9ef66f0ba4449e81cdc6f94598240004.ppt
- Количество слайдов: 63

Polychromatic Flow Cytometry and Flow Applications Basic Concepts Kevin P. Weller Technical Applications Specialist
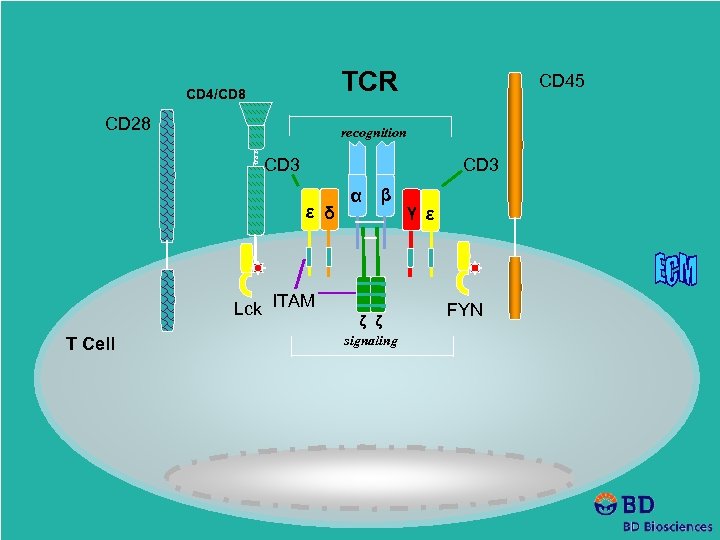
TCR CD 4/CD 8 CD 28 CD 45 recognition CD 3 ε δ Lck ITAM T Cell α β ζ ζ signaling γ ε FYN
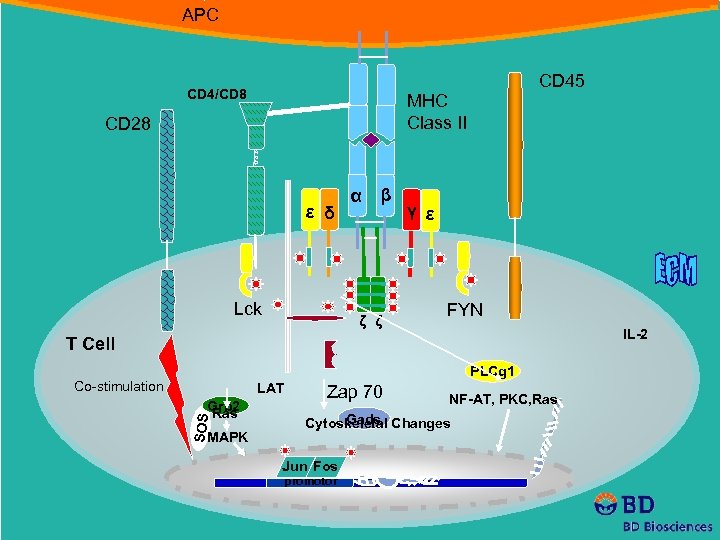
APC CD 4/CD 8 CD 45 MHC Class II CD 28 ε δ Lck α β ζ ζ γ ε FYN IL-2 T Cell PLCg 1 Co-stimulation LAT SOS Grb 2 Ras MAPK Zap 70 NF-AT, PKC, Ras Gads Cytoskeletal Changes Jun Fos promotor

Polychromatic Flow Cytometry • What Do We Need For PFC? – Chemistry – the fluorescent dyes • Must be bright (S/N) • Minimal spectral overlap • Straightforward conjugation to antibodies – Instrumentation • • More Light Sources: Multi-laser (2 - 4 or more) More Detectors: 6 to 16 or more parameters More Efficient Optical Pathway: Higher sensitivity Higher resolution & fast complex data handling : Digital Electronics & New Graphical User Interface
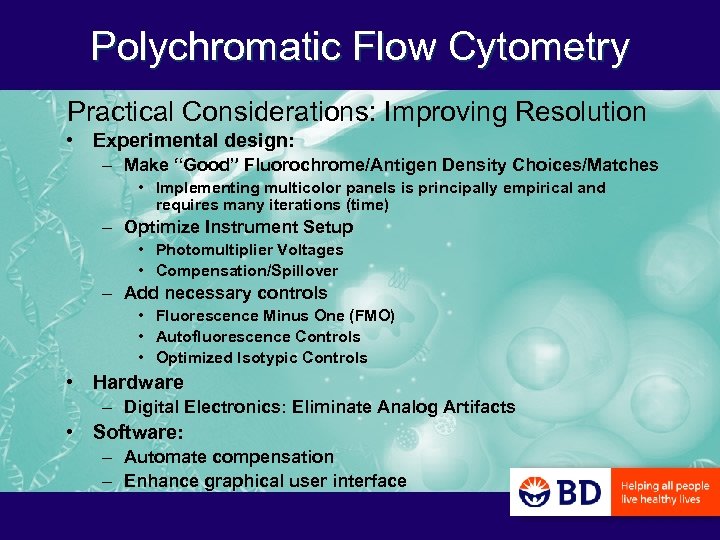
Polychromatic Flow Cytometry Practical Considerations: Improving Resolution • Experimental design: – Make “Good” Fluorochrome/Antigen Density Choices/Matches • Implementing multicolor panels is principally empirical and requires many iterations (time) – Optimize Instrument Setup • Photomultiplier Voltages • Compensation/Spillover – Add necessary controls • Fluorescence Minus One (FMO) • Autofluorescence Controls • Optimized Isotypic Controls • Hardware – Digital Electronics: Eliminate Analog Artifacts • Software: – Automate compensation – Enhance graphical user interface
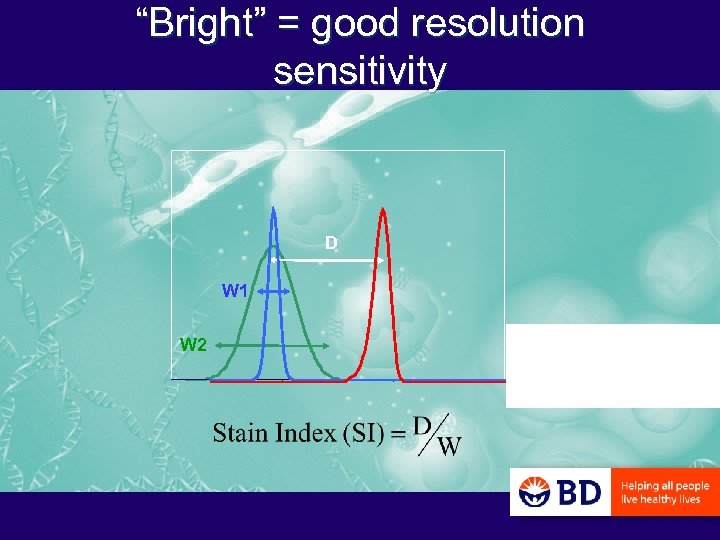
“Bright” = good resolution sensitivity D W 1 W 2
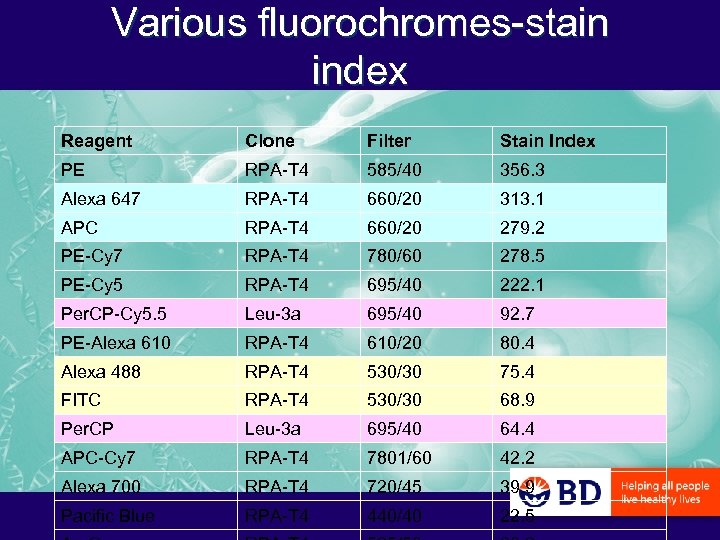
Various fluorochromes-stain index Reagent Clone Filter Stain Index PE RPA-T 4 585/40 356. 3 Alexa 647 RPA-T 4 660/20 313. 1 APC RPA-T 4 660/20 279. 2 PE-Cy 7 RPA-T 4 780/60 278. 5 PE-Cy 5 RPA-T 4 695/40 222. 1 Per. CP-Cy 5. 5 Leu-3 a 695/40 92. 7 PE-Alexa 610 RPA-T 4 610/20 80. 4 Alexa 488 RPA-T 4 530/30 75. 4 FITC RPA-T 4 530/30 68. 9 Per. CP Leu-3 a 695/40 64. 4 APC-Cy 7 RPA-T 4 7801/60 42. 2 Alexa 700 RPA-T 4 720/45 39. 9 Pacific Blue RPA-T 4 440/40 22. 5
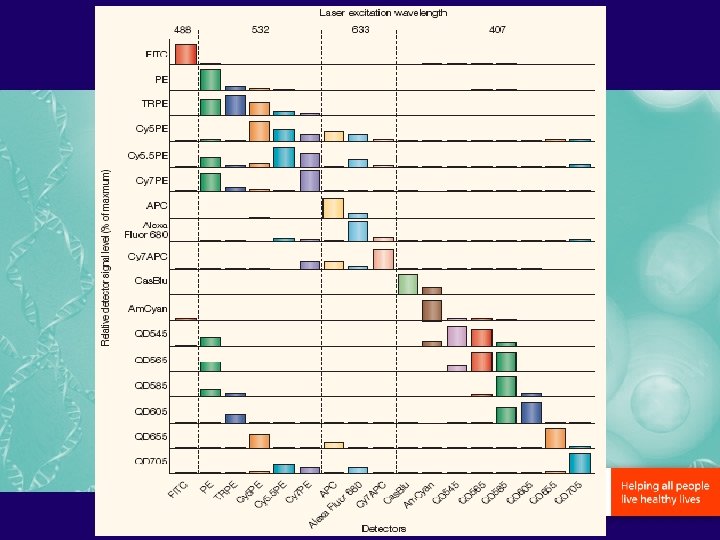
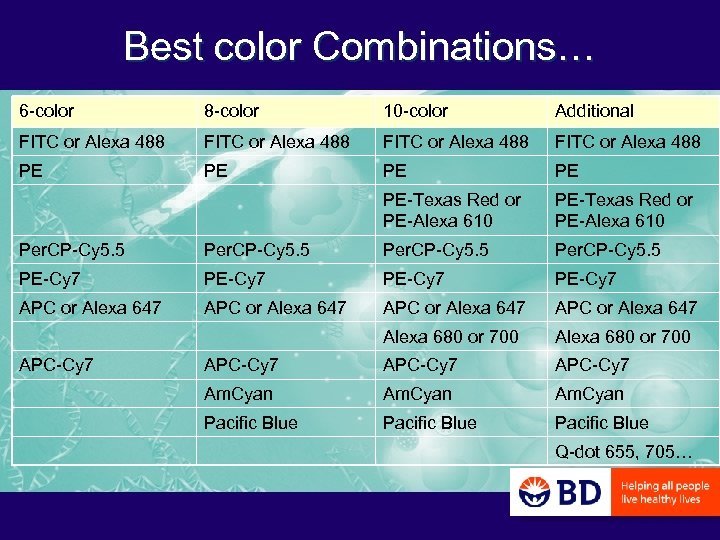
Best color Combinations… 6 -color 8 -color 10 -color Additional FITC or Alexa 488 PE PE PE-Texas Red or PE-Alexa 610 Per. CP-Cy 5. 5 PE-Cy 7 APC or Alexa 647 Alexa 680 or 700 APC-Cy 7 Am. Cyan Pacific Blue APC-Cy 7 Q-dot 655, 705…
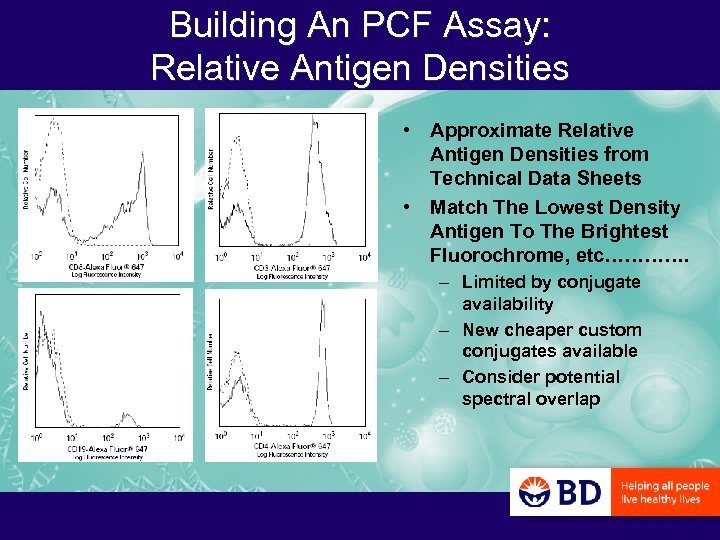
Building An PCF Assay: Relative Antigen Densities • Approximate Relative Antigen Densities from Technical Data Sheets • Match The Lowest Density Antigen To The Brightest Fluorochrome, etc…………. – Limited by conjugate availability – New cheaper custom conjugates available – Consider potential spectral overlap

Building An PCF Assay: Laser Choices Optimize Your PCF Assay By: • Using Multiple Laser Lines • Don’t “Pack” A Laser Line • Choose “Optimal” Laser/Fluorochrome Combinations: – To Minimize Spillover Background – To Optimize Signal : Noise • Optimize Filter Choices to Minimize Spillover – Use JAVA Applet on BD Website

Building An PCF Assay: Effect of Spillover on Double Stained Cells CD 45 -FITC Dim CD 4 -PE CD 45 - Per. CP Dim CD 4 -PE Compensated analog data: CD 45 FITC makes dim CD 4 difficult to measure due to FITC spillover into PE and resultant “spread” Compensated analog data: CD 45 Per. CP allows same dim CD 4 cells to be separated from bkg. – little spillover into PE

Top 4 Sources of Problems In Multi-Color Analyses 1. ) Compensation 2. ) Compensation 3. ) Compensation 4. ) Compensation
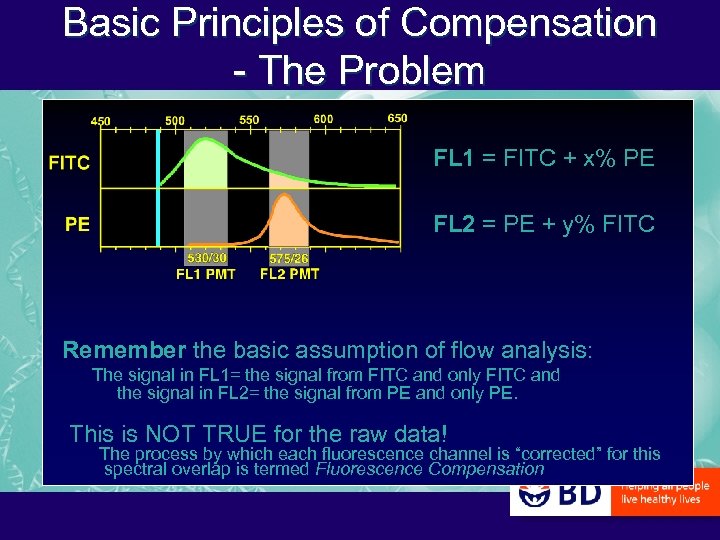
Basic Principles of Compensation - The Problem FL 1 = FITC + x% PE FL 2 = PE + y% FITC Remember the basic assumption of flow analysis: The signal in FL 1= the signal from FITC and only FITC and the signal in FL 2= the signal from PE and only PE. This is NOT TRUE for the raw data! The process by which each fluorescence channel is “corrected” for this spectral overlap is termed Fluorescence Compensation
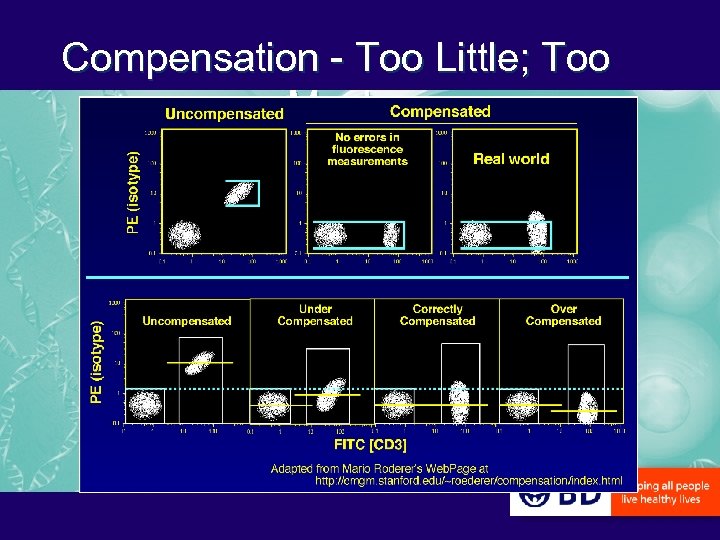
Compensation - Too Little; Too Much
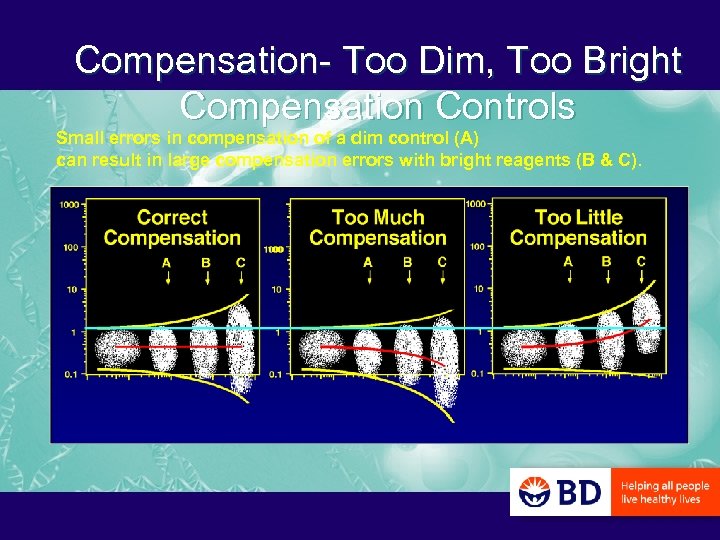
Compensation- Too Dim, Too Bright Compensation Controls Small errors in compensation of a dim control (A) can result in large compensation errors with bright reagents (B & C).
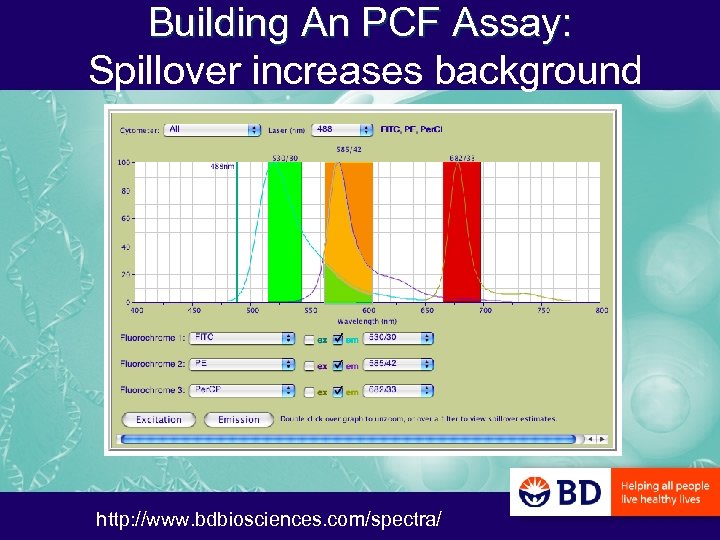
Building An PCF Assay: Spillover increases background http: //www. bdbiosciences. com/spectra/
Building An PCF Assay: FMO (Fluorescence Minus One) • Compensated data exhibits spread • Bright single positives may change threshold levels between dim and background in other dimensions • Use where autofluorescence and/or isotypic controls are NOT useful for determining threshold over background • The best control is one stained with all reagents except the one of interest

Building An PCF Assay: FMO (Fluorescence Minus One) PBMC were stained as shown in a 4 -color experiment. Compensation was properly set for all spillovers Courtesy Mario Roederer
Setting Up For A PCF Assay: Compensation: BDTM Comp. Beads • Three Specificities – Anti-mouse Ig, kappa – Anti-rat/hamster Ig, kappa • Negative Control Bead • Supplied in sets: Positive & Negative Bead • Stain with reagents used for PCF Assay – Optimal Spillover Control – 50% positive/50% negative Control

Setting Up For A PCF Assay: Compensation: BDTM Comp. Beads Method: For Each Conjugate: 1. Add 1 drop (60 ul) of positive bead and 1 drop of negative bead to 100 ul of staining buffer in a tube or well 2. Add optimally titered antibody 3. Incubate 15 -30 minutes RT 4. Wash with staining buffer 5. Resuspend pellet in staining buffer 6. Run according to instructions for automated spillover algorithm
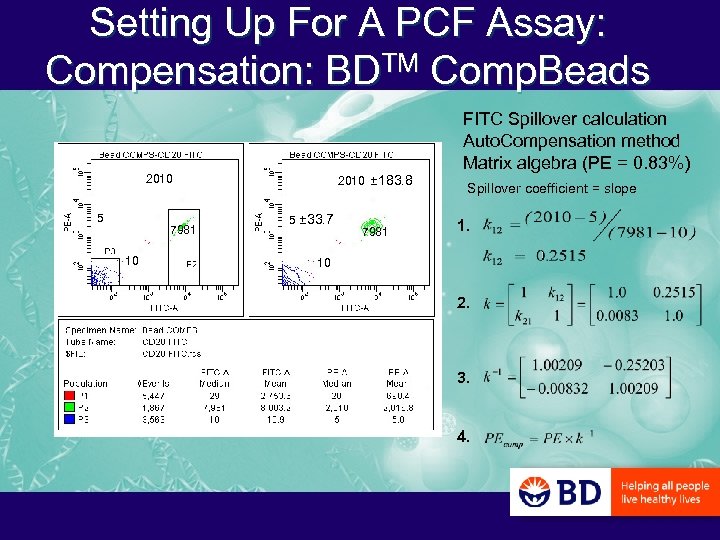
Setting Up For A PCF Assay: Compensation: BDTM Comp. Beads 2010 ± 183. 8 2010 5 7981 10 5 ± 33. 7 7981 FITC Spillover calculation Auto. Compensation method Matrix algebra (PE = 0. 83%) Spillover coefficient = slope 1. 10 2. 3. 4.
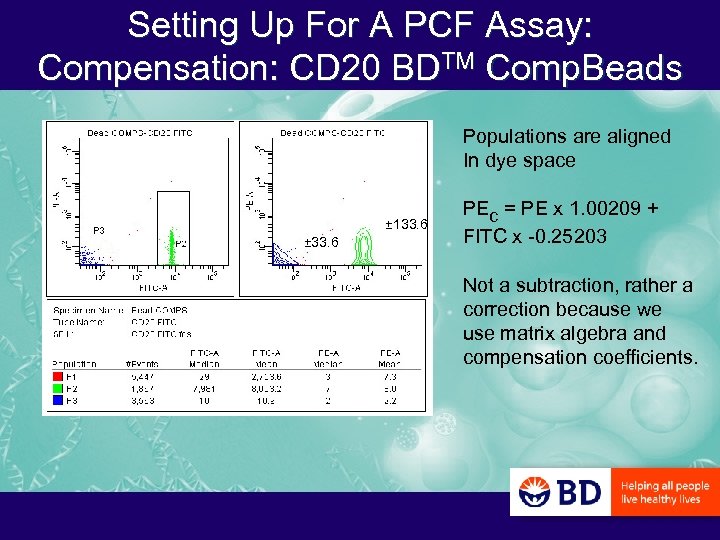
Setting Up For A PCF Assay: Compensation: CD 20 BDTM Comp. Beads Populations are aligned In dye space ± 133. 6 ± 33. 6 PEc = PE x 1. 00209 + FITC x -0. 25203 Not a subtraction, rather a correction because we use matrix algebra and compensation coefficients.
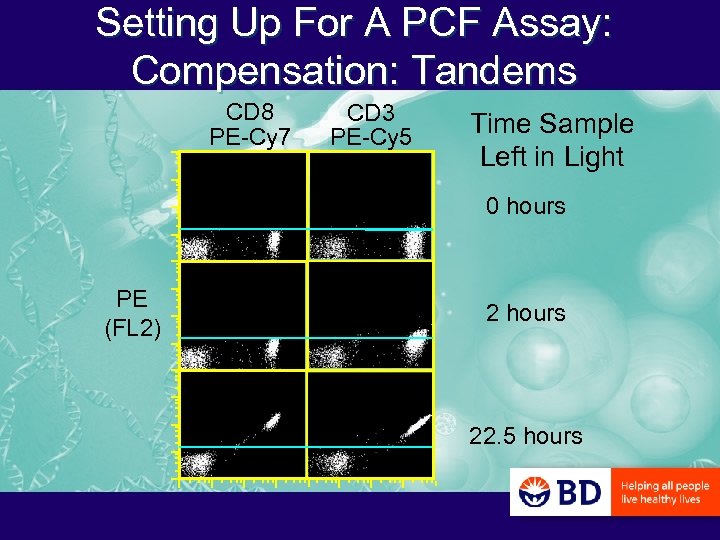
Setting Up For A PCF Assay: Compensation: Tandems CD 8 PE-Cy 7 CD 3 PE-Cy 5 Time Sample Left in Light 0 hours PE (FL 2) 2 hours 22. 5 hours
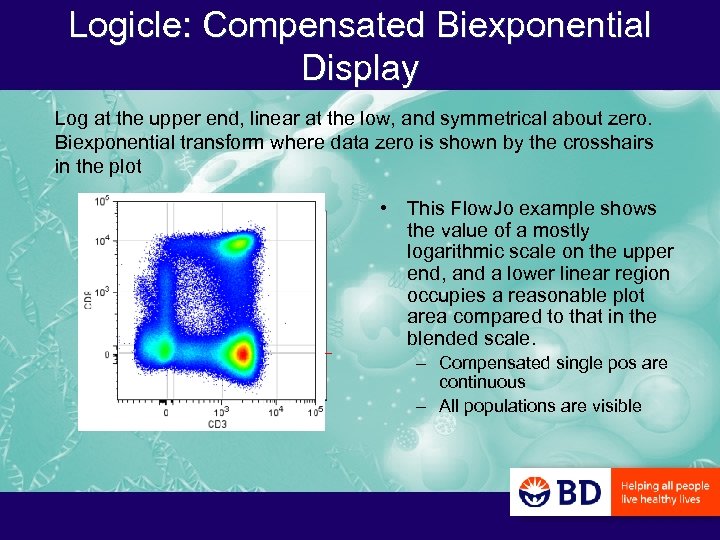
Logicle: Compensated Biexponential Display Log at the upper end, linear at the low, and symmetrical about zero. Biexponential transform where data zero is shown by the crosshairs in the plot • This Flow. Jo example shows the value of a mostly logarithmic scale on the upper end, and a lower linear region occupies a reasonable plot area compared to that in the blended scale. – Compensated single pos are continuous – All populations are visible
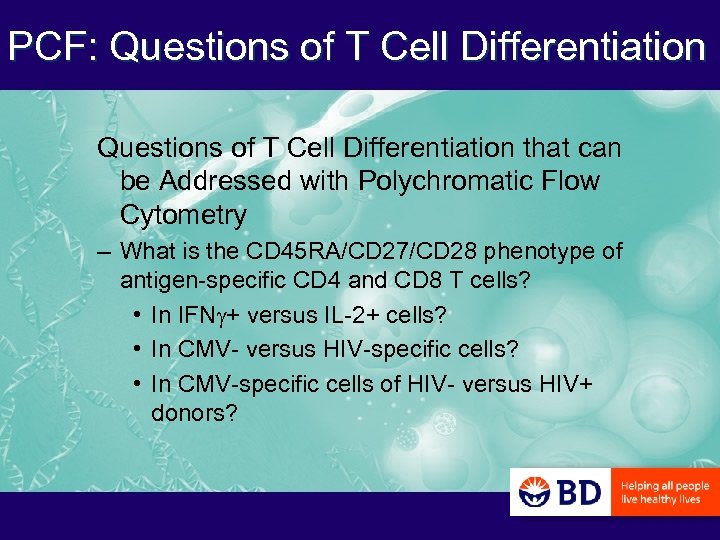
PCF: Questions of T Cell Differentiation that can be Addressed with Polychromatic Flow Cytometry – What is the CD 45 RA/CD 27/CD 28 phenotype of antigen-specific CD 4 and CD 8 T cells? • In IFNg+ versus IL-2+ cells? • In CMV- versus HIV-specific cells? • In CMV-specific cells of HIV- versus HIV+ donors?
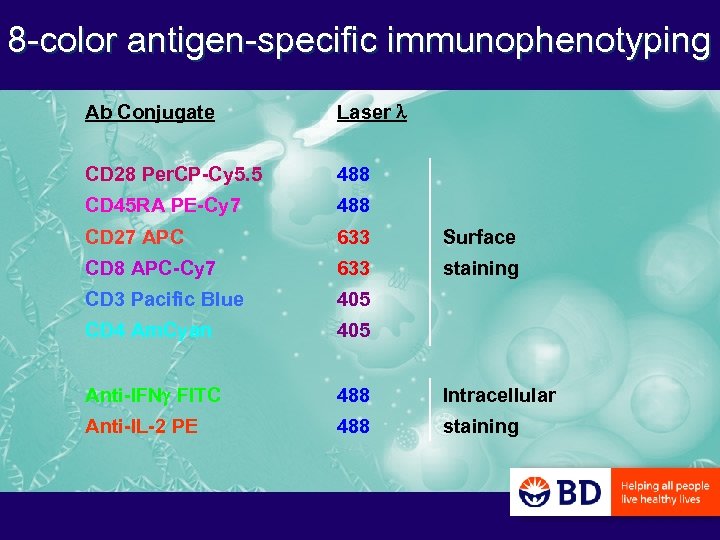
8 -color antigen-specific immunophenotyping Ab Conjugate Laser l CD 28 Per. CP-Cy 5. 5 488 CD 45 RA PE-Cy 7 488 CD 27 APC 633 Surface CD 8 APC-Cy 7 633 staining CD 3 Pacific Blue 405 CD 4 Am. Cyan 405 Anti-IFNg FITC 488 Intracellular Anti-IL-2 PE 488 staining
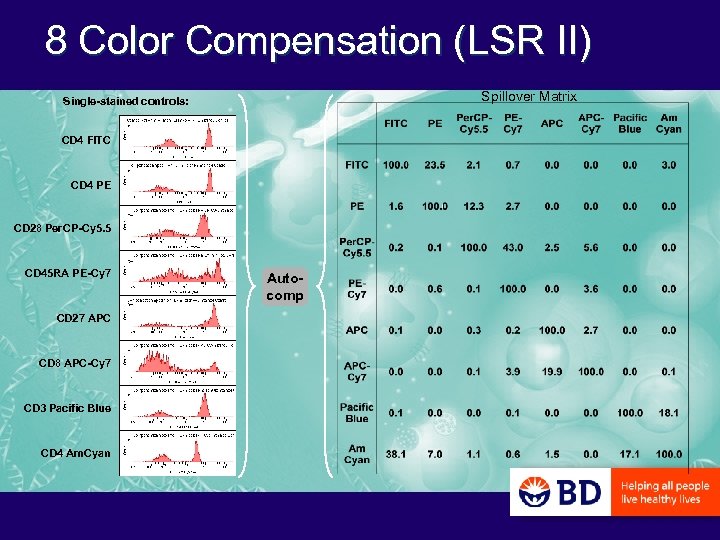
8 Color Compensation (LSR II) Spillover Matrix Single-stained controls: CD 4 FITC CD 4 PE CD 28 Per. CP-Cy 5. 5 CD 45 RA PE-Cy 7 CD 27 APC CD 8 APC-Cy 7 CD 3 Pacific Blue CD 4 Am. Cyan Autocomp
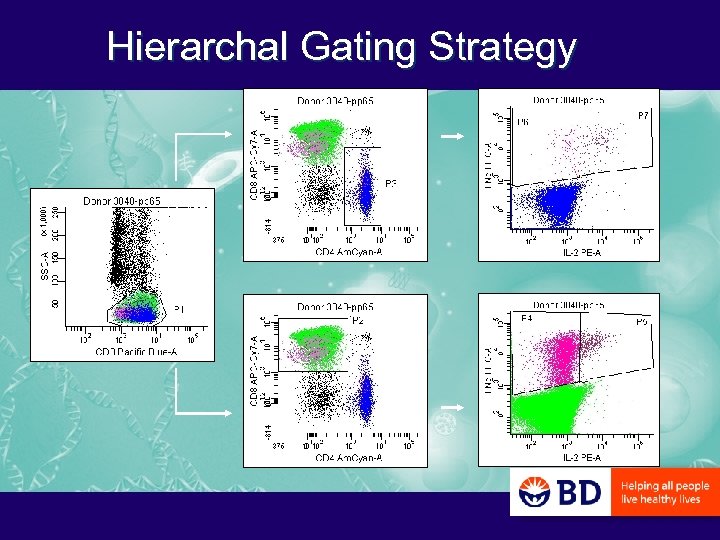
Hierarchal Gating Strategy
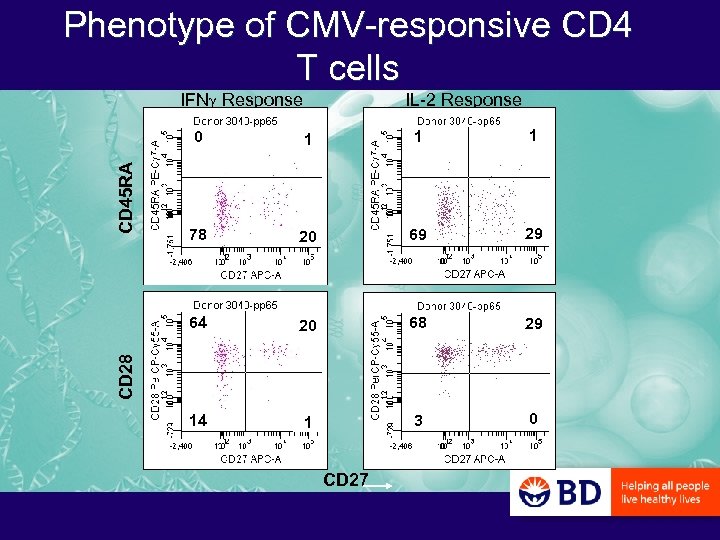
Phenotype of CMV-responsive CD 4 T cells IFNg Response IL-2 Response 1 1 78 20 69 29 20 68 29 14 1 3 0 CD 28 1 64 CD 45 RA 0 CD 27
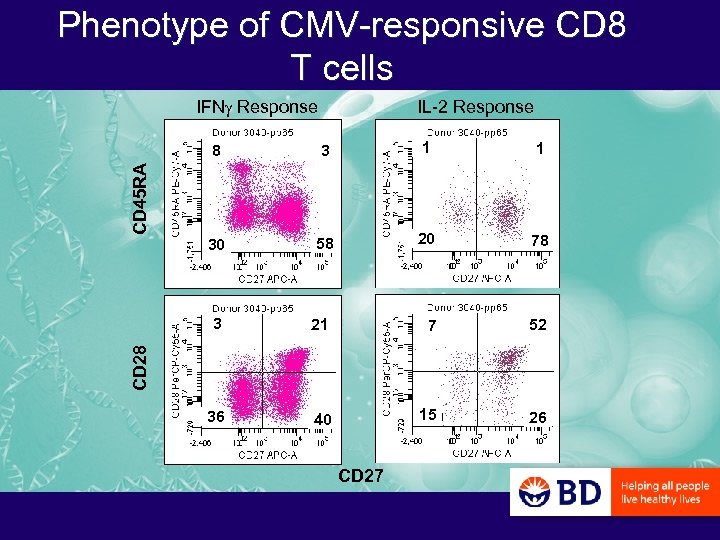
Phenotype of CMV-responsive CD 8 T cells IFNg Response IL-2 Response 3 1 1 30 58 20 78 CD 45 RA 8 21 7 52 36 40 15 26 CD 28 3 CD 27
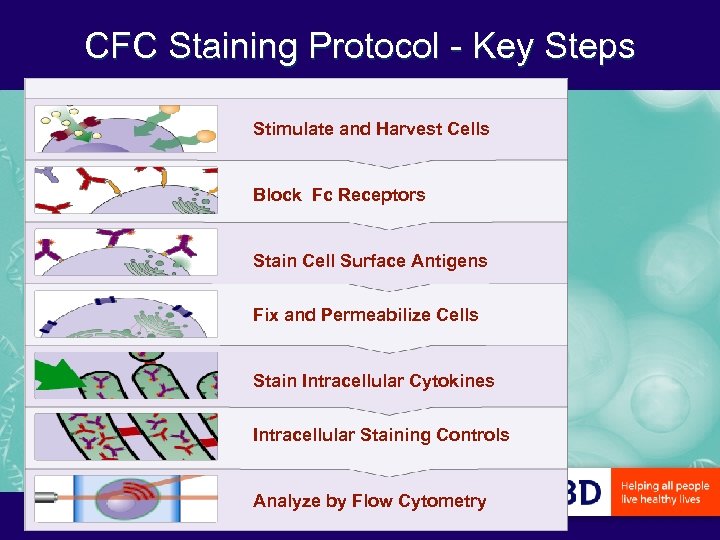
CFC Staining Protocol - Key Steps Stimulate and Harvest Cells Block Fc Receptors Stain Cell Surface Antigens Fix and Permeabilize Cells Stain Intracellular Cytokines Intracellular Staining Controls Analyze by Flow Cytometry
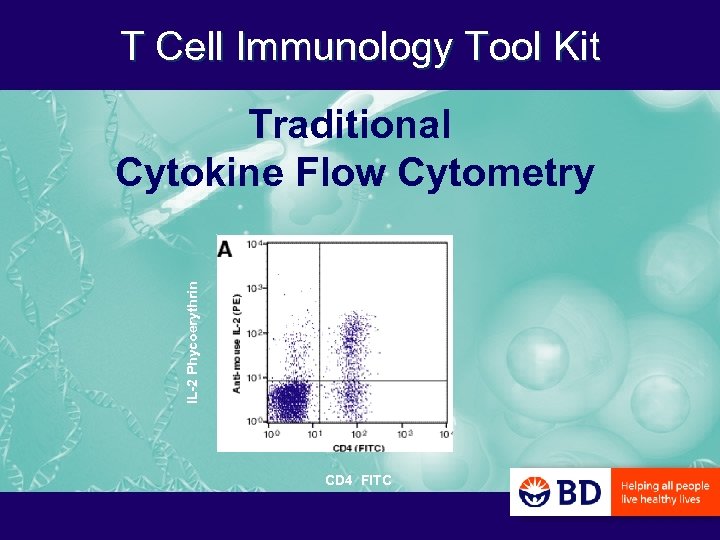
T Cell Immunology Tool Kit IL-2 Phycoerythrin Traditional Cytokine Flow Cytometry CD 4 FITC
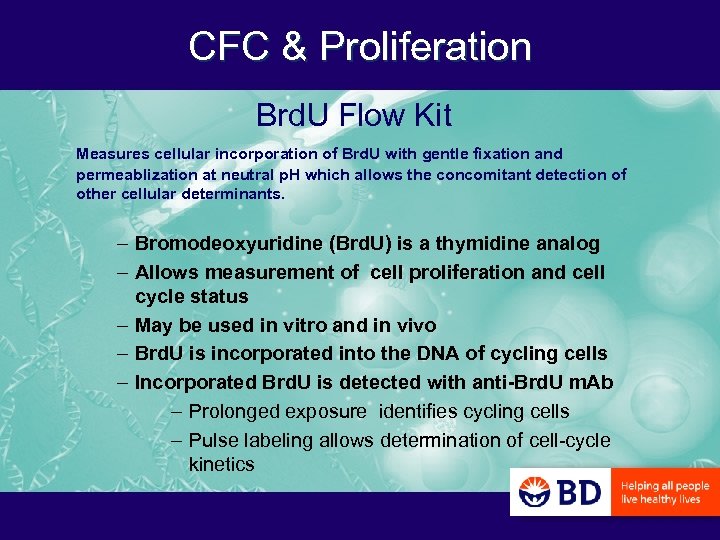
CFC & Proliferation Brd. U Flow Kit Measures cellular incorporation of Brd. U with gentle fixation and permeablization at neutral p. H which allows the concomitant detection of other cellular determinants. – Bromodeoxyuridine (Brd. U) is a thymidine analog – Allows measurement of cell proliferation and cell cycle status – May be used in vitro and in vivo – Brd. U is incorporated into the DNA of cycling cells – Incorporated Brd. U is detected with anti-Brd. U m. Ab – Prolonged exposure identifies cycling cells – Pulse labeling allows determination of cell-cycle kinetics
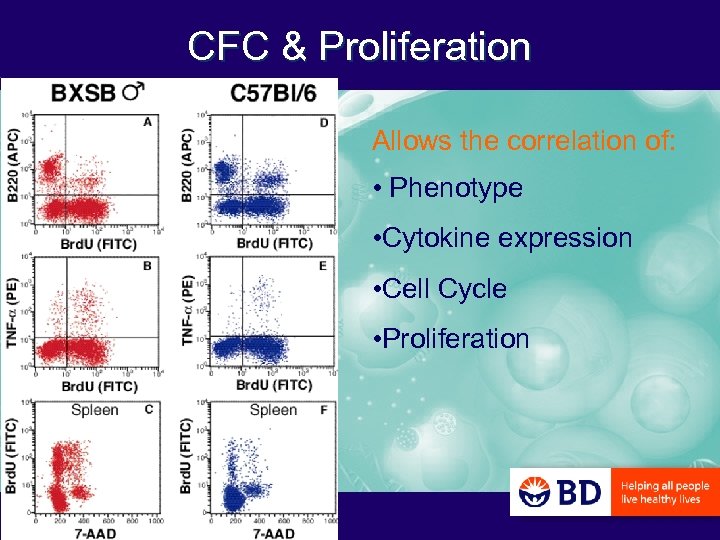
CFC & Proliferation Allows the correlation of: • Phenotype • Cytokine expression • Cell Cycle • Proliferation
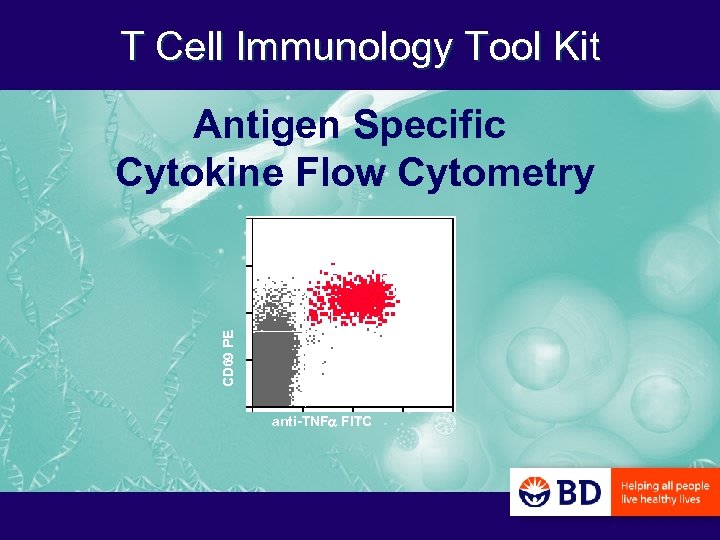
T Cell Immunology Tool Kit CD 69 PE Antigen Specific Cytokine Flow Cytometry anti-TNF FITC
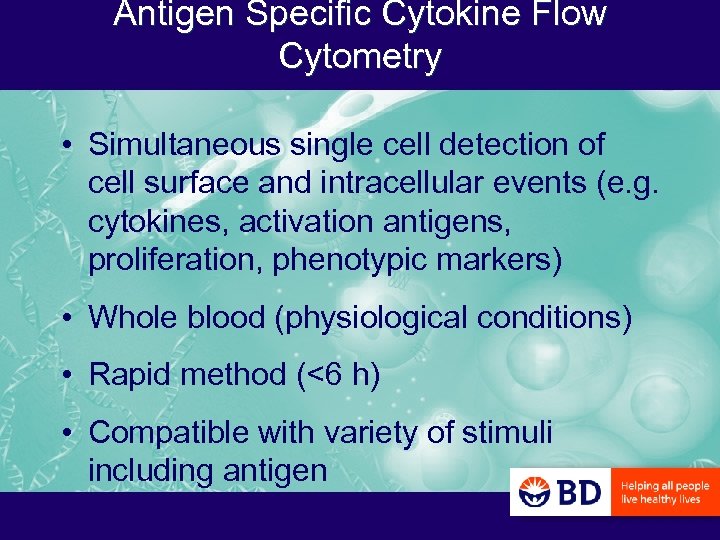
Antigen Specific Cytokine Flow Cytometry • Simultaneous single cell detection of cell surface and intracellular events (e. g. cytokines, activation antigens, proliferation, phenotypic markers) • Whole blood (physiological conditions) • Rapid method (<6 h) • Compatible with variety of stimuli including antigen
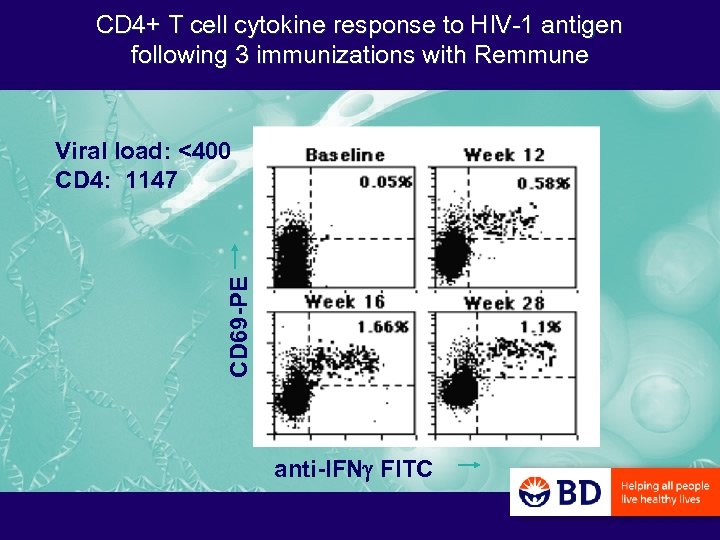
CD 4+ T cell cytokine response to HIV-1 antigen following 3 immunizations with Remmune CD 69 -PE Viral load: <400 CD 4: 1147 anti-IFNg FITC
What is a Cytometric Bead Array (CBA) ELISA =
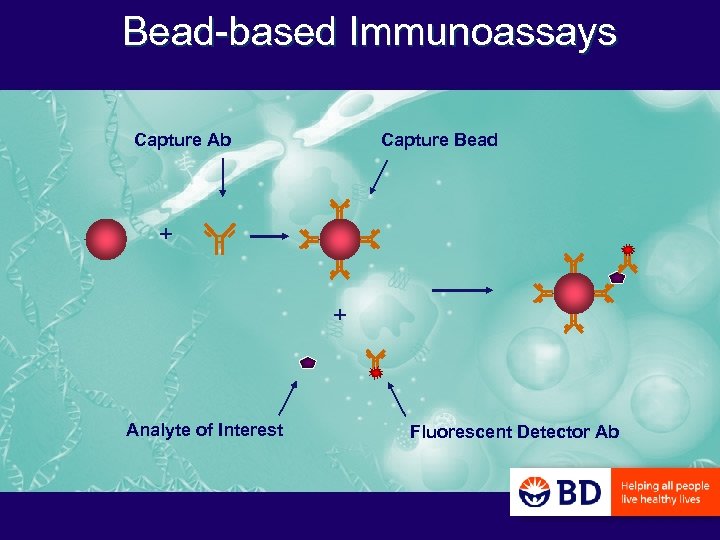
Bead-based Immunoassays Capture Ab Capture Bead + + Analyte of Interest Fluorescent Detector Ab

Beads and Flow Cytometry – A Powerful Tool IL-8 103 FL 3 (670 LP) + Bead Intensity 104 IL-6 102 101 100 101 102 103 104 Detector Ab Intensity FL 2 (585/42 BP)
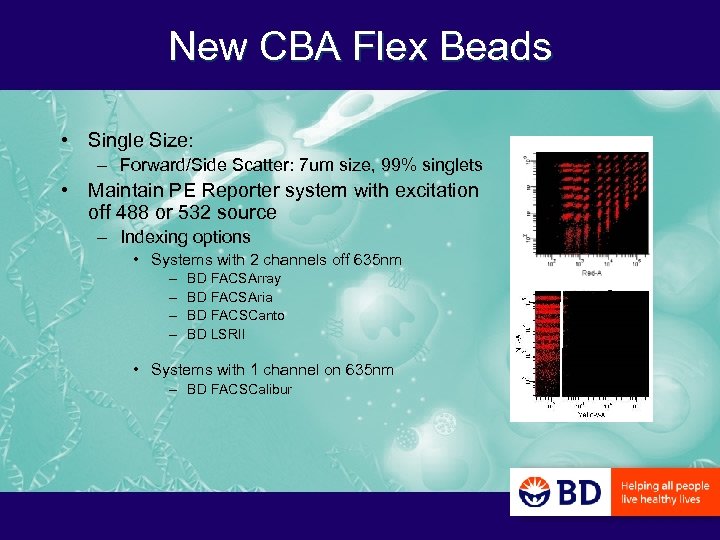
New CBA Flex Beads • Single Size: – Forward/Side Scatter: 7 um size, 99% singlets • Maintain PE Reporter system with excitation off 488 or 532 source – Indexing options • Systems with 2 channels off 635 nm – – BD FACSArray BD FACSAria BD FACSCanto BD LSRII • Systems with 1 channel on 635 nm – BD FACSCalibur A B C D E F G H I 1 2 3 45 6 7 8 9
Plex definition: clustering Double click or drag to assign
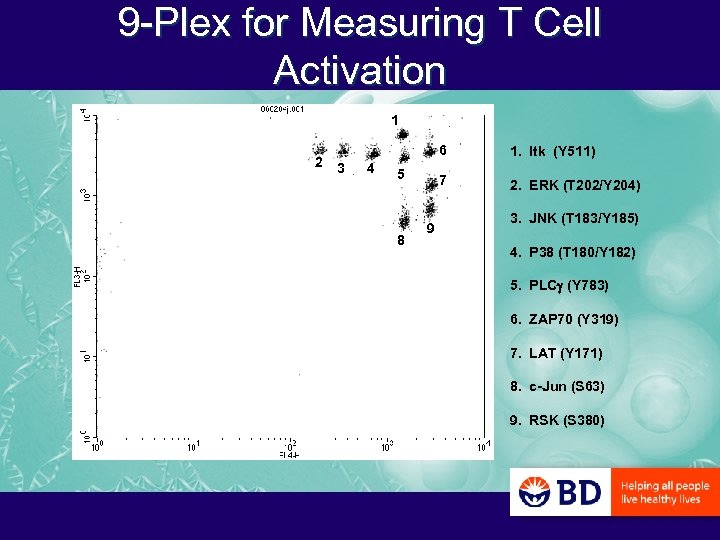
9 -Plex for Measuring T Cell Activation 1 2 6 3 4 7 5 8 9 1. Itk (Y 511) 2. ERK (T 202/Y 204) 3. JNK (T 183/Y 185) 4. P 38 (T 180/Y 182) 5. PLCg (Y 783) 6. ZAP 70 (Y 319) 7. LAT (Y 171) 8. c-Jun (S 63) 9. RSK (S 380)
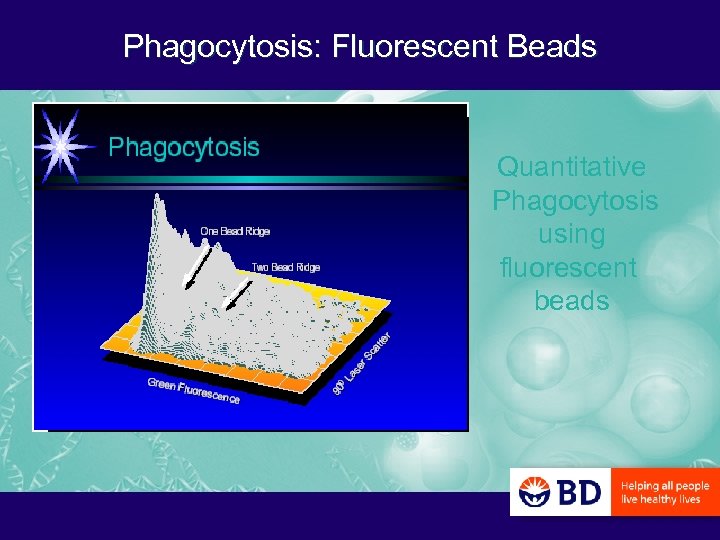
Phagocytosis: Fluorescent Beads Quantitative Phagocytosis using fluorescent beads
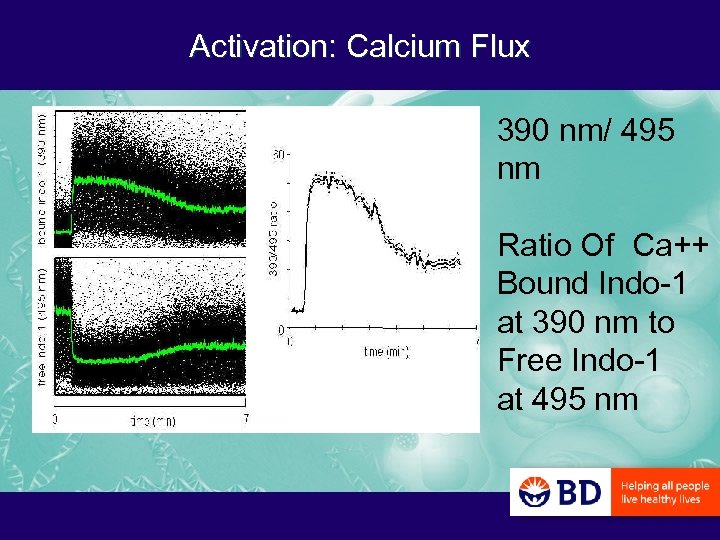
Activation: Calcium Flux 390 nm/ 495 nm Ratio Of Ca++ Bound Indo-1 at 390 nm to Free Indo-1 at 495 nm
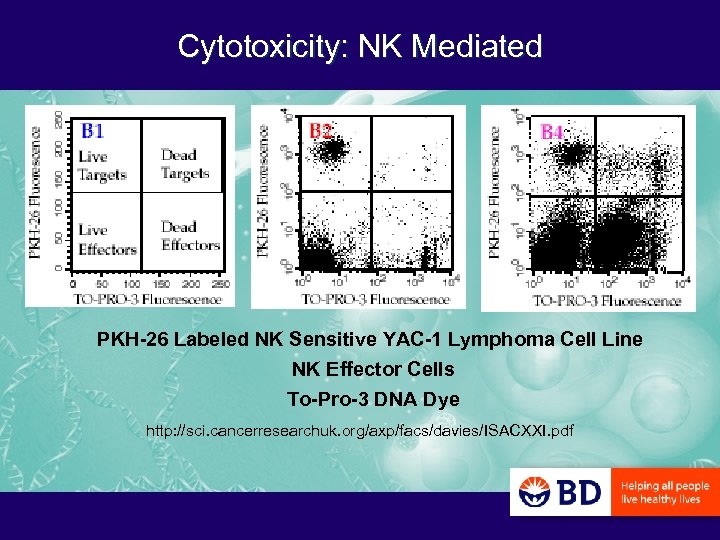
Cytotoxicity: NK Mediated PKH-26 Labeled NK Sensitive YAC-1 Lymphoma Cell Line NK Effector Cells To-Pro-3 DNA Dye http: //sci. cancerresearchuk. org/axp/facs/davies/ISACXXI. pdf
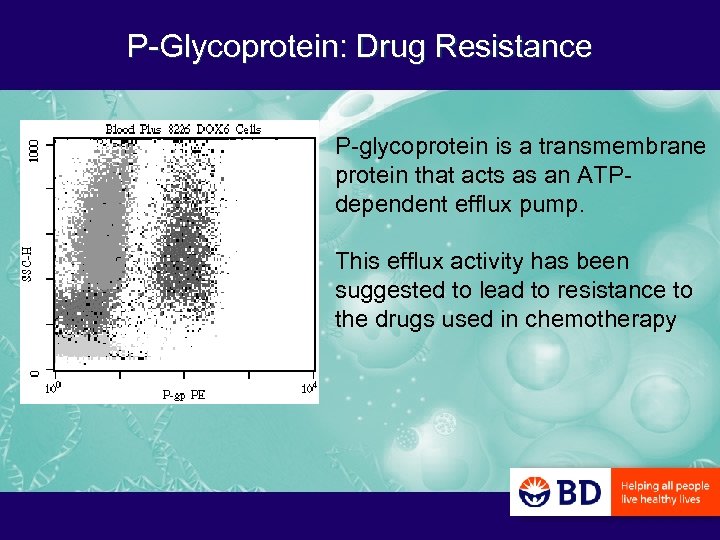
P-Glycoprotein: Drug Resistance P-glycoprotein is a transmembrane protein that acts as an ATPdependent efflux pump. This efflux activity has been suggested to lead to resistance to the drugs used in chemotherapy
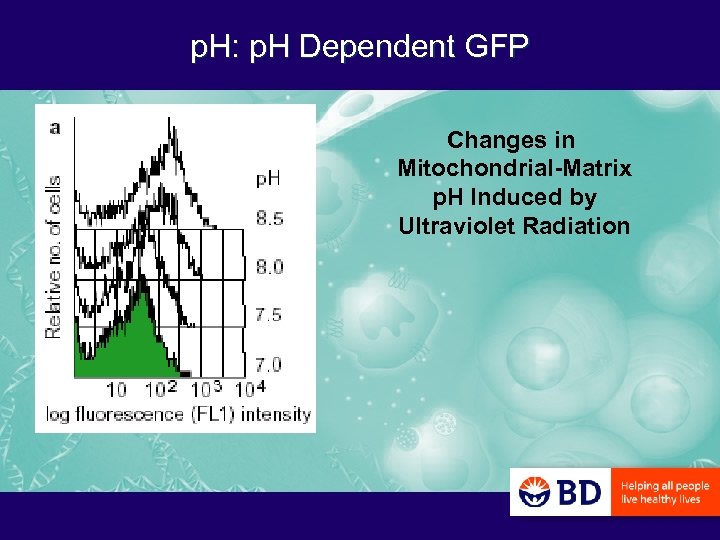
p. H: p. H Dependent GFP Changes in Mitochondrial-Matrix p. H Induced by Ultraviolet Radiation
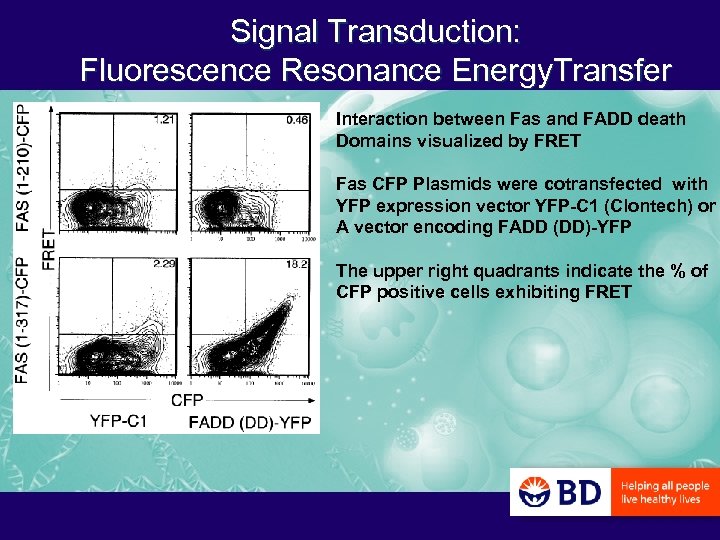
Signal Transduction: Fluorescence Resonance Energy. Transfer Interaction between Fas and FADD death Domains visualized by FRET Fas CFP Plasmids were cotransfected with YFP expression vector YFP-C 1 (Clontech) or A vector encoding FADD (DD)-YFP The upper right quadrants indicate the % of CFP positive cells exhibiting FRET
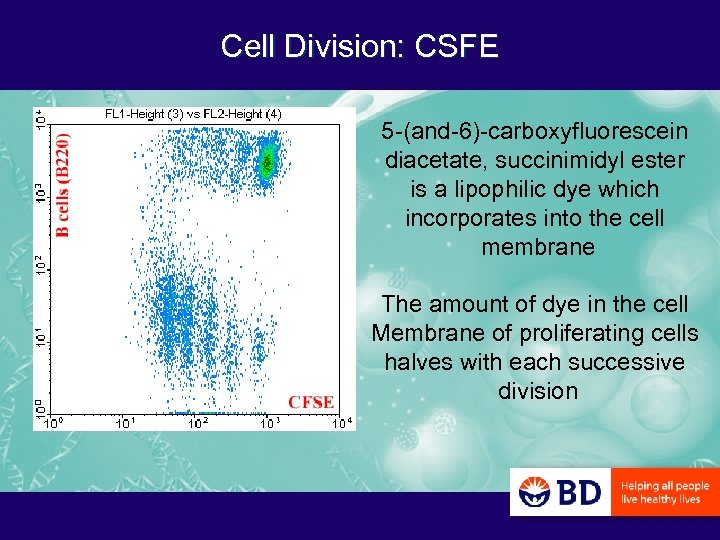
Cell Division: CSFE 5 -(and-6)-carboxyfluorescein diacetate, succinimidyl ester is a lipophilic dye which incorporates into the cell membrane The amount of dye in the cell Membrane of proliferating cells halves with each successive division
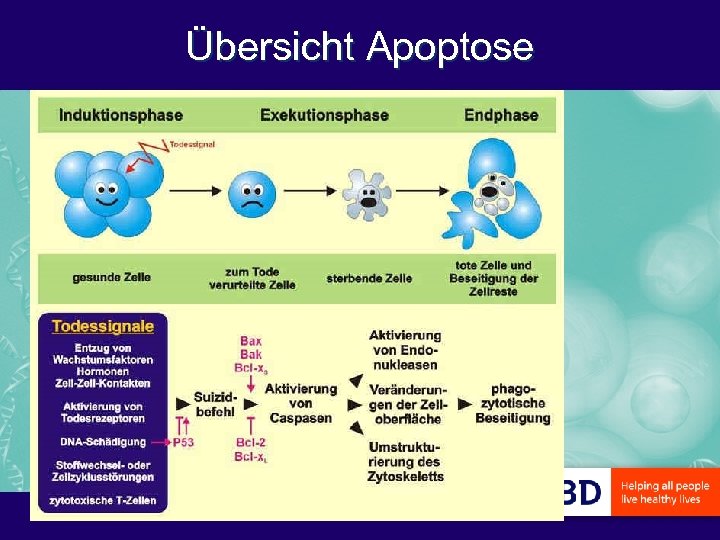
Übersicht Apoptose
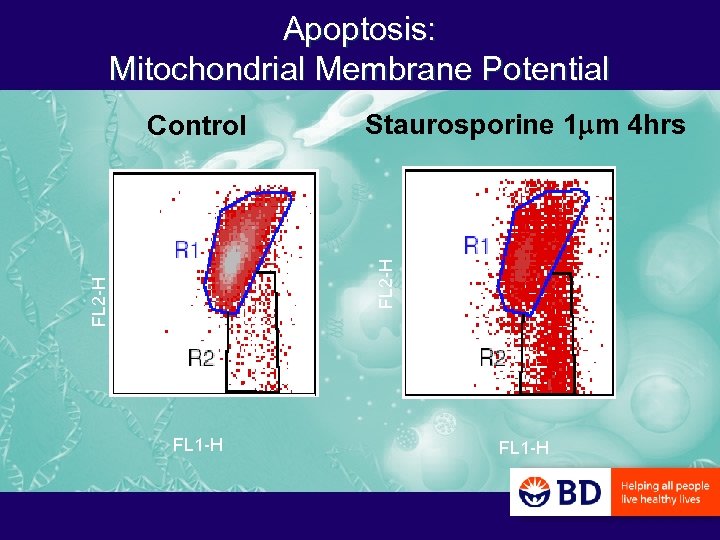
Apoptosis: Mitochondrial Membrane Potential Staurosporine 1 mm 4 hrs FL 2 -H Control FL 1 -H
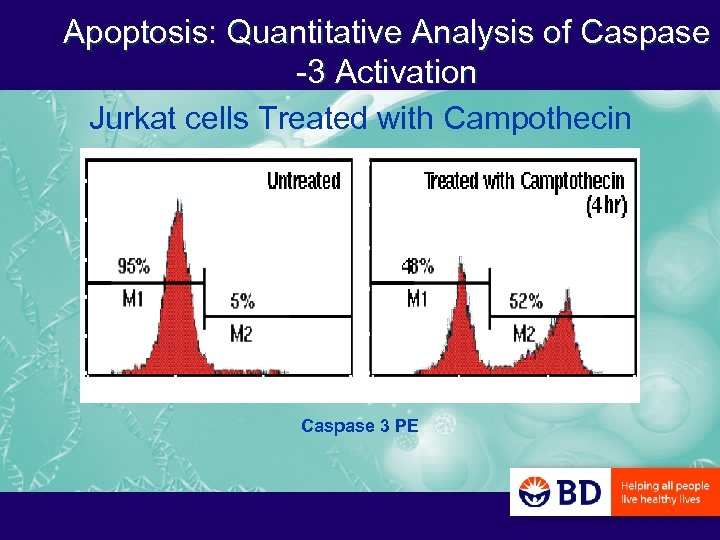
Apoptosis: Quantitative Analysis of Caspase -3 Activation Jurkat cells Treated with Campothecin Caspase 3 PE
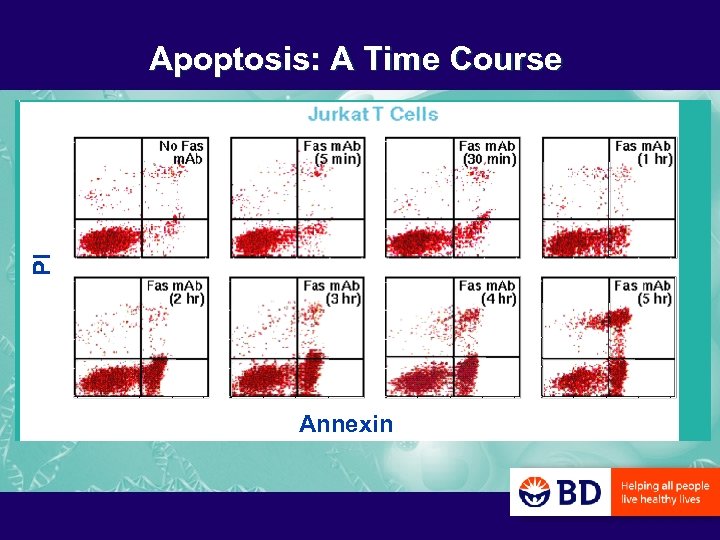
PI Apoptosis: A Time Course Annexin
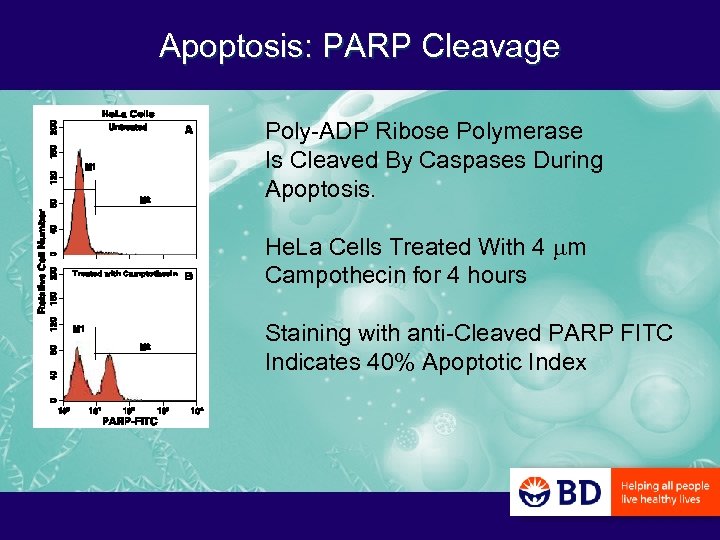
Apoptosis: PARP Cleavage Poly-ADP Ribose Polymerase Is Cleaved By Caspases During Apoptosis. He. La Cells Treated With 4 mm Campothecin for 4 hours Staining with anti-Cleaved PARP FITC Indicates 40% Apoptotic Index
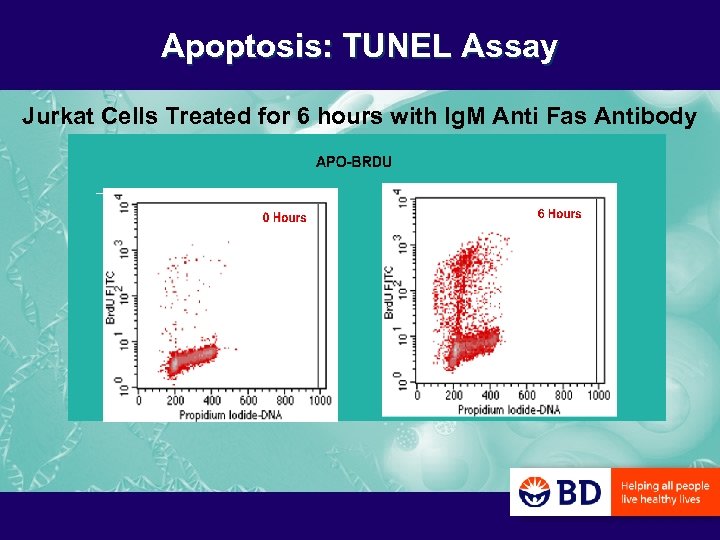
Apoptosis: TUNEL Assay Jurkat Cells Treated for 6 hours with Ig. M Anti Fas Antibody
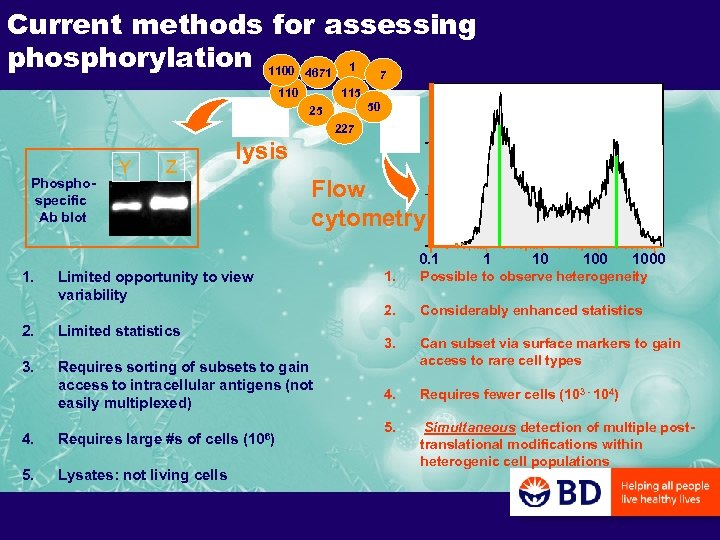
Current methods for assessing phosphorylation 1100 4671 1 7 110 115 50 25 227 Phospecific Ab blot Y Z lysis Flow cytometry 0. 1 1. Limited opportunity to view variability 2. Limited statistics 3. Requires sorting of subsets to gain access to intracellular antigens (not easily multiplexed) 4. Requires large #s of cells (106) 5. Lysates: not living cells 1 10 1000 1. Possible to observe heterogeneity 2. Considerably enhanced statistics 3. Can subset via surface markers to gain access to rare cell types 4. Requires fewer cells (103 - 104) 5. Simultaneous detection of multiple posttranslational modifications within heterogenic cell populations
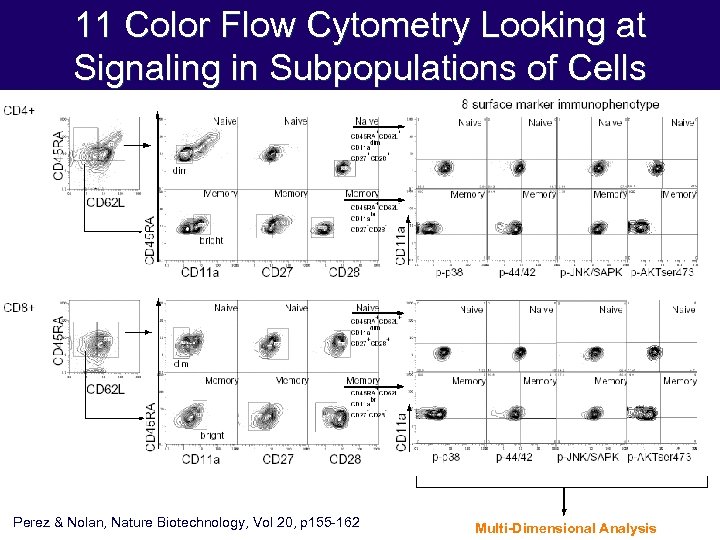
11 Color Flow Cytometry Looking at Signaling in Subpopulations of Cells Perez & Nolan, Nature Biotechnology, Vol 20, p 155 -162 Multi-Dimensional Analysis
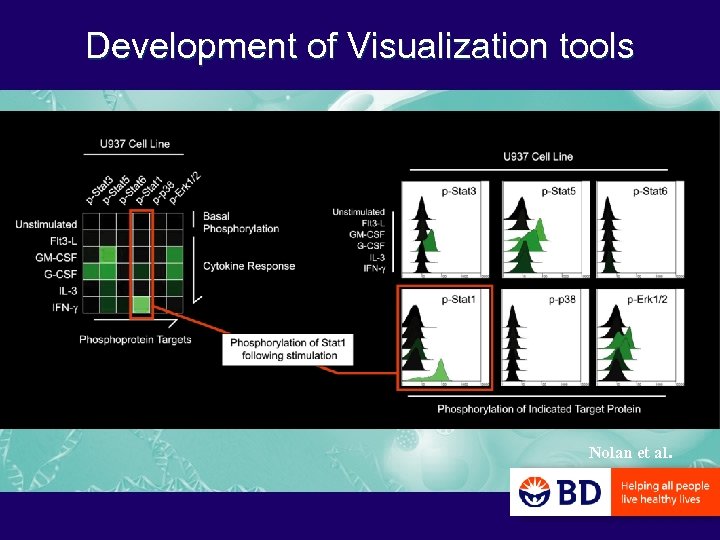
Development of Visualization tools Nolan et al.
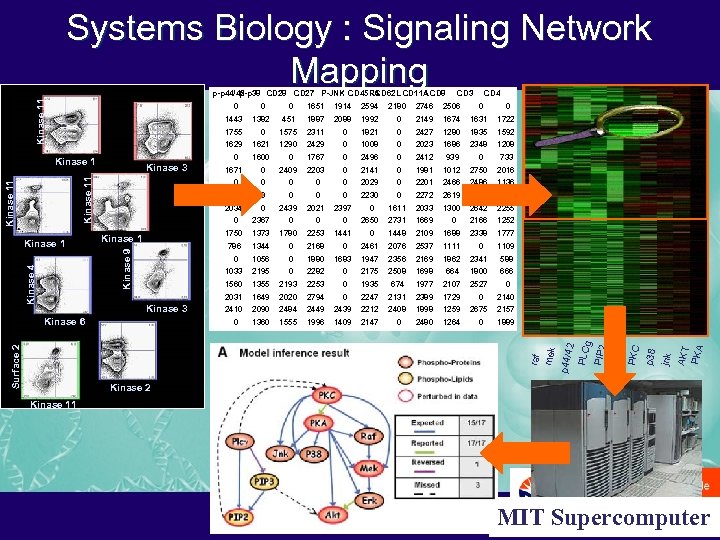
Systems Biology : Signaling Network Mapping 0 1443 1755 1629 1600 2594 451 1887 2088 1992 1575 2311 0 1290 2429 0 1767 2203 0 0 2149 1674 1631 1722 1821 0 2427 1280 1835 1592 1008 0 2023 1686 2348 1208 0 2496 0 2412 939 0 0 733 0 2141 0 1981 1012 2750 2016 0 2029 0 2201 2466 2486 1136 0 0 2230 0 2272 2619 2471 1023 1611 2033 1300 2642 2255 2731 1669 2166 1252 2338 1777 2367 2439 0 0 1056 0 1880 1033 2195 0 2282 1560 1355 2193 1649 2410 1441 1448 2109 1688 2461 2076 2537 1111 1947 2356 2169 1862 2341 588 0 2175 2508 1698 664 1800 666 2253 0 1935 674 1977 2107 2527 2020 2794 0 2247 2131 2389 1729 2090 2484 2449 2439 2212 2408 1898 1259 1360 1555 1996 1409 2147 2480 1264 0 0 2675 0 1109 0 2140 2157 1889 raf mek 1683 0 0 AKT PKA 2168 0 2253 2650 jnk 0 0 0 p 38 1344 0 Kinase 6 1373 786 2397 PKC 1750 Kinase 1 1780 2021 PIP 3 0 Kinase 9 Kinase 4 0 0 Kinase 3 Surface 2 CD 4 2506 0 2031 Kinase 1 CD 3 2746 0 0 2409 2180 0 2034 0 1914 0 Kinase 11 1671 0 1621 1651 PIP 2 Kinase 3 1382 0 2 PLCg Kinase 1 0 0 p 44/4 Kinase 11 p-p 44/42 p-p 38 CD 27 P-JNK CD 45 RA CD 62 L CD 11 A CD 8 Kinase 2 Kinase 11 MIT Supercomputer
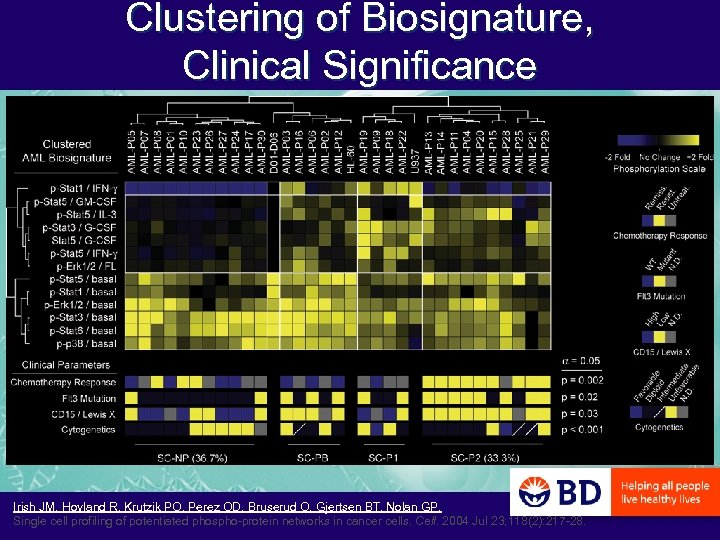
Clustering of Biosignature, Clinical Significance Irish JM, Hovland R, Krutzik PO, Perez OD, Bruserud O, Gjertsen BT, Nolan GP. Single cell profiling of potentiated phospho-protein networks in cancer cells. Cell. 2004 Jul 23; 118(2): 217 -28.

• Thank You for your time
9ef66f0ba4449e81cdc6f94598240004.ppt