0844ef4fb7a9f4c719a9bc152d113f4f.ppt
- Количество слайдов: 27
 PLETHICO PHARMACEUTICALS LIMITED
PLETHICO PHARMACEUTICALS LIMITED
 Agenda 1. Company Overview 2. Expansion Project & Status 3. Growth Strategy 4. Journey Ahead 5. Summary 2
Agenda 1. Company Overview 2. Expansion Project & Status 3. Growth Strategy 4. Journey Ahead 5. Summary 2
 Company Overview 3
Company Overview 3
 Background - Company § Plethico was incorporated in 1991 to undertake manufacture of Formulations and undertake focused marketing of ethical/ prescriptions market. § Focused on innovation and pioneered introduction of: î Doxycycline based unique anti-biotic formulation î Co-trimoxazole based unique anti-bacterial formulation § Introduced for the first time in India, novel ayurvedic / herbal preparation (Single Herb Fenugreek extract) § Adopted the “Branded Generic” model for marketing formulations in India & abroad. 4
Background - Company § Plethico was incorporated in 1991 to undertake manufacture of Formulations and undertake focused marketing of ethical/ prescriptions market. § Focused on innovation and pioneered introduction of: î Doxycycline based unique anti-biotic formulation î Co-trimoxazole based unique anti-bacterial formulation § Introduced for the first time in India, novel ayurvedic / herbal preparation (Single Herb Fenugreek extract) § Adopted the “Branded Generic” model for marketing formulations in India & abroad. 4
 Background - Company § TODAY: The company has made it’s presence felt Internationally in niche segments like: î Herbal & Nutraceuticals preparations including Food supplements î OTC / Consumer Products î NDDS 5
Background - Company § TODAY: The company has made it’s presence felt Internationally in niche segments like: î Herbal & Nutraceuticals preparations including Food supplements î OTC / Consumer Products î NDDS 5
 Background - Company § Operations in 45 countries including India § Export targets at the semi-regulated / un-regulated markets of îEastern Europe (including the Common Wealth of Independent States (CIS)) îThe African nations îTHIRD FRONT: · South East Asian (SEA) nations · Gulf Co-operation Council (GCC) · Latin American Countries (LAC) § Over 300 sales representatives and front line-managers are employed across markets including 143 in India. 6
Background - Company § Operations in 45 countries including India § Export targets at the semi-regulated / un-regulated markets of îEastern Europe (including the Common Wealth of Independent States (CIS)) îThe African nations îTHIRD FRONT: · South East Asian (SEA) nations · Gulf Co-operation Council (GCC) · Latin American Countries (LAC) § Over 300 sales representatives and front line-managers are employed across markets including 143 in India. 6
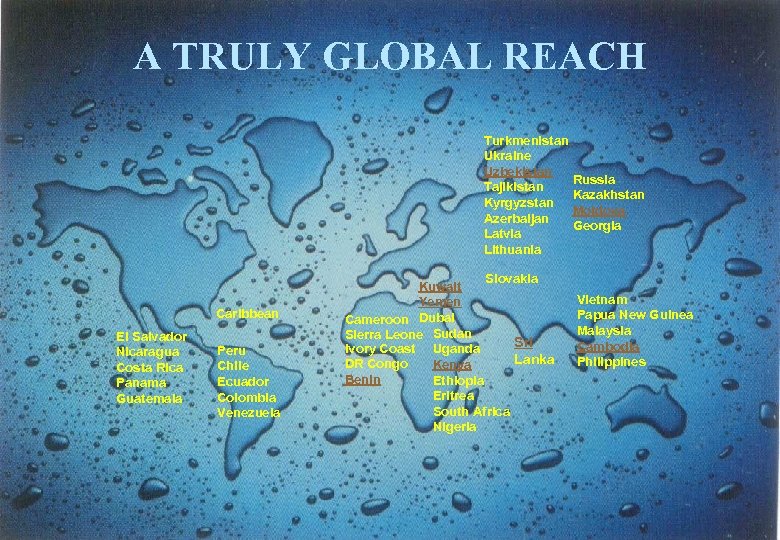 A TRULY GLOBAL REACH Turkmenistan Ukraine Uzbekistan Russia Tajikistan Kazakhstan Kyrgyzstan Moldova Azerbaijan Georgia Latvia Lithuania Caribbean El Salvador Nicaragua Costa Rica Panama Guatemala Peru Chile Ecuador Colombia Venezuela Slovakia Kuwait Yemen Cameroon Dubai Sierra Leone Sudan Sri Ivory Coast Uganda Lanka DR Congo Kenya Benin Ethiopia Eritrea South Africa Nigeria Vietnam Papua New Guinea Malaysia Cambodia Philippines
A TRULY GLOBAL REACH Turkmenistan Ukraine Uzbekistan Russia Tajikistan Kazakhstan Kyrgyzstan Moldova Azerbaijan Georgia Latvia Lithuania Caribbean El Salvador Nicaragua Costa Rica Panama Guatemala Peru Chile Ecuador Colombia Venezuela Slovakia Kuwait Yemen Cameroon Dubai Sierra Leone Sudan Sri Ivory Coast Uganda Lanka DR Congo Kenya Benin Ethiopia Eritrea South Africa Nigeria Vietnam Papua New Guinea Malaysia Cambodia Philippines
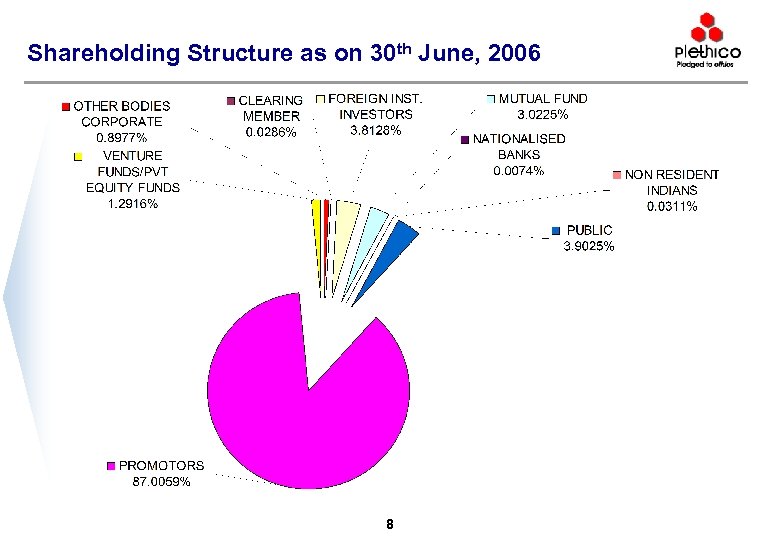 Shareholding Structure as on 30 th June, 2006 8
Shareholding Structure as on 30 th June, 2006 8
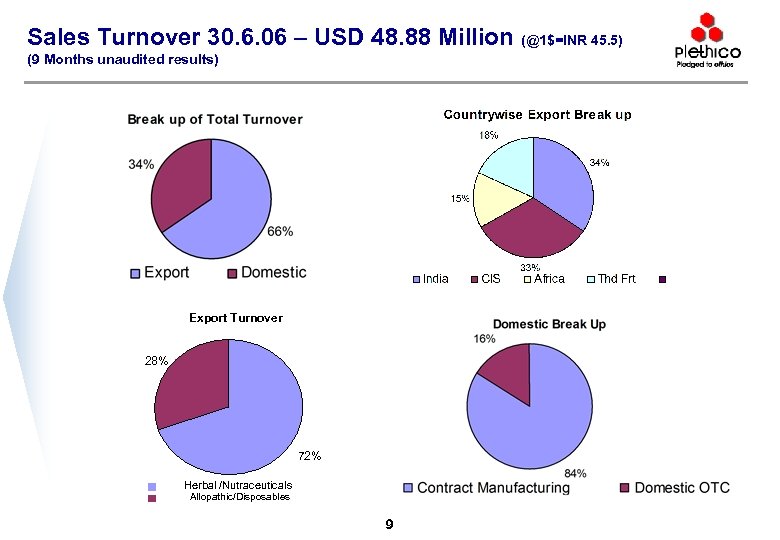 Sales Turnover 30. 6. 06 – USD 48. 88 Million (@1$=INR 45. 5) (9 Months unaudited results) Export Turnover 28% 72% Herbal /Nutraceuticals Allopathic/Disposables 9
Sales Turnover 30. 6. 06 – USD 48. 88 Million (@1$=INR 45. 5) (9 Months unaudited results) Export Turnover 28% 72% Herbal /Nutraceuticals Allopathic/Disposables 9
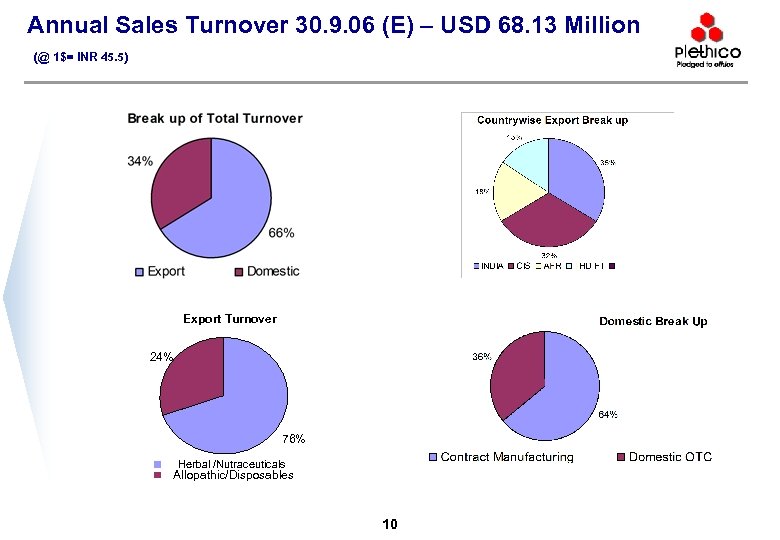 Annual Sales Turnover 30. 9. 06 (E) – USD 68. 13 Million (@ 1$= INR 45. 5) Export Turnover 24% 76% Herbal /Nutraceuticals Allopathic/Disposables 10
Annual Sales Turnover 30. 9. 06 (E) – USD 68. 13 Million (@ 1$= INR 45. 5) Export Turnover 24% 76% Herbal /Nutraceuticals Allopathic/Disposables 10
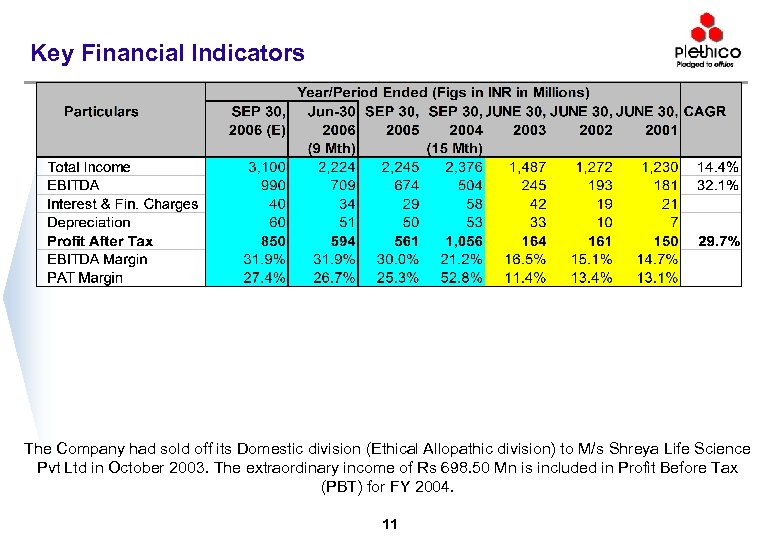 Key Financial Indicators The Company had sold off its Domestic division (Ethical Allopathic division) to M/s Shreya Life Science Pvt Ltd in October 2003. The extraordinary income of Rs 698. 50 Mn is included in Profit Before Tax (PBT) for FY 2004. 11
Key Financial Indicators The Company had sold off its Domestic division (Ethical Allopathic division) to M/s Shreya Life Science Pvt Ltd in October 2003. The extraordinary income of Rs 698. 50 Mn is included in Profit Before Tax (PBT) for FY 2004. 11
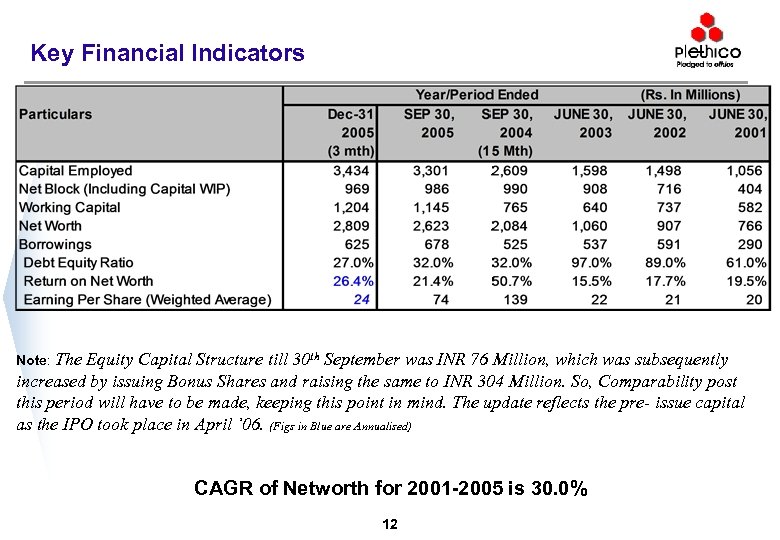 Key Financial Indicators Note: The Equity Capital Structure till 30 th September was INR 76 Million, which was subsequently increased by issuing Bonus Shares and raising the same to INR 304 Million. So, Comparability post this period will have to be made, keeping this point in mind. The update reflects the pre- issue capital as the IPO took place in April ’ 06. (Figs in Blue are Annualised) CAGR of Networth for 2001 -2005 is 30. 0% 12
Key Financial Indicators Note: The Equity Capital Structure till 30 th September was INR 76 Million, which was subsequently increased by issuing Bonus Shares and raising the same to INR 304 Million. So, Comparability post this period will have to be made, keeping this point in mind. The update reflects the pre- issue capital as the IPO took place in April ’ 06. (Figs in Blue are Annualised) CAGR of Networth for 2001 -2005 is 30. 0% 12
 Expansion Project & Status 13
Expansion Project & Status 13
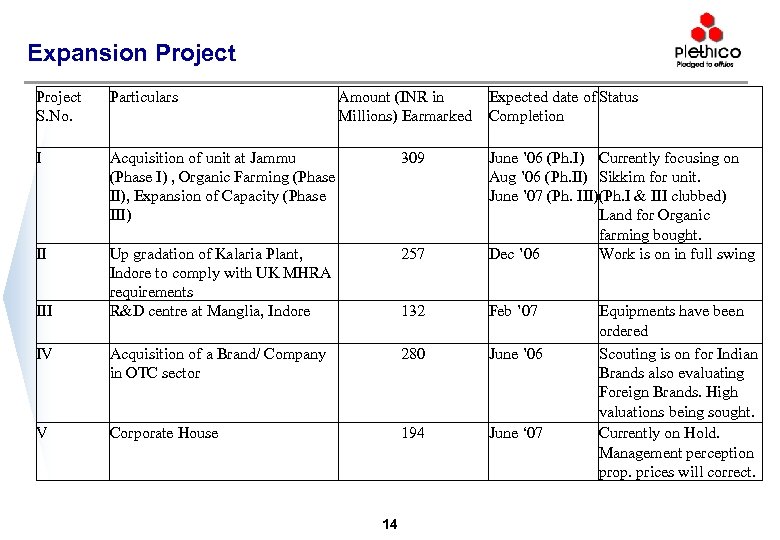 Expansion Project S. No. Particulars I Acquisition of unit at Jammu (Phase I) , Organic Farming (Phase II), Expansion of Capacity (Phase III) 309 II Up gradation of Kalaria Plant, Indore to comply with UK MHRA requirements R&D centre at Manglia, Indore 257 June ’ 06 (Ph. I) Currently focusing on Aug ’ 06 (Ph. II) Sikkim for unit. June ’ 07 (Ph. III)(Ph. I & III clubbed) Land for Organic farming bought. Dec ’ 06 Work is on in full swing 132 Feb ’ 07 IV Acquisition of a Brand/ Company in OTC sector 280 June ’ 06 V Corporate House 194 June ‘ 07 III Amount (INR in Millions) Earmarked 14 Expected date of Status Completion Equipments have been ordered Scouting is on for Indian Brands also evaluating Foreign Brands. High valuations being sought. Currently on Hold. Management perception prop. prices will correct.
Expansion Project S. No. Particulars I Acquisition of unit at Jammu (Phase I) , Organic Farming (Phase II), Expansion of Capacity (Phase III) 309 II Up gradation of Kalaria Plant, Indore to comply with UK MHRA requirements R&D centre at Manglia, Indore 257 June ’ 06 (Ph. I) Currently focusing on Aug ’ 06 (Ph. II) Sikkim for unit. June ’ 07 (Ph. III)(Ph. I & III clubbed) Land for Organic farming bought. Dec ’ 06 Work is on in full swing 132 Feb ’ 07 IV Acquisition of a Brand/ Company in OTC sector 280 June ’ 06 V Corporate House 194 June ‘ 07 III Amount (INR in Millions) Earmarked 14 Expected date of Status Completion Equipments have been ordered Scouting is on for Indian Brands also evaluating Foreign Brands. High valuations being sought. Currently on Hold. Management perception prop. prices will correct.
 Growth Strategy
Growth Strategy
 Growth Strategy A. INVESTMENT STRATEGY: The Company has invested in future growth by building a two pronged investment footprint. î “Organic growth footprint” - Build robust facilities & nurture brands. î “Inorganic Growth footprint” - Invest strategically into strong setup’s · Marketing & Distribution outfit (called M&D Strategy) · Manufacturing cum M&D outfit (Called MM&D Strategy). 16
Growth Strategy A. INVESTMENT STRATEGY: The Company has invested in future growth by building a two pronged investment footprint. î “Organic growth footprint” - Build robust facilities & nurture brands. î “Inorganic Growth footprint” - Invest strategically into strong setup’s · Marketing & Distribution outfit (called M&D Strategy) · Manufacturing cum M&D outfit (Called MM&D Strategy). 16
 Growth Strategy (Cont’d) The Company has implemented the M&D strategy for consolidation & growth in the Common Wealth of Independent States (CIS) in the following Countries. The stake held by the Company is as under: îKazakhstan – 75%* îKyrgyzstan – 75%* îMoldova – 63%* îRussia – 51% îUkraine – 51% îAzerbaijan – 51%* NOTE: Total amount invested is approx USD 26 Million till date. It has completed all the requisite formalities in 4* Countries. For Russia & Ukraine all formalities are expected to be completed latest by Dec ’ 06. 17
Growth Strategy (Cont’d) The Company has implemented the M&D strategy for consolidation & growth in the Common Wealth of Independent States (CIS) in the following Countries. The stake held by the Company is as under: îKazakhstan – 75%* îKyrgyzstan – 75%* îMoldova – 63%* îRussia – 51% îUkraine – 51% îAzerbaijan – 51%* NOTE: Total amount invested is approx USD 26 Million till date. It has completed all the requisite formalities in 4* Countries. For Russia & Ukraine all formalities are expected to be completed latest by Dec ’ 06. 17
 Growth Strategy (Cont’d) The Company has implemented the MM&D strategy for consolidation & growth in Africa îKenya – Plethico Africa Ltd - via joint venture for setting up world class manufacturing unit with liquid and ointment plant, allowing access to 17 COMESA Countries (Common Market For Eastern & Southern Africa) î This unit is expected to cater to the World Bank supported Tender Market in Africa as a “African entity” and also undertake manufacture of Ethical formulations for Kenyan markets to begin with. î The Marketing & Distribution outfit will have two wings; one the Ethical division and second the OTC division î Plethico (India) is interested in marketing it’s Herbal & Nutraceutical formulations through the OTC division. 18
Growth Strategy (Cont’d) The Company has implemented the MM&D strategy for consolidation & growth in Africa îKenya – Plethico Africa Ltd - via joint venture for setting up world class manufacturing unit with liquid and ointment plant, allowing access to 17 COMESA Countries (Common Market For Eastern & Southern Africa) î This unit is expected to cater to the World Bank supported Tender Market in Africa as a “African entity” and also undertake manufacture of Ethical formulations for Kenyan markets to begin with. î The Marketing & Distribution outfit will have two wings; one the Ethical division and second the OTC division î Plethico (India) is interested in marketing it’s Herbal & Nutraceutical formulations through the OTC division. 18
 Growth Strategy (Cont’d) B. BACKWARD INTEGRATION: · § EXECUTED: Backward Integration by installation of State of the art: î “Klockner Hansell GMBH” machinery for manufacturing Lozenges î “Aoki Japan” machine for PET bottles. · § CURRENTLY WORKING ON: Entry into regulated markets with herbal products via Organic Farming in Jammu. 19
Growth Strategy (Cont’d) B. BACKWARD INTEGRATION: · § EXECUTED: Backward Integration by installation of State of the art: î “Klockner Hansell GMBH” machinery for manufacturing Lozenges î “Aoki Japan” machine for PET bottles. · § CURRENTLY WORKING ON: Entry into regulated markets with herbal products via Organic Farming in Jammu. 19
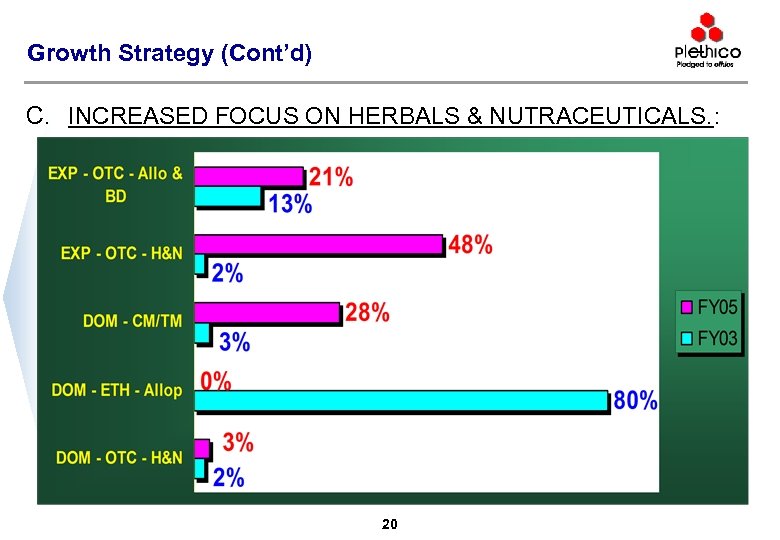 Growth Strategy (Cont’d) C. INCREASED FOCUS ON HERBALS & NUTRACEUTICALS. : 20
Growth Strategy (Cont’d) C. INCREASED FOCUS ON HERBALS & NUTRACEUTICALS. : 20
 Growth Strategy (Cont’d) D. OTHER INITIATIVES. : § Entry into regulated markets of UK/ Europe. § Innovative products like NDDS for diabetes and cancer patients. § Focussed approach to Lat Am, GCC and SEA countries. § Pan India reach through confectionary, foot care and oral care products in OTC India. § Domestic reach: îfield force to increase from 100 to 500 over 2 years § Strategic shift to Sikkim to cater to IT planning till 2022. § Fillip to operations through manufacturing JV in Kenya. 21
Growth Strategy (Cont’d) D. OTHER INITIATIVES. : § Entry into regulated markets of UK/ Europe. § Innovative products like NDDS for diabetes and cancer patients. § Focussed approach to Lat Am, GCC and SEA countries. § Pan India reach through confectionary, foot care and oral care products in OTC India. § Domestic reach: îfield force to increase from 100 to 500 over 2 years § Strategic shift to Sikkim to cater to IT planning till 2022. § Fillip to operations through manufacturing JV in Kenya. 21
 Journey Ahead 22
Journey Ahead 22
 The Journey Ahead…. . 1. Compliance with UK MHRA requirements at Kalaria, MP, India site. 2. Acquire a Company with few Brands in Europe / UK, having a strong distribution setup in place. This unit will seek manufacture of these brands in Kalaria plant, which will be in “Approvable status”. 3. This will trigger an Inspection at Kalaria. (We expect approvals by March ’ 08. ) 4. Kalaria unit will be earmarked for Regulated Countries. 5. Kandla & Manglia unit will be earmarked Semi / unregulated Countries. 6. November ’ 08 may see Plethico back in the Ethical market in the Lifestyle drug segment. 7. Explore Inorganic growth opportunities at all points of time. 23
The Journey Ahead…. . 1. Compliance with UK MHRA requirements at Kalaria, MP, India site. 2. Acquire a Company with few Brands in Europe / UK, having a strong distribution setup in place. This unit will seek manufacture of these brands in Kalaria plant, which will be in “Approvable status”. 3. This will trigger an Inspection at Kalaria. (We expect approvals by March ’ 08. ) 4. Kalaria unit will be earmarked for Regulated Countries. 5. Kandla & Manglia unit will be earmarked Semi / unregulated Countries. 6. November ’ 08 may see Plethico back in the Ethical market in the Lifestyle drug segment. 7. Explore Inorganic growth opportunities at all points of time. 23
 Summary 24
Summary 24
 Summary § Non infringing model of Business § State of art manufacturing units in Indore possessing capability of manufacturing all possible dosage forms. § Strong formulation development technique including NDDS like effervescent tablets. § Strong M&D tie up in Russia, CIS & Cambodia § Strong management team and motivated workforce § Foray into Backward Integration for cultivating essential critical herbs for captive consumption thus enabling high end finger printing technology for herbal formulations § Strong system oriented company § Product list exceeding 400, transcending more than 39 therapeutic categories exporting to more than 45 countries across the globe 25
Summary § Non infringing model of Business § State of art manufacturing units in Indore possessing capability of manufacturing all possible dosage forms. § Strong formulation development technique including NDDS like effervescent tablets. § Strong M&D tie up in Russia, CIS & Cambodia § Strong management team and motivated workforce § Foray into Backward Integration for cultivating essential critical herbs for captive consumption thus enabling high end finger printing technology for herbal formulations § Strong system oriented company § Product list exceeding 400, transcending more than 39 therapeutic categories exporting to more than 45 countries across the globe 25
 THANK YOU 26
THANK YOU 26
 PLETHICO PHARMACEUTICALS LIMITED Q&A
PLETHICO PHARMACEUTICALS LIMITED Q&A


