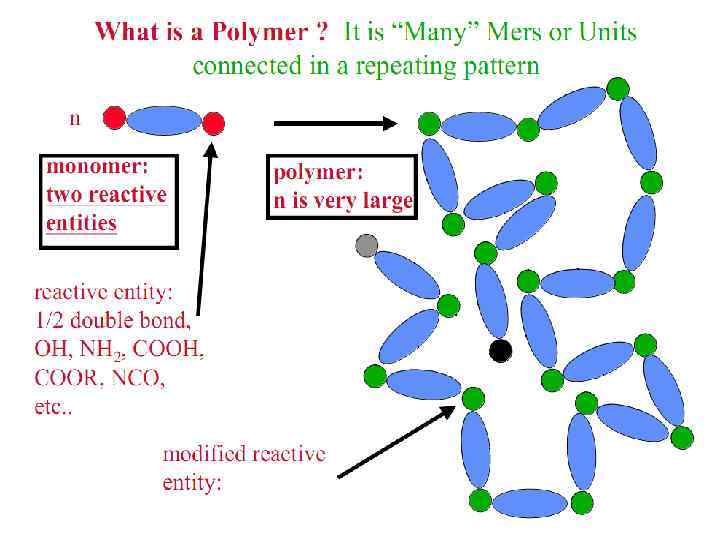
Polymers are substances whose molecules have high molar masses and are composed of a large number of repeating units. There are both naturally occurring and synthetic polymers. Among naturally occurring polymers are proteins, starches, cellulose, and latex. Synthetic polymers are produced commercially on a very large scale and have a wide range of properties and uses. The materials commonly called plastics are all synthetic polymers. Polymers are formed by chemical reactions in which a large number of molecules called monomers are joined sequentially, forming a chain. In many polymers, only one monomer is used. In others, two or three different monomers may be combined:

Why are "Polymers" so important ? § § Replace materials such as wood, aluminum, steel, glass. Polymers are organic materials of low density (ca. 1 g. cm-3) Versatility of Synthesis-Processing-Property relationships. Raw materials and processing are cost effective and recycling or disposal are practical (in principle). § Some of the Innumerable Applications: • Packaging, Clothing, Structural Materials, Adhesives, Paints. • Electronic (conducting and electroluminescent polymers). • Actuators: Piezo and Pyrolectric Activity. • Medicine (drug delivery, prostheses, membranes, sutures). • Oil recovery (viscosity modifier).

Polymer Engineering: Introduction and Scope § Polymer : Long Chain Molecules (macromolecules based on some repeating units) § Polymer Science and Engineering: • Organic Chemistry: Polymer Synthesis (monomer to polymer) • Physical Chemistry: Polymer Characterization • Chemical Engineering: Polymer Processing • Materials Science: Structural & Solid State Studies • Mechanical Engineering: Mechanical Properties, Tribology • Engineering Science and Mechanics: Composites, Adhesion • Wood Science and Forestry: Lignin, Cellulose
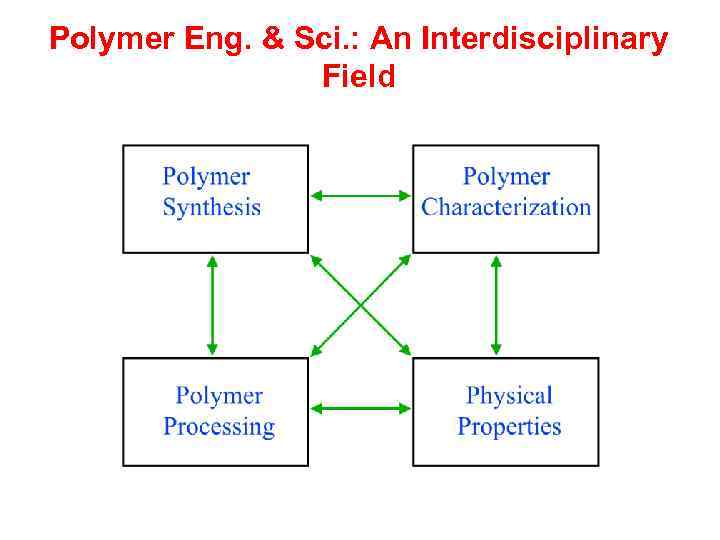
Polymer Eng. & Sci. : An Interdisciplinary Field

Five Unique Features of Polymeric Materials 1. Synthesis (Step and Chain Polymerizations) 2. Average Molar Mass and Molar Mass Distribution 3. Type of Bonding (Covalent and Van der Waals) 4. Semicrystalline Morphology (Amorphous and Crystalline) 5. Viscoelasticity (Temperature and time dependence)
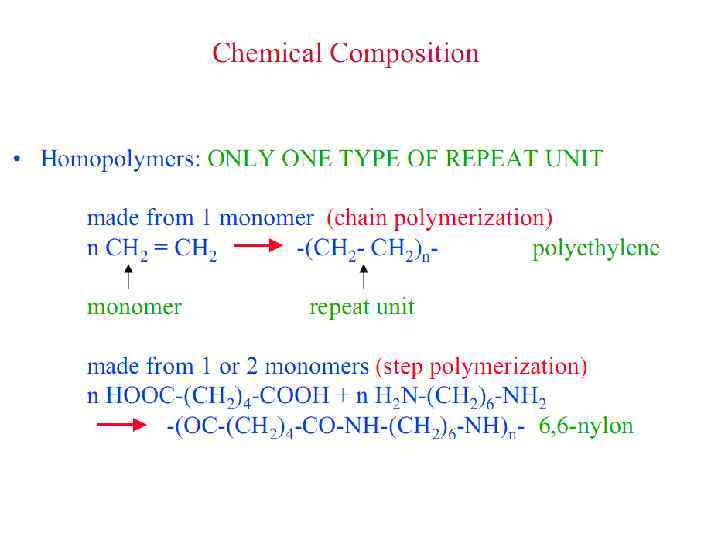
Polymers are classified by the characteristics of the reactions by which they are formed. If all atoms in the monomers are incorporated into the polymer, the polymer is called an addition polymer. If some of the atoms of the monomers are released into small molecules, such as water, the polymer is called a condensation polymer. Most addition polymers are made from monomers containing a double bond between carbon atoms. Such monomers are called olefins, and most commercial addition polymers are polyolefins. Condensation polymers are made from monomers that have two different groups of atoms which can join together to form, for example, ester or amide links. Polyesters are an important class of commercial polymers, as are polyamides (nylon).
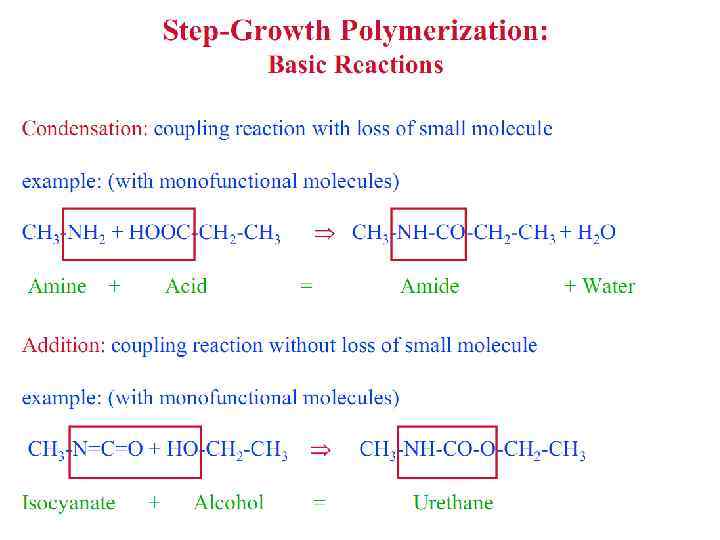
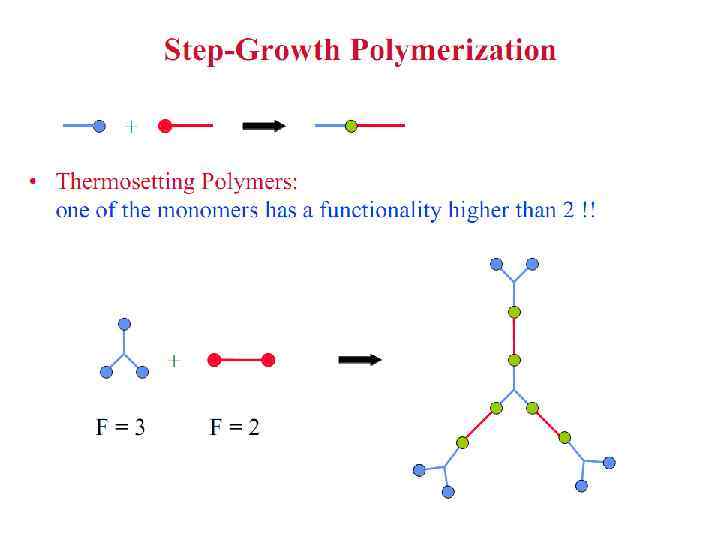
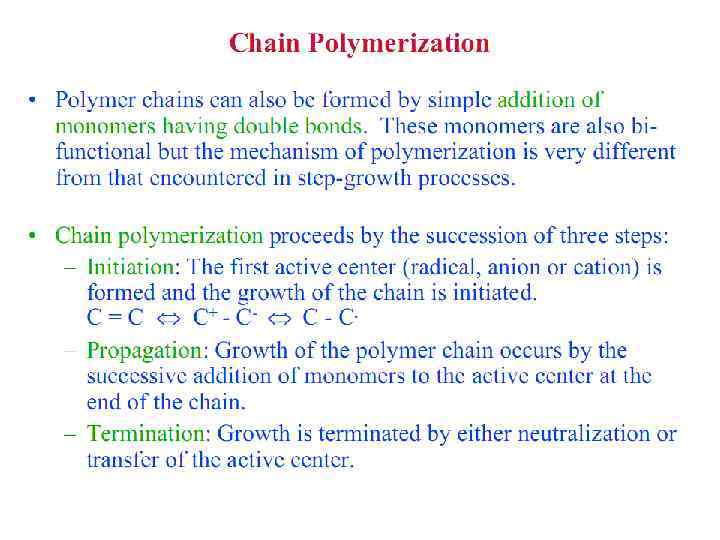
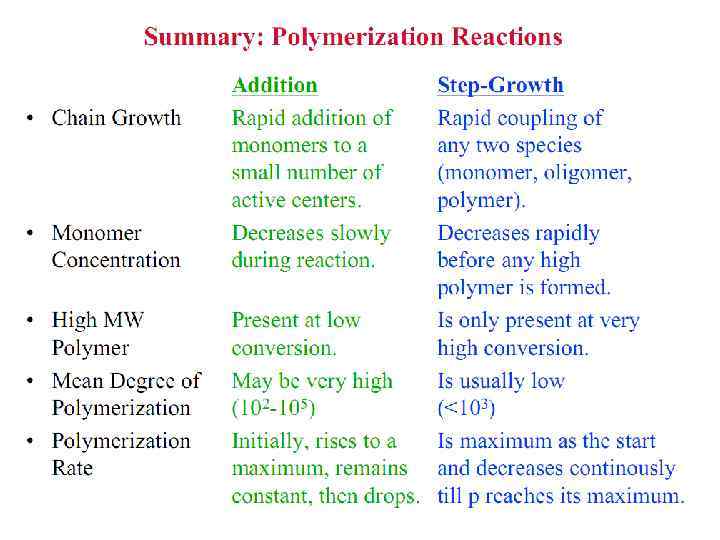
Polymerization Reactions § Polymerization is a reaction to convert monomers to a polymer. § Two types of polymerization reaction: • Step-Growth Polymerization (Old = Condensation Polym. ) applies to monomers with functional groups such as: -COOH, COOR, -COOOC-, -COCl, -OH, -NH 2, -CHO, -NCO, epoxy. • Chain Polymerization (Old = Addition Polym. ) applies to monomers having double bonds or ring structure. § Both classes of reaction can lead to the formation of either linear polymers or polymer networks. Whether the linear chains or polymer networks are obtained only depends on the number of reactive entities per monomer.
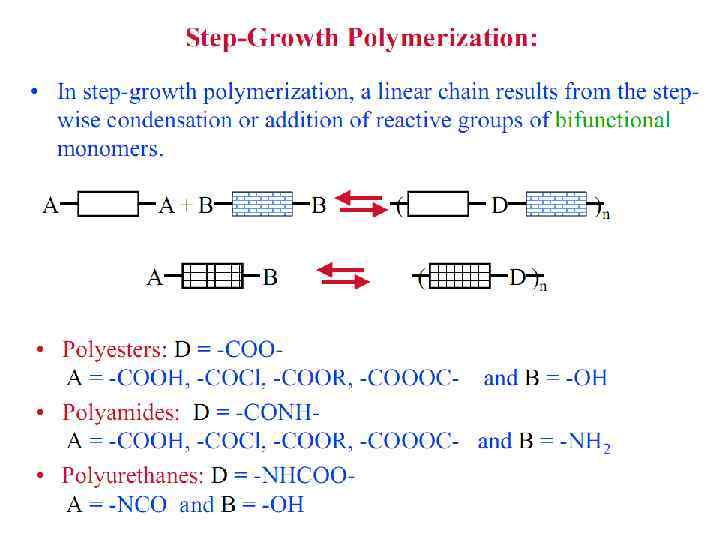
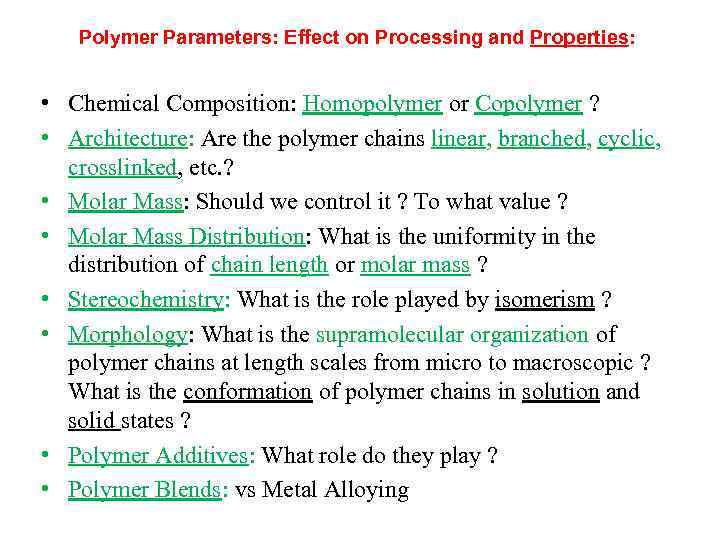
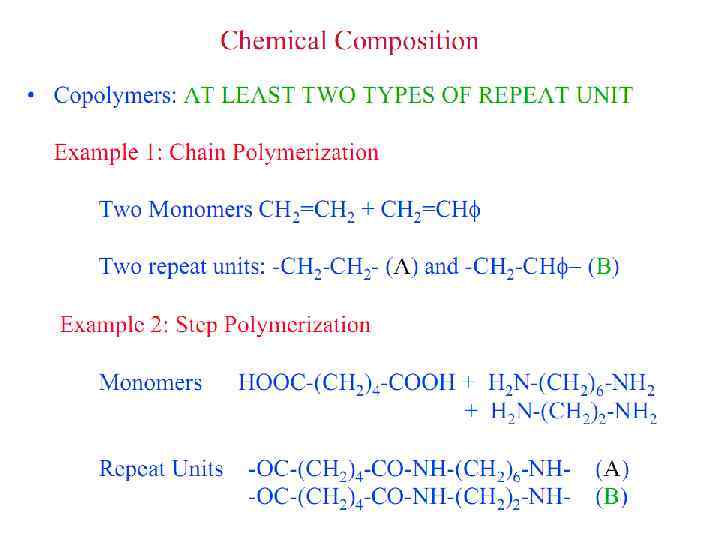
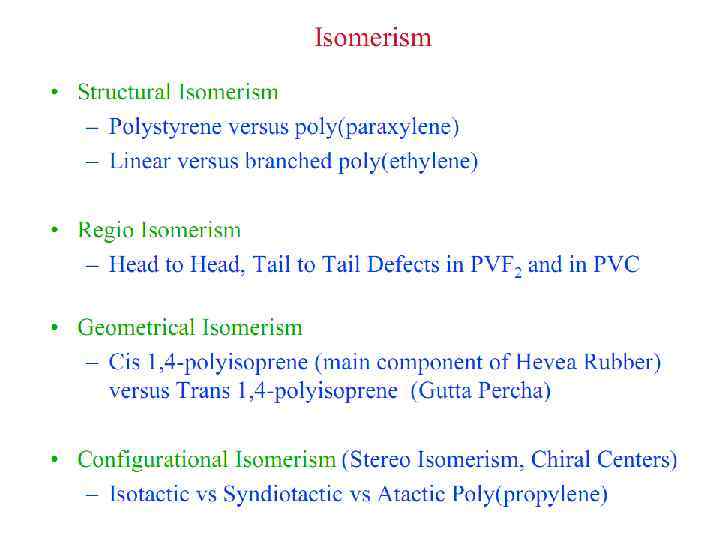
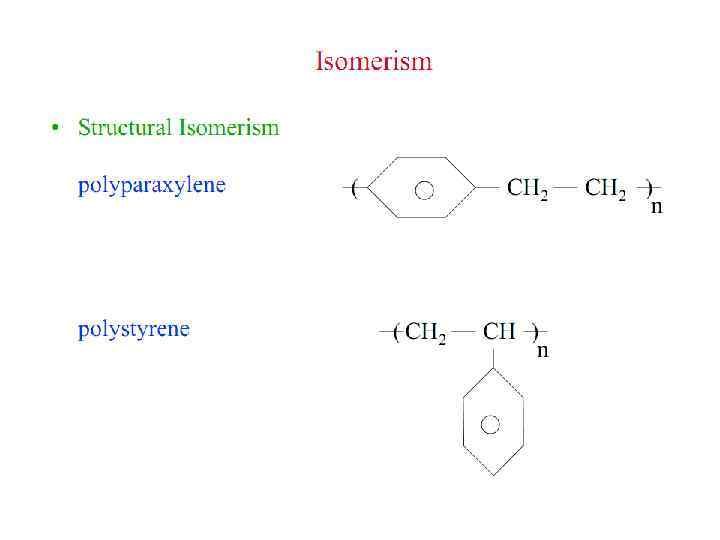
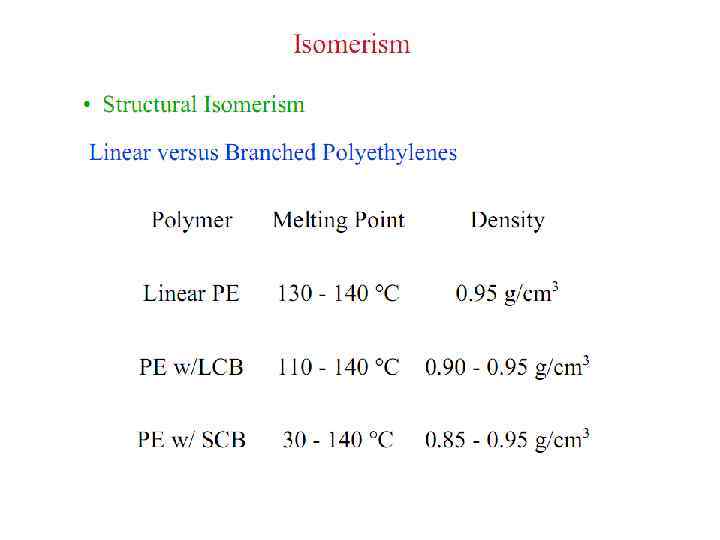
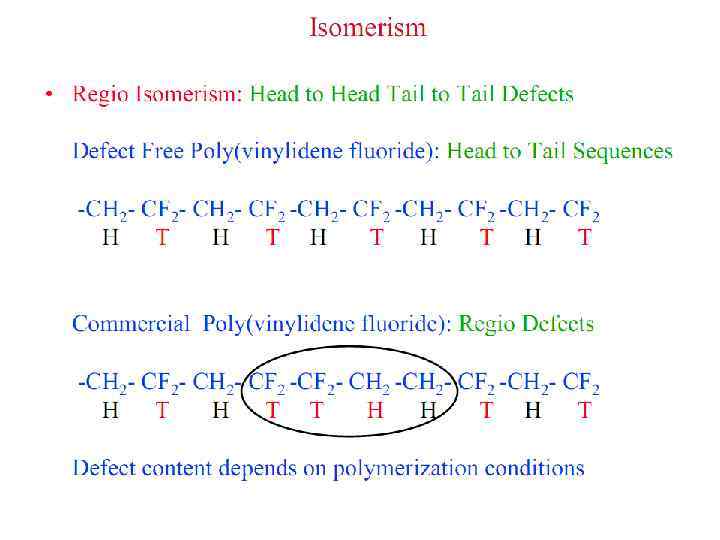
Polymer Parameters: Effect on Processing and Properties: • Chemical Composition: Homopolymer or Copolymer ? • Architecture: Are the polymer chains linear, branched, cyclic, crosslinked, etc. ? • Molar Mass: Should we control it ? To what value ? • Molar Mass Distribution: What is the uniformity in the distribution of chain length or molar mass ? • Stereochemistry: What is the role played by isomerism ? • Morphology: What is the supramolecular organization of polymer chains at length scales from micro to macroscopic ? What is the conformation of polymer chains in solution and solid states ? • Polymer Additives: What role do they play ? • Polymer Blends: vs Metal Alloying
BBBBBBB I AAAAAAAAAAAAAAAAA I BBBBBBBB
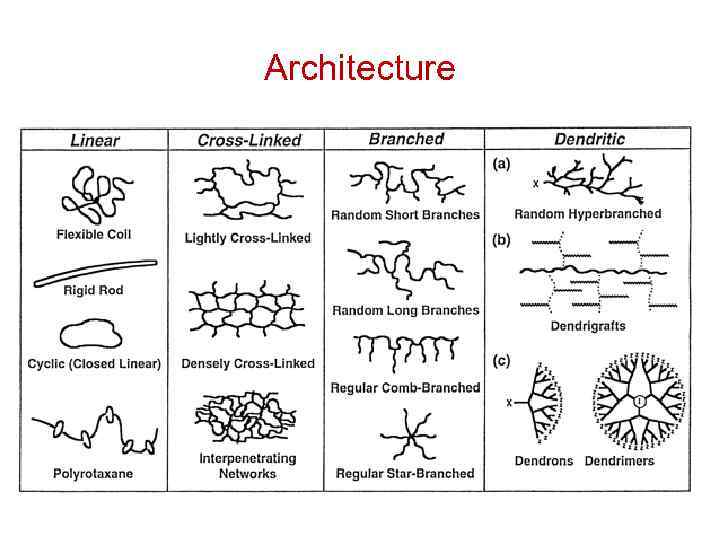
Architecture
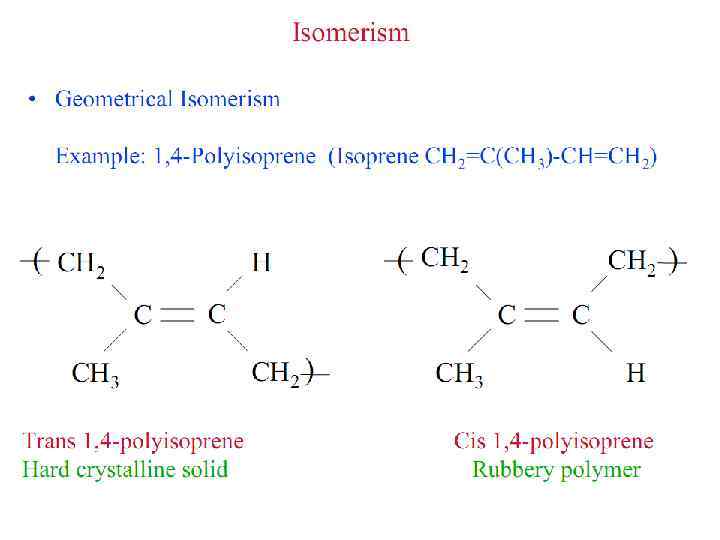
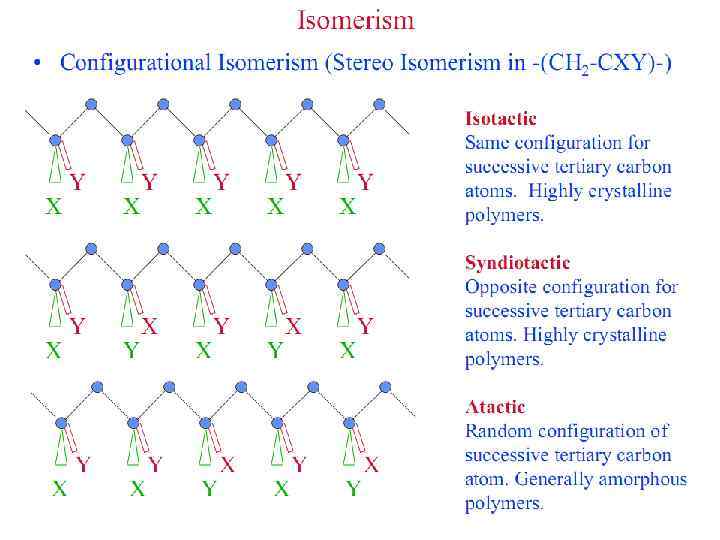

HOW DO WE NAME POLYMERS?

Source-based names • Source-based name when the polymer is derived from a single (original or hypothetical) monomer; or random co-/ter-polymers – Poly(vinyl alcohola) – Poly(styrene-co-butadiene) – Polyformaldehyde (not polyoxymethylene)b – Poly(ethylene oxide) (not poly(ethylene glycol)b a – when the name is long, parentheses are used to separate the name from ‘poly’ b - actually the second name is quite common 2/14/2018 Chapter 1. Primer/introduction 28
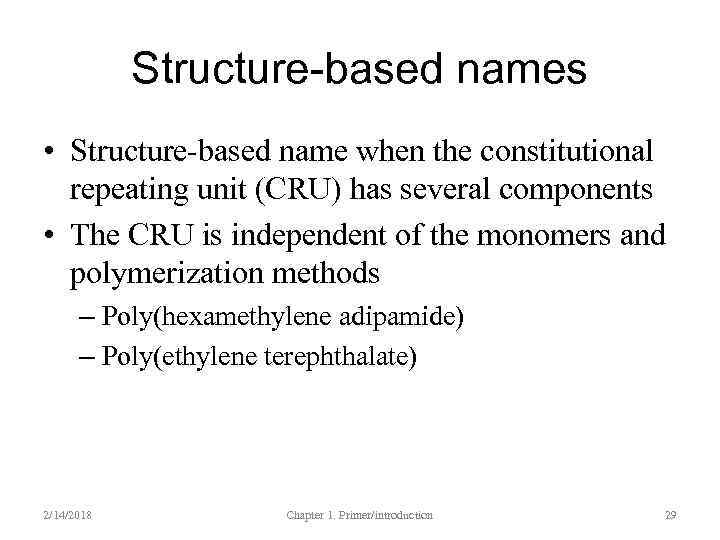
Structure-based names • Structure-based name when the constitutional repeating unit (CRU) has several components • The CRU is independent of the monomers and polymerization methods – Poly(hexamethylene adipamide) – Poly(ethylene terephthalate) 2/14/2018 Chapter 1. Primer/introduction 29
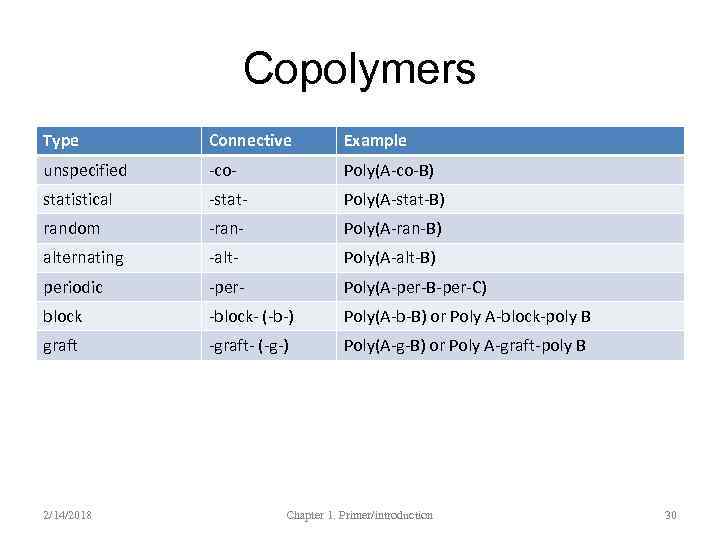
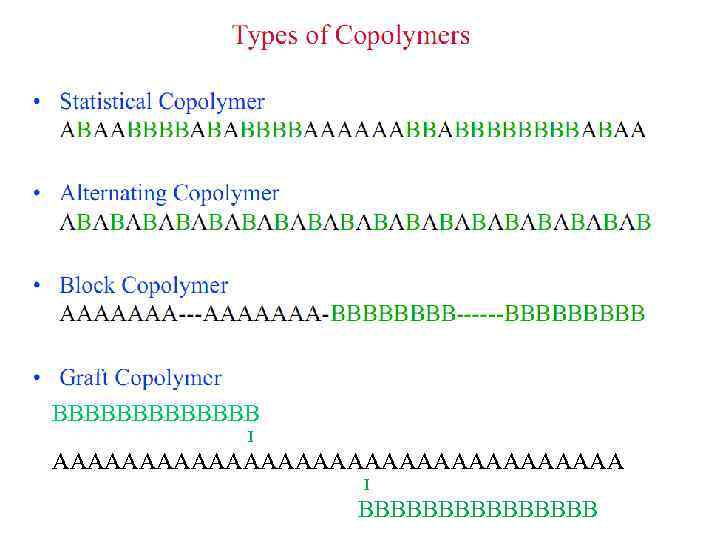
Copolymers Type Connective Example unspecified -co- Poly(A-co-B) statistical -stat- Poly(A-stat-B) random -ran- Poly(A-ran-B) alternating -alt- Poly(A-alt-B) periodic -per- Poly(A-per-B-per-C) block -block- (-b-) Poly(A-b-B) or Poly A-block-poly B graft -graft- (-g-) Poly(A-g-B) or Poly A-graft-poly B 2/14/2018 Chapter 1. Primer/introduction 30
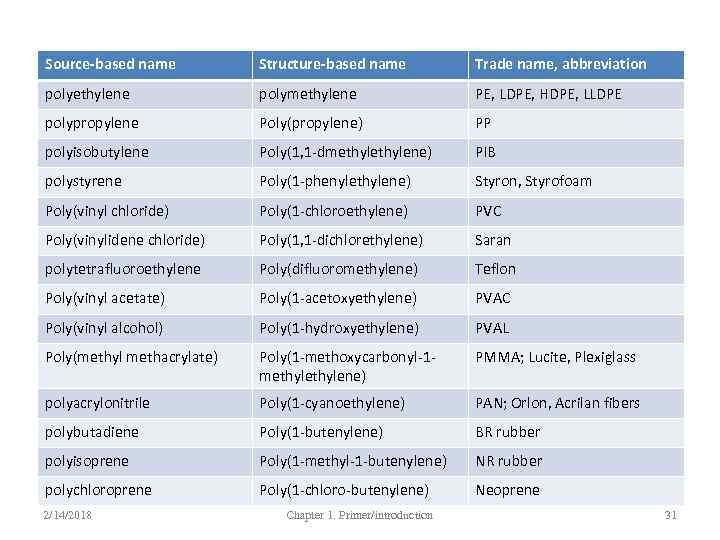
Source-based name Structure-based name Trade name, abbreviation polyethylene polymethylene PE, LDPE, HDPE, LLDPE polypropylene Poly(propylene) PP polyisobutylene Poly(1, 1 -dmethylene) PIB polystyrene Poly(1 -phenylethylene) Styron, Styrofoam Poly(vinyl chloride) Poly(1 -chloroethylene) PVC Poly(vinylidene chloride) Poly(1, 1 -dichlorethylene) Saran polytetrafluoroethylene Poly(difluoromethylene) Teflon Poly(vinyl acetate) Poly(1 -acetoxyethylene) PVAC Poly(vinyl alcohol) Poly(1 -hydroxyethylene) PVAL Poly(methyl methacrylate) Poly(1 -methoxycarbonyl-1 methylene) PMMA; Lucite, Plexiglass polyacrylonitrile Poly(1 -cyanoethylene) PAN; Orlon, Acrilan fibers polybutadiene Poly(1 -butenylene) BR rubber polyisoprene Poly(1 -methyl-1 -butenylene) NR rubber polychloroprene Poly(1 -chloro-butenylene) Neoprene 2/14/2018 Chapter 1. Primer/introduction 31
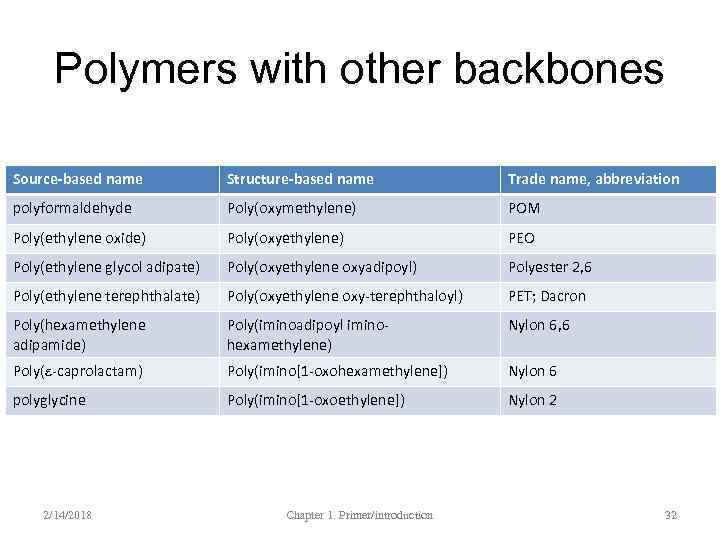
Polymers with other backbones Source-based name Structure-based name Trade name, abbreviation polyformaldehyde Poly(oxymethylene) POM Poly(ethylene oxide) Poly(oxyethylene) PEO Poly(ethylene glycol adipate) Poly(oxyethylene oxyadipoyl) Polyester 2, 6 Poly(ethylene terephthalate) Poly(oxyethylene oxy-terephthaloyl) PET; Dacron Poly(hexamethylene adipamide) Poly(iminoadipoyl iminohexamethylene) Nylon 6, 6 Poly(e-caprolactam) Poly(imino[1 -oxohexamethylene]) Nylon 6 polyglycine Poly(imino[1 -oxoethylene]) Nylon 2 2/14/2018 Chapter 1. Primer/introduction 32
Polymers.ppt