2916d95c92a0c73f69fb15778f644038.ppt
- Количество слайдов: 59
 Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content. Select slides from the original presentation are omitted where Research To Practice was unable to obtain permission from the publication source and/or author. Links to view the actual reference materials have been provided for your use in place of any omitted slides.
Please note, these are the actual video-recorded proceedings from the live CME event and may include the use of trade names and other raw, unedited content. Select slides from the original presentation are omitted where Research To Practice was unable to obtain permission from the publication source and/or author. Links to view the actual reference materials have been provided for your use in place of any omitted slides.
 Prostate Cancer: New Agents and Emerging Clinical Algorithms Thursday, October 11, 2012 7: 30 PM – 8: 30 PM ET
Prostate Cancer: New Agents and Emerging Clinical Algorithms Thursday, October 11, 2012 7: 30 PM – 8: 30 PM ET
 Neil Love, MD Research To Practice Miami, Florida Christopher J Logothetis, MD Chairman/Professor Genitourinary Medical Oncology The University of Texas MD Anderson Cancer Center Houston, Texas A Oliver Sartor, MD Medical Director Tulane Cancer Center Laborde Professor of Cancer Research Professor of Medicine and Urology Tulane Medical School New Orleans, Louisiana
Neil Love, MD Research To Practice Miami, Florida Christopher J Logothetis, MD Chairman/Professor Genitourinary Medical Oncology The University of Texas MD Anderson Cancer Center Houston, Texas A Oliver Sartor, MD Medical Director Tulane Cancer Center Laborde Professor of Cancer Research Professor of Medicine and Urology Tulane Medical School New Orleans, Louisiana
 Agenda — Emerging Systemic Treatments for Prostate Cancer • Optimal Sequencing of Endocrine Therapy – Case from Dr Sartor: 75 -year-old retired attorney – Continuous vs intermittent ADT – COU-AA-301, COU-AA-302: Abiraterone – AFFIRM: Enzalutamide – Early data with TAK-700 (Orteronel) • Bone-Targeted Treatment – ALSYMPCA: Radium-223 • Tolerability of Next-Generation Hormonal Agents – Faculty cases • Other New Agents of Interest – Cabozantinib, dasatinib
Agenda — Emerging Systemic Treatments for Prostate Cancer • Optimal Sequencing of Endocrine Therapy – Case from Dr Sartor: 75 -year-old retired attorney – Continuous vs intermittent ADT – COU-AA-301, COU-AA-302: Abiraterone – AFFIRM: Enzalutamide – Early data with TAK-700 (Orteronel) • Bone-Targeted Treatment – ALSYMPCA: Radium-223 • Tolerability of Next-Generation Hormonal Agents – Faculty cases • Other New Agents of Interest – Cabozantinib, dasatinib
 Current Controversies, Recent Developments and Emerging Strategies in the Management of Prostate Cancer A Clinical Investigator Think Tank Faculty Tomasz M Beer, MD Robert Dreicer, MD, MS Mario A Eisenberger, MD William K Oh, MD Daniel P Petrylak, MD A Oliver Sartor, MD Susan F Slovin, MD, Ph. D Matthew R Smith, MD, Ph. D Moderator Neil Love, MD
Current Controversies, Recent Developments and Emerging Strategies in the Management of Prostate Cancer A Clinical Investigator Think Tank Faculty Tomasz M Beer, MD Robert Dreicer, MD, MS Mario A Eisenberger, MD William K Oh, MD Daniel P Petrylak, MD A Oliver Sartor, MD Susan F Slovin, MD, Ph. D Matthew R Smith, MD, Ph. D Moderator Neil Love, MD


 Faculty Case: Dr Sartor • A 75 -year-old retired attorney with an active lifestyle • Underwent radical prostatectomy 5 years prior – Recurrence followed by salvage radiation therapy • PSA-only recurrence: – PSADT = 8 months – PSA 4. 6 ng/m. L • Patient experiencing “PSA anxiety”
Faculty Case: Dr Sartor • A 75 -year-old retired attorney with an active lifestyle • Underwent radical prostatectomy 5 years prior – Recurrence followed by salvage radiation therapy • PSA-only recurrence: – PSADT = 8 months – PSA 4. 6 ng/m. L • Patient experiencing “PSA anxiety”
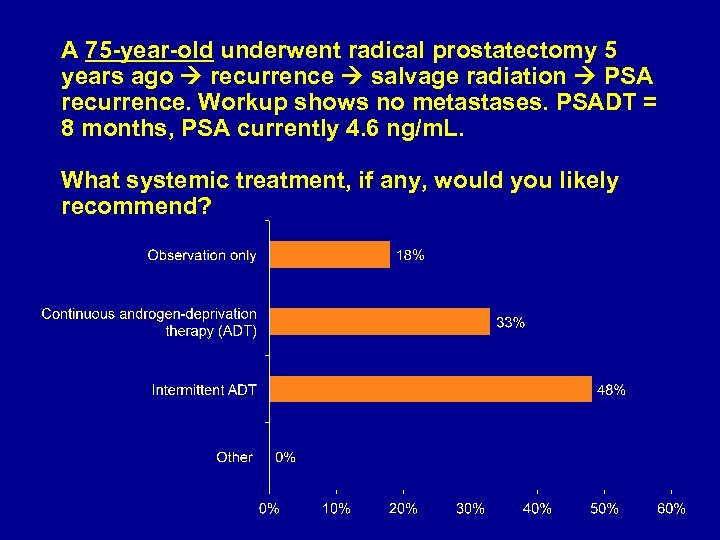 A 75 -year-old underwent radical prostatectomy 5 years ago recurrence salvage radiation PSA recurrence. Workup shows no metastases. PSADT = 8 months, PSA currently 4. 6 ng/m. L. What systemic treatment, if any, would you likely recommend?
A 75 -year-old underwent radical prostatectomy 5 years ago recurrence salvage radiation PSA recurrence. Workup shows no metastases. PSADT = 8 months, PSA currently 4. 6 ng/m. L. What systemic treatment, if any, would you likely recommend?
 Current Controversies, Recent Developments and Emerging Strategies in the Management of Prostate Cancer — A Clinical Investigator Think Tank September 7, 2012 75 -year-old with PSA-only disease • Robert Dreicer, MD, MS: “There’s no right answer; I would not have initiated therapy. ” • Mario A Eisenberger, MD: “No, I don’t think we would start treatment. We would wait” • Susan F Slovin, MD, Ph. D: “I would not initiate therapy. ”
Current Controversies, Recent Developments and Emerging Strategies in the Management of Prostate Cancer — A Clinical Investigator Think Tank September 7, 2012 75 -year-old with PSA-only disease • Robert Dreicer, MD, MS: “There’s no right answer; I would not have initiated therapy. ” • Mario A Eisenberger, MD: “No, I don’t think we would start treatment. We would wait” • Susan F Slovin, MD, Ph. D: “I would not initiate therapy. ”

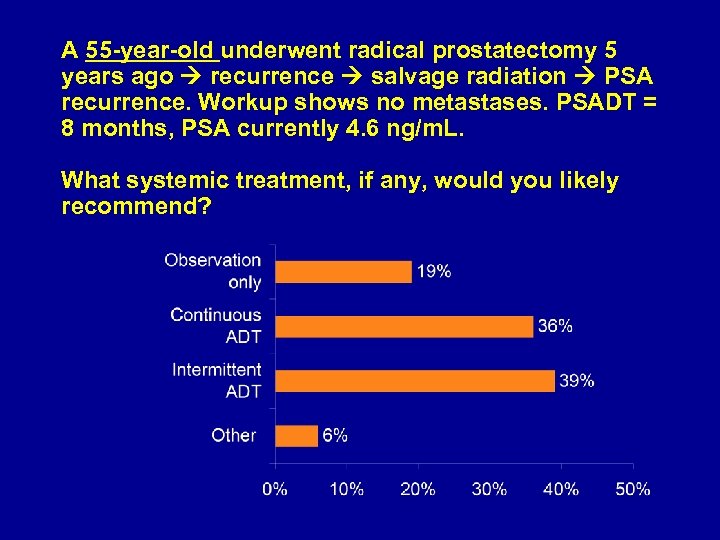 A 55 -year-old underwent radical prostatectomy 5 years ago recurrence salvage radiation PSA recurrence. Workup shows no metastases. PSADT = 8 months, PSA currently 4. 6 ng/m. L. What systemic treatment, if any, would you likely recommend?
A 55 -year-old underwent radical prostatectomy 5 years ago recurrence salvage radiation PSA recurrence. Workup shows no metastases. PSADT = 8 months, PSA currently 4. 6 ng/m. L. What systemic treatment, if any, would you likely recommend?
 Intermittent (IAD) versus Continuous Androgen Deprivation (CAD) in Hormone Sensitive Metastatic Prostate Cancer (HSM 1 PC) Patients (pts): Results of S 9346 (INT-0162), an International Phase III Trial Hussain M et al. Proc ASCO 2012; Abstract 4.
Intermittent (IAD) versus Continuous Androgen Deprivation (CAD) in Hormone Sensitive Metastatic Prostate Cancer (HSM 1 PC) Patients (pts): Results of S 9346 (INT-0162), an International Phase III Trial Hussain M et al. Proc ASCO 2012; Abstract 4.
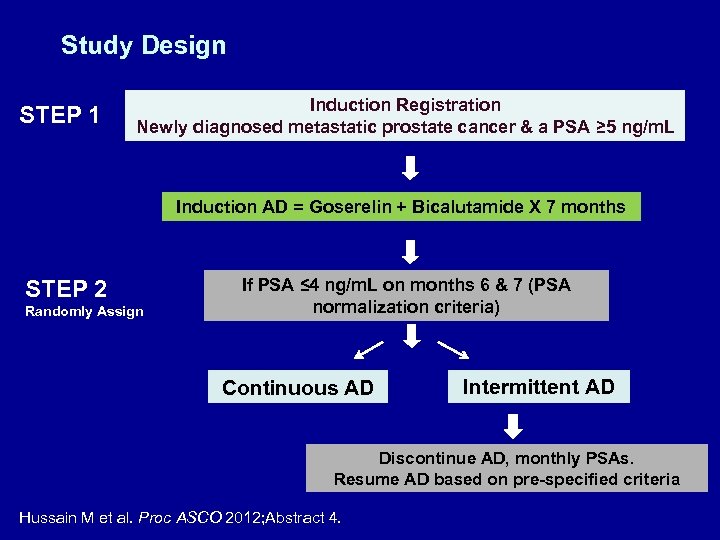 Study Design STEP 1 Induction Registration Newly diagnosed metastatic prostate cancer & a PSA ≥ 5 ng/m. L Induction AD = Goserelin + Bicalutamide X 7 months STEP 2 Randomly Assign If PSA ≤ 4 ng/m. L on months 6 & 7 (PSA normalization criteria) Continuous AD Intermittent AD Discontinue AD, monthly PSAs. Resume AD based on pre-specified criteria Hussain M et al. Proc ASCO 2012; Abstract 4.
Study Design STEP 1 Induction Registration Newly diagnosed metastatic prostate cancer & a PSA ≥ 5 ng/m. L Induction AD = Goserelin + Bicalutamide X 7 months STEP 2 Randomly Assign If PSA ≤ 4 ng/m. L on months 6 & 7 (PSA normalization criteria) Continuous AD Intermittent AD Discontinue AD, monthly PSAs. Resume AD based on pre-specified criteria Hussain M et al. Proc ASCO 2012; Abstract 4.
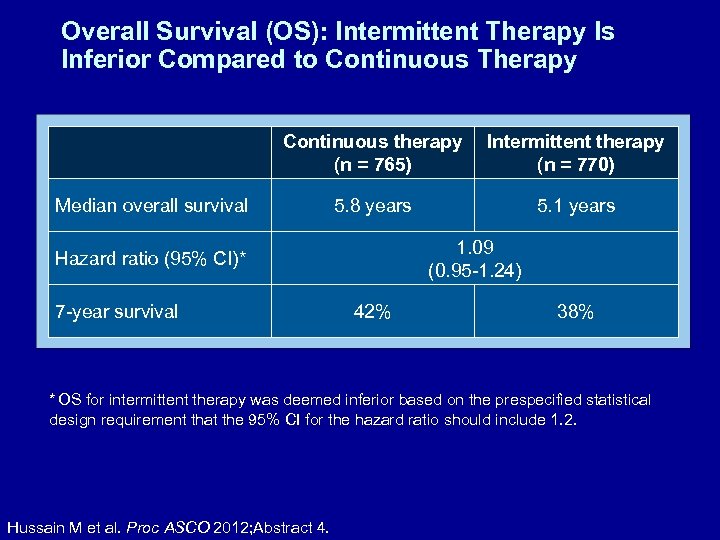 Overall Survival (OS): Intermittent Therapy Is Inferior Compared to Continuous Therapy Continuous therapy (n = 765) Intermittent therapy (n = 770) 5. 8 years 5. 1 years Median overall survival 1. 09 (0. 95 -1. 24) Hazard ratio (95% CI)* 7 -year survival 42% 38% * OS for intermittent therapy was deemed inferior based on the prespecified statistical design requirement that the 95% CI for the hazard ratio should include 1. 2. Hussain M et al. Proc ASCO 2012; Abstract 4.
Overall Survival (OS): Intermittent Therapy Is Inferior Compared to Continuous Therapy Continuous therapy (n = 765) Intermittent therapy (n = 770) 5. 8 years 5. 1 years Median overall survival 1. 09 (0. 95 -1. 24) Hazard ratio (95% CI)* 7 -year survival 42% 38% * OS for intermittent therapy was deemed inferior based on the prespecified statistical design requirement that the 95% CI for the hazard ratio should include 1. 2. Hussain M et al. Proc ASCO 2012; Abstract 4.

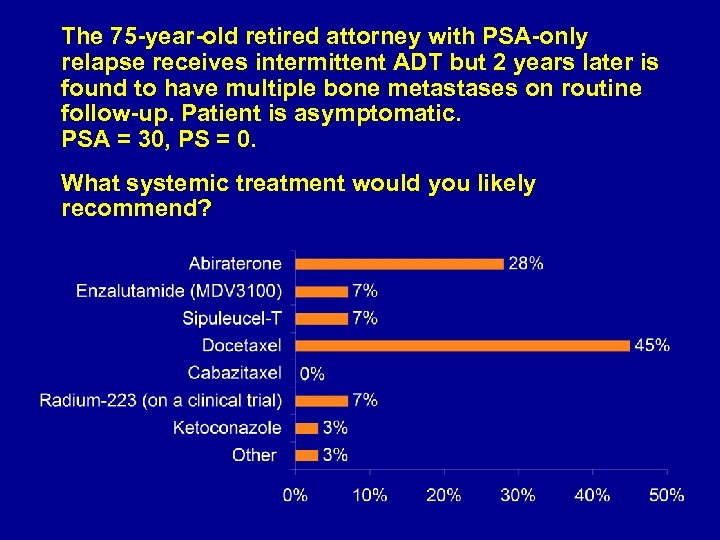 The 75 -year-old retired attorney with PSA-only relapse receives intermittent ADT but 2 years later is found to have multiple bone metastases on routine follow-up. Patient is asymptomatic. PSA = 30, PS = 0. What systemic treatment would you likely recommend?
The 75 -year-old retired attorney with PSA-only relapse receives intermittent ADT but 2 years later is found to have multiple bone metastases on routine follow-up. Patient is asymptomatic. PSA = 30, PS = 0. What systemic treatment would you likely recommend?
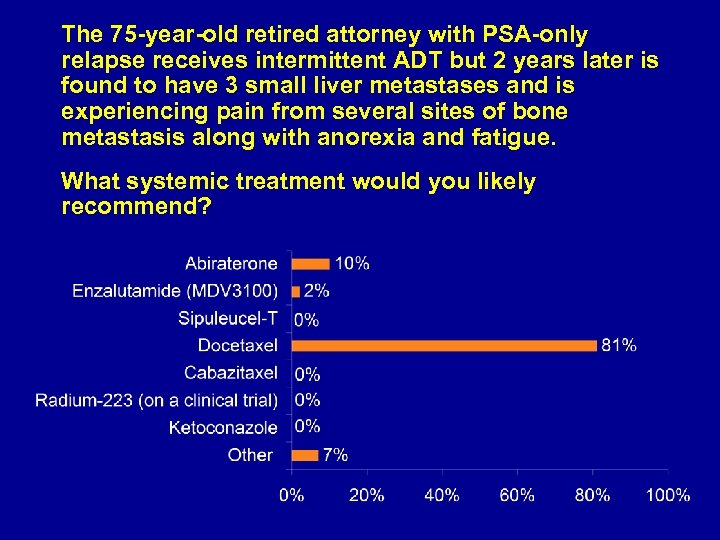 The 75 -year-old retired attorney with PSA-only relapse receives intermittent ADT but 2 years later is found to have 3 small liver metastases and is experiencing pain from several sites of bone metastasis along with anorexia and fatigue. What systemic treatment would you likely recommend?
The 75 -year-old retired attorney with PSA-only relapse receives intermittent ADT but 2 years later is found to have 3 small liver metastases and is experiencing pain from several sites of bone metastasis along with anorexia and fatigue. What systemic treatment would you likely recommend?
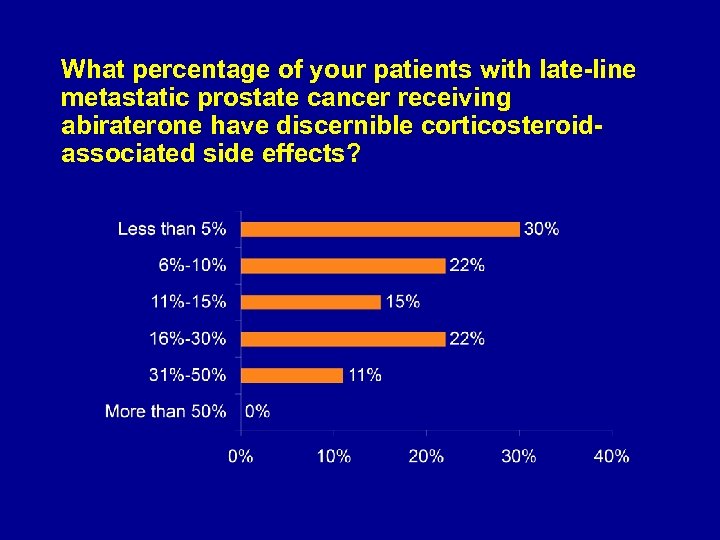 What percentage of your patients with late-line metastatic prostate cancer receiving abiraterone have discernible corticosteroidassociated side effects?
What percentage of your patients with late-line metastatic prostate cancer receiving abiraterone have discernible corticosteroidassociated side effects?
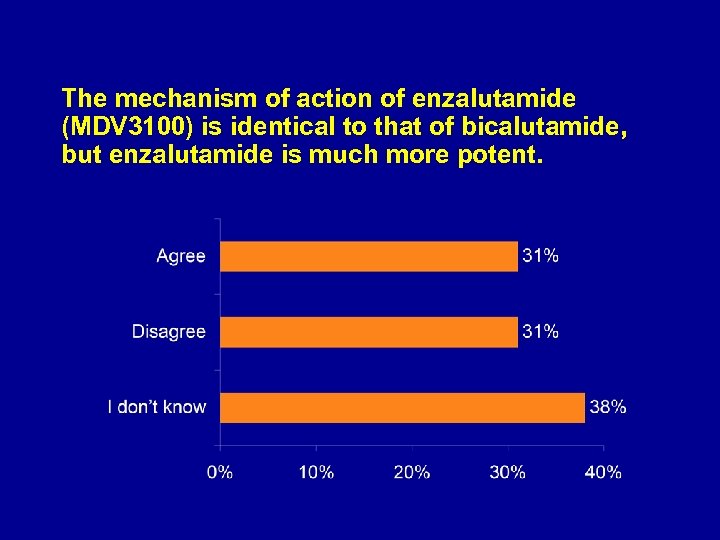 The mechanism of action of enzalutamide (MDV 3100) is identical to that of bicalutamide, but enzalutamide is much more potent.
The mechanism of action of enzalutamide (MDV 3100) is identical to that of bicalutamide, but enzalutamide is much more potent.
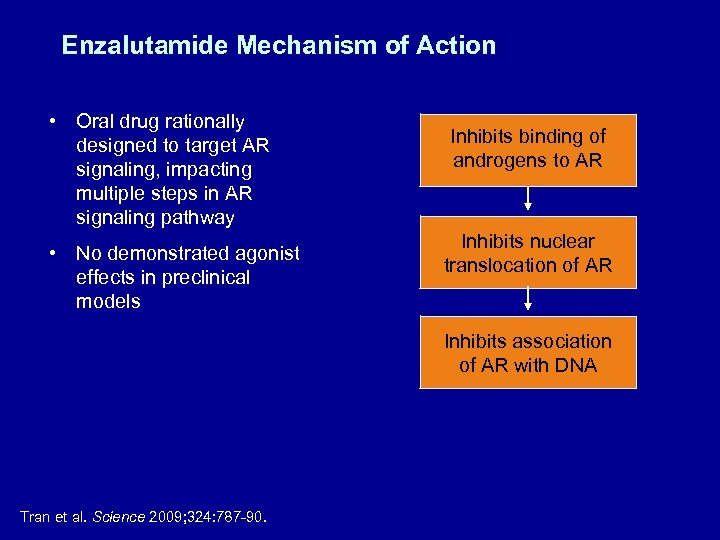 Enzalutamide Mechanism of Action • Oral drug rationally designed to target AR signaling, impacting multiple steps in AR signaling pathway • No demonstrated agonist effects in preclinical models Inhibits binding of androgens to AR Inhibits nuclear translocation of AR Inhibits association of AR with DNA Tran et al. Science 2009; 324: 787 -90.
Enzalutamide Mechanism of Action • Oral drug rationally designed to target AR signaling, impacting multiple steps in AR signaling pathway • No demonstrated agonist effects in preclinical models Inhibits binding of androgens to AR Inhibits nuclear translocation of AR Inhibits association of AR with DNA Tran et al. Science 2009; 324: 787 -90.
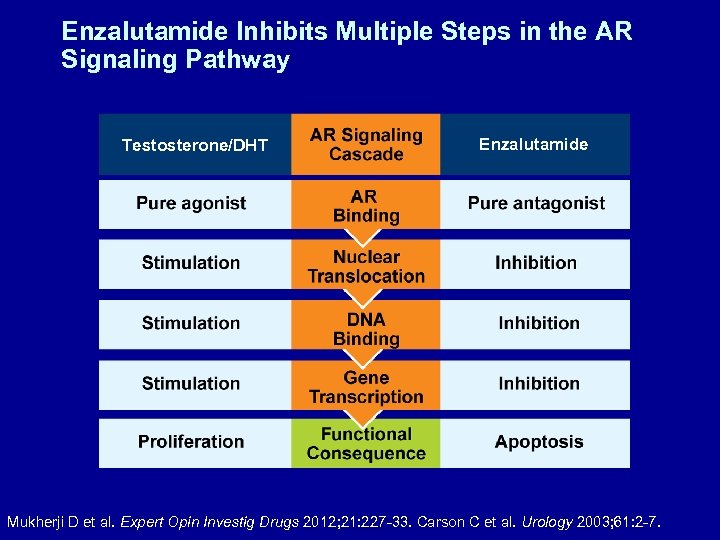 Enzalutamide Inhibits Multiple Steps in the AR Signaling Pathway Testosterone/DHT Enzalutamide Mukherji D et al. Expert Opin Investig Drugs 2012; 21: 227 -33. Carson C et al. Urology 2003; 61: 2 -7.
Enzalutamide Inhibits Multiple Steps in the AR Signaling Pathway Testosterone/DHT Enzalutamide Mukherji D et al. Expert Opin Investig Drugs 2012; 21: 227 -33. Carson C et al. Urology 2003; 61: 2 -7.
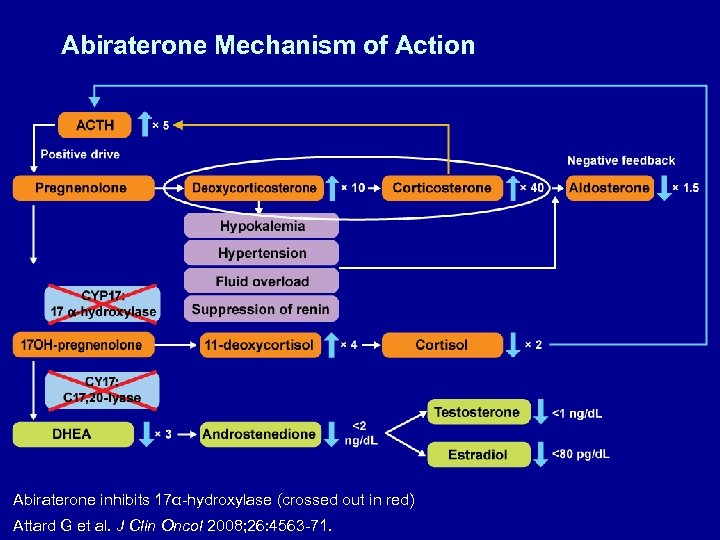 Abiraterone Mechanism of Action Abiraterone inhibits 17α-hydroxylase (crossed out in red) Attard G et al. J Clin Oncol 2008; 26: 4563 -71.
Abiraterone Mechanism of Action Abiraterone inhibits 17α-hydroxylase (crossed out in red) Attard G et al. J Clin Oncol 2008; 26: 4563 -71.
 Current Controversies, Recent Developments and Emerging Strategies in the Management of Prostate Cancer — A Clinical Investigator Think Tank September 7, 2012 Sequence of enzalutamide and abiraterone in metastatic disease • Tomasz M Beer, MD: “Given no data, go with enzalutamide first. ” • Robert Dreicer, MD, MS: “With all the caveats, enzalutamide. ” • Mario A Eisenberger, MD: “Enzalutamide. It’s easier to use. It’s less toxic. No need for steroids. ” • William K Oh, MD: “Probably enzalutamide…less toxicity” • Daniel P Petrylak, MD: “Since we’re trying to sequence these drugs, if you believe that corticosteroids may have an abrogating effect on sipuleucel-T, I would tend to go with enzalutamide first. ” • Susan F Slovin, MD, Ph. D: “Enzalutamide, with one caveat…in patients where I want to go to a completely different category of drugs I would use abiraterone” • Matthew R Smith, MD, Ph. D: “Prefer to use abiraterone first for now in the prechemotherapy setting, since I have longer experience with it. It’s a very transient answer, and we await the results of the PREVAIL trial. ”
Current Controversies, Recent Developments and Emerging Strategies in the Management of Prostate Cancer — A Clinical Investigator Think Tank September 7, 2012 Sequence of enzalutamide and abiraterone in metastatic disease • Tomasz M Beer, MD: “Given no data, go with enzalutamide first. ” • Robert Dreicer, MD, MS: “With all the caveats, enzalutamide. ” • Mario A Eisenberger, MD: “Enzalutamide. It’s easier to use. It’s less toxic. No need for steroids. ” • William K Oh, MD: “Probably enzalutamide…less toxicity” • Daniel P Petrylak, MD: “Since we’re trying to sequence these drugs, if you believe that corticosteroids may have an abrogating effect on sipuleucel-T, I would tend to go with enzalutamide first. ” • Susan F Slovin, MD, Ph. D: “Enzalutamide, with one caveat…in patients where I want to go to a completely different category of drugs I would use abiraterone” • Matthew R Smith, MD, Ph. D: “Prefer to use abiraterone first for now in the prechemotherapy setting, since I have longer experience with it. It’s a very transient answer, and we await the results of the PREVAIL trial. ”
 Interim Analysis (IA) Results of COUAA-302, a Randomized, Phase III Study of Abiraterone Acetate (AA) in Chemotherapy-Naïve Patients (pts) with Metastatic Castration-Resistant Prostate Cancer (m. CRPC) Ryan CJ et al. Proc ASCO 2012; Abstract LBA 4518.
Interim Analysis (IA) Results of COUAA-302, a Randomized, Phase III Study of Abiraterone Acetate (AA) in Chemotherapy-Naïve Patients (pts) with Metastatic Castration-Resistant Prostate Cancer (m. CRPC) Ryan CJ et al. Proc ASCO 2012; Abstract LBA 4518.
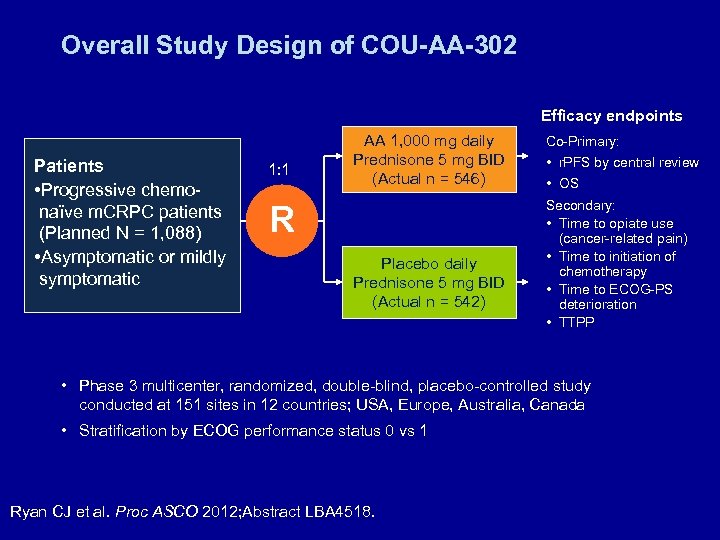 Overall Study Design of COU-AA-302 Efficacy endpoints Patients • Progressive chemonaïve m. CRPC patients (Planned N = 1, 088) • Asymptomatic or mildly symptomatic 1: 1 AA 1, 000 mg daily Prednisone 5 mg BID (Actual n = 546) R Placebo daily Prednisone 5 mg BID (Actual n = 542) Co-Primary: • r. PFS by central review • OS Secondary: • Time to opiate use (cancer-related pain) • Time to initiation of chemotherapy • Time to ECOG-PS deterioration • TTPP • Phase 3 multicenter, randomized, double-blind, placebo-controlled study conducted at 151 sites in 12 countries; USA, Europe, Australia, Canada • Stratification by ECOG performance status 0 vs 1 Ryan CJ et al. Proc ASCO 2012; Abstract LBA 4518.
Overall Study Design of COU-AA-302 Efficacy endpoints Patients • Progressive chemonaïve m. CRPC patients (Planned N = 1, 088) • Asymptomatic or mildly symptomatic 1: 1 AA 1, 000 mg daily Prednisone 5 mg BID (Actual n = 546) R Placebo daily Prednisone 5 mg BID (Actual n = 542) Co-Primary: • r. PFS by central review • OS Secondary: • Time to opiate use (cancer-related pain) • Time to initiation of chemotherapy • Time to ECOG-PS deterioration • TTPP • Phase 3 multicenter, randomized, double-blind, placebo-controlled study conducted at 151 sites in 12 countries; USA, Europe, Australia, Canada • Stratification by ECOG performance status 0 vs 1 Ryan CJ et al. Proc ASCO 2012; Abstract LBA 4518.
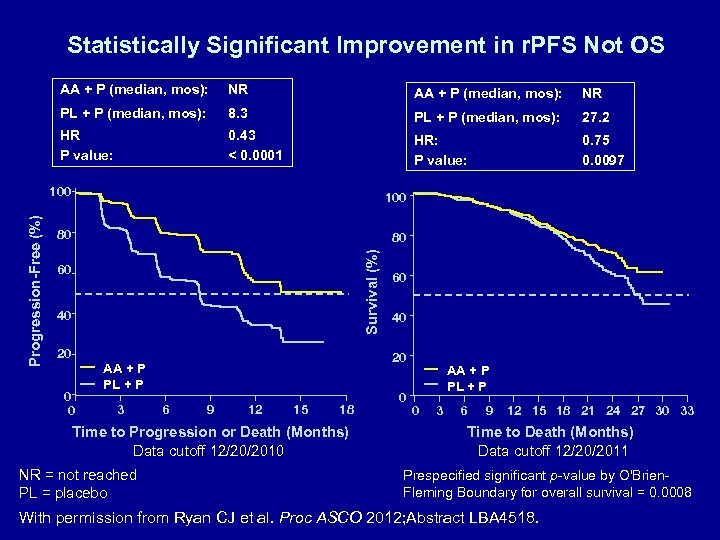 Statistically Significant Improvement in r. PFS Not OS AA + P (median, mos): NR PL + P (median, mos): 8. 3 PL + P (median, mos): 27. 2 HR P value: 0. 43 < 0. 0001 HR: P value: 0. 75 0. 0097 100 80 80 Survival (%) Progression-Free (%) 100 60 40 20 0 0 6 9 12 15 18 Time to Progression or Death (Months) Data cutoff 12/20/2010 NR = not reached PL = placebo 40 20 AA + P PL + P 3 60 0 AA + P PL + P 0 3 6 9 12 15 18 21 24 27 30 33 Time to Death (Months) Data cutoff 12/20/2011 Prespecified significant p-value by O'Brien. Fleming Boundary for overall survival = 0. 0008 With permission from Ryan CJ et al. Proc ASCO 2012; Abstract LBA 4518.
Statistically Significant Improvement in r. PFS Not OS AA + P (median, mos): NR PL + P (median, mos): 8. 3 PL + P (median, mos): 27. 2 HR P value: 0. 43 < 0. 0001 HR: P value: 0. 75 0. 0097 100 80 80 Survival (%) Progression-Free (%) 100 60 40 20 0 0 6 9 12 15 18 Time to Progression or Death (Months) Data cutoff 12/20/2010 NR = not reached PL = placebo 40 20 AA + P PL + P 3 60 0 AA + P PL + P 0 3 6 9 12 15 18 21 24 27 30 33 Time to Death (Months) Data cutoff 12/20/2011 Prespecified significant p-value by O'Brien. Fleming Boundary for overall survival = 0. 0008 With permission from Ryan CJ et al. Proc ASCO 2012; Abstract LBA 4518.
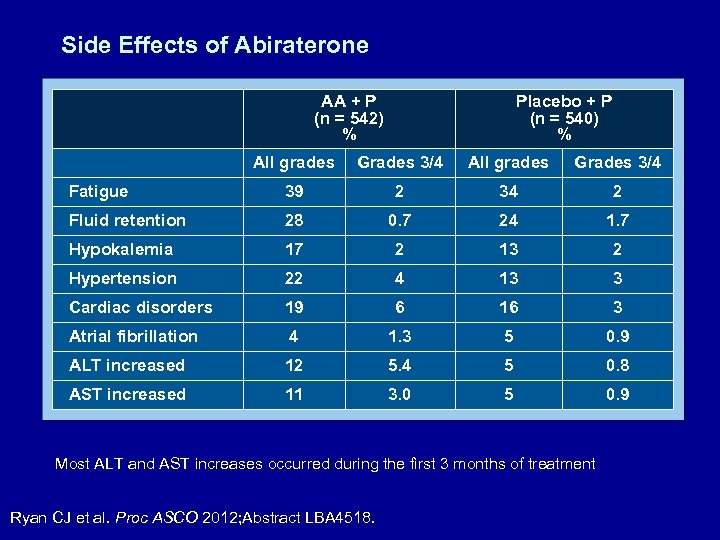 Side Effects of Abiraterone AA + P (n = 542) % Placebo + P (n = 540) % All grades Grades 3/4 Fatigue 39 2 34 2 Fluid retention 28 0. 7 24 1. 7 Hypokalemia 17 2 13 2 Hypertension 22 4 13 3 Cardiac disorders 19 6 16 3 Atrial fibrillation 4 1. 3 5 0. 9 ALT increased 12 5. 4 5 0. 8 AST increased 11 3. 0 5 0. 9 Most ALT and AST increases occurred during the first 3 months of treatment Ryan CJ et al. Proc ASCO 2012; Abstract LBA 4518.
Side Effects of Abiraterone AA + P (n = 542) % Placebo + P (n = 540) % All grades Grades 3/4 Fatigue 39 2 34 2 Fluid retention 28 0. 7 24 1. 7 Hypokalemia 17 2 13 2 Hypertension 22 4 13 3 Cardiac disorders 19 6 16 3 Atrial fibrillation 4 1. 3 5 0. 9 ALT increased 12 5. 4 5 0. 8 AST increased 11 3. 0 5 0. 9 Most ALT and AST increases occurred during the first 3 months of treatment Ryan CJ et al. Proc ASCO 2012; Abstract LBA 4518.
 Increased Survival with Enzalutamide in Prostate Cancer After Chemotherapy Scher HI et al. N Engl J Med 2012; 367: 1187 -97.
Increased Survival with Enzalutamide in Prostate Cancer After Chemotherapy Scher HI et al. N Engl J Med 2012; 367: 1187 -97.
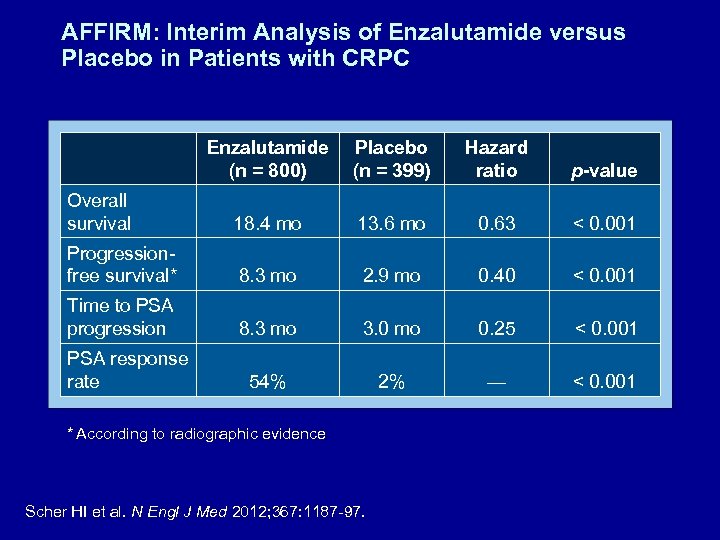 AFFIRM: Interim Analysis of Enzalutamide versus Placebo in Patients with CRPC Enzalutamide (n = 800) Placebo (n = 399) Hazard ratio p-value Overall survival 18. 4 mo 13. 6 mo 0. 63 < 0. 001 Progressionfree survival* 8. 3 mo 2. 9 mo 0. 40 < 0. 001 Time to PSA progression 8. 3 mo 3. 0 mo 0. 25 < 0. 001 54% 2% — < 0. 001 PSA response rate * According to radiographic evidence Scher HI et al. N Engl J Med 2012; 367: 1187 -97.
AFFIRM: Interim Analysis of Enzalutamide versus Placebo in Patients with CRPC Enzalutamide (n = 800) Placebo (n = 399) Hazard ratio p-value Overall survival 18. 4 mo 13. 6 mo 0. 63 < 0. 001 Progressionfree survival* 8. 3 mo 2. 9 mo 0. 40 < 0. 001 Time to PSA progression 8. 3 mo 3. 0 mo 0. 25 < 0. 001 54% 2% — < 0. 001 PSA response rate * According to radiographic evidence Scher HI et al. N Engl J Med 2012; 367: 1187 -97.
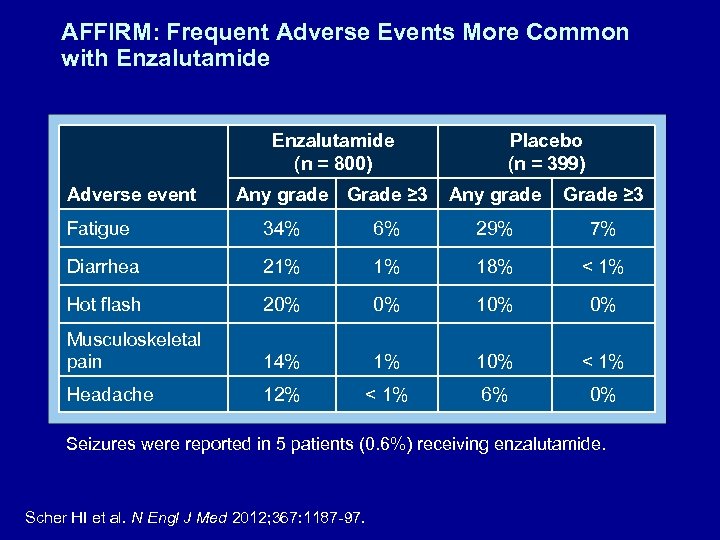 AFFIRM: Frequent Adverse Events More Common with Enzalutamide (n = 800) Adverse event Any grade Grade ≥ 3 Placebo (n = 399) Any grade Grade ≥ 3 Fatigue 34% 6% 29% 7% Diarrhea 21% 1% 18% < 1% Hot flash 20% 0% 10% 0% Musculoskeletal pain 14% 1% 10% < 1% Headache 12% < 1% 6% 0% Seizures were reported in 5 patients (0. 6%) receiving enzalutamide. Scher HI et al. N Engl J Med 2012; 367: 1187 -97.
AFFIRM: Frequent Adverse Events More Common with Enzalutamide (n = 800) Adverse event Any grade Grade ≥ 3 Placebo (n = 399) Any grade Grade ≥ 3 Fatigue 34% 6% 29% 7% Diarrhea 21% 1% 18% < 1% Hot flash 20% 0% 10% 0% Musculoskeletal pain 14% 1% 10% < 1% Headache 12% < 1% 6% 0% Seizures were reported in 5 patients (0. 6%) receiving enzalutamide. Scher HI et al. N Engl J Med 2012; 367: 1187 -97.
 Safety and Activity of the Investigational Agent Orteronel without Prednisone in Men with Nonmetastatic Castration-Resistant Prostate Cancer and Rising Prostate-Specific Antigen: Updated Results of a Phase II Study George DJ et al. Proc ASCO 2012; Abstract 4549.
Safety and Activity of the Investigational Agent Orteronel without Prednisone in Men with Nonmetastatic Castration-Resistant Prostate Cancer and Rising Prostate-Specific Antigen: Updated Results of a Phase II Study George DJ et al. Proc ASCO 2012; Abstract 4549.
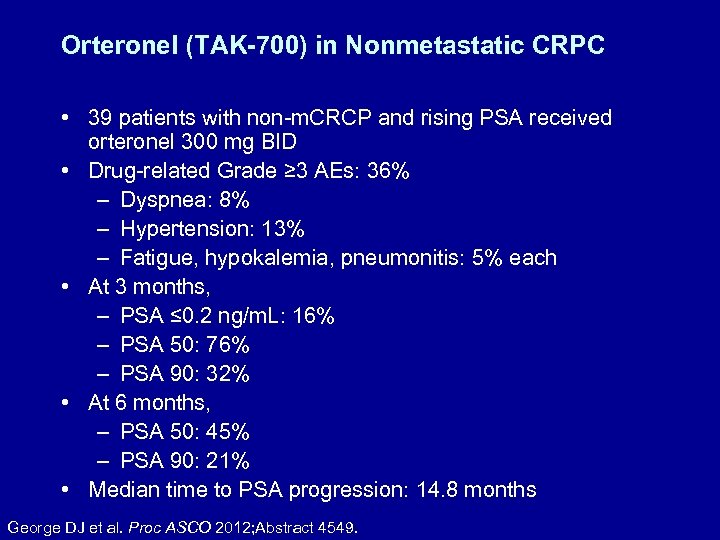 Orteronel (TAK-700) in Nonmetastatic CRPC • 39 patients with non-m. CRCP and rising PSA received orteronel 300 mg BID • Drug-related Grade ≥ 3 AEs: 36% – Dyspnea: 8% – Hypertension: 13% – Fatigue, hypokalemia, pneumonitis: 5% each • At 3 months, – PSA ≤ 0. 2 ng/m. L: 16% – PSA 50: 76% – PSA 90: 32% • At 6 months, – PSA 50: 45% – PSA 90: 21% • Median time to PSA progression: 14. 8 months George DJ et al. Proc ASCO 2012; Abstract 4549.
Orteronel (TAK-700) in Nonmetastatic CRPC • 39 patients with non-m. CRCP and rising PSA received orteronel 300 mg BID • Drug-related Grade ≥ 3 AEs: 36% – Dyspnea: 8% – Hypertension: 13% – Fatigue, hypokalemia, pneumonitis: 5% each • At 3 months, – PSA ≤ 0. 2 ng/m. L: 16% – PSA 50: 76% – PSA 90: 32% • At 6 months, – PSA 50: 45% – PSA 90: 21% • Median time to PSA progression: 14. 8 months George DJ et al. Proc ASCO 2012; Abstract 4549.
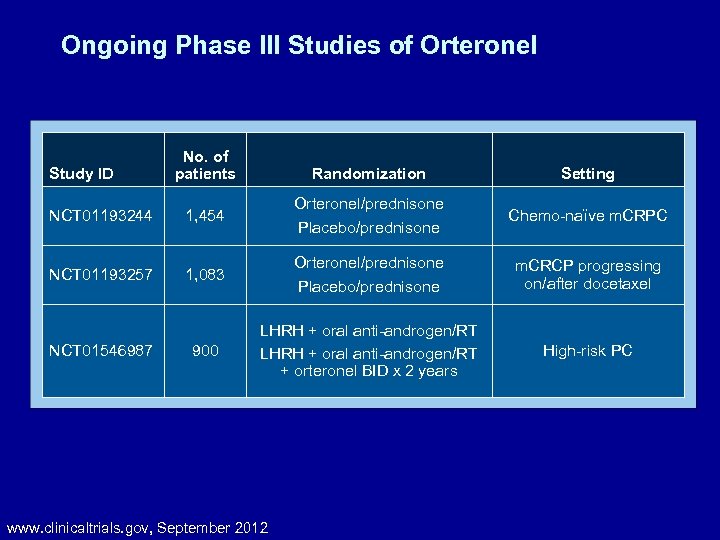 Ongoing Phase III Studies of Orteronel Study ID No. of patients Randomization Setting NCT 01193244 1, 454 Orteronel/prednisone Placebo/prednisone Chemo-naïve m. CRPC NCT 01193257 1, 083 Orteronel/prednisone Placebo/prednisone m. CRCP progressing on/after docetaxel 900 LHRH + oral anti-androgen/RT + orteronel BID x 2 years High-risk PC NCT 01546987 www. clinicaltrials. gov, September 2012
Ongoing Phase III Studies of Orteronel Study ID No. of patients Randomization Setting NCT 01193244 1, 454 Orteronel/prednisone Placebo/prednisone Chemo-naïve m. CRPC NCT 01193257 1, 083 Orteronel/prednisone Placebo/prednisone m. CRCP progressing on/after docetaxel 900 LHRH + oral anti-androgen/RT + orteronel BID x 2 years High-risk PC NCT 01546987 www. clinicaltrials. gov, September 2012
 A Phase III, Randomized, Double-Blind, Multicenter Trial Comparing the Investigational Agent Orteronel (TAK 700) plus Prednisone (P) with Placebo plus P in Patients with Metastatic Castration-Resistant Prostate Cancer (m. CRPC) That Has Progressed During or Following Docetaxel-Based Therapy Dreicer R et al. Proc ASCO 2012; Abstract TPS 4693.
A Phase III, Randomized, Double-Blind, Multicenter Trial Comparing the Investigational Agent Orteronel (TAK 700) plus Prednisone (P) with Placebo plus P in Patients with Metastatic Castration-Resistant Prostate Cancer (m. CRPC) That Has Progressed During or Following Docetaxel-Based Therapy Dreicer R et al. Proc ASCO 2012; Abstract TPS 4693.

 Radium Targets Osteoblastic Bone Metastases by Acting as a Calcium Mimetic • Radium-223 acts as a calcium mimetic • Naturally self-targets to bone metastases Cheetham PJ et al. Oncology 2012; 26(4): 330 -7.
Radium Targets Osteoblastic Bone Metastases by Acting as a Calcium Mimetic • Radium-223 acts as a calcium mimetic • Naturally self-targets to bone metastases Cheetham PJ et al. Oncology 2012; 26(4): 330 -7.
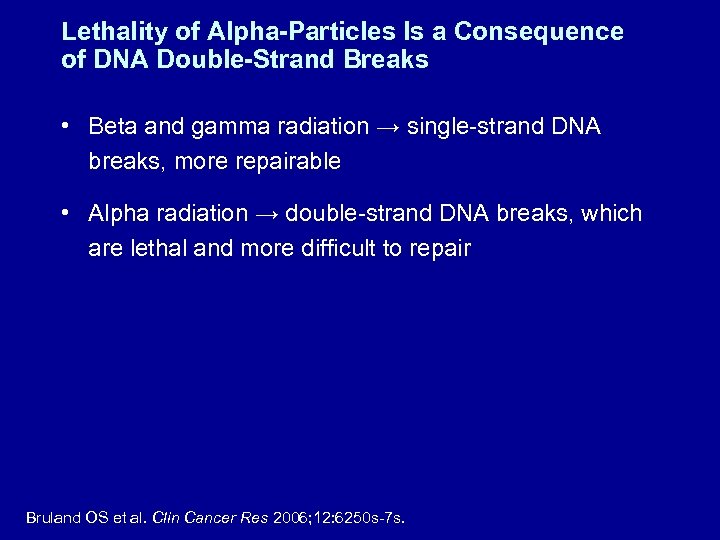 Lethality of Alpha-Particles Is a Consequence of DNA Double-Strand Breaks • Beta and gamma radiation → single-strand DNA breaks, more repairable • Alpha radiation → double-strand DNA breaks, which are lethal and more difficult to repair Bruland OS et al. Clin Cancer Res 2006; 12: 6250 s-7 s.
Lethality of Alpha-Particles Is a Consequence of DNA Double-Strand Breaks • Beta and gamma radiation → single-strand DNA breaks, more repairable • Alpha radiation → double-strand DNA breaks, which are lethal and more difficult to repair Bruland OS et al. Clin Cancer Res 2006; 12: 6250 s-7 s.
 Radium-223 Targets Bone Metastases • Alpha-particles induce double-strand DNA breaks in adjacent tumor cells • Short penetration of alpha emitters (2 -10 cell diameters) = highly localized tumor cell killing and minimal damage to surrounding normal tissue Perez et al. Principles and Practice of Radiation Oncology. 5 th ed. Lippincott Williams & Wilkins; 2007: 103.
Radium-223 Targets Bone Metastases • Alpha-particles induce double-strand DNA breaks in adjacent tumor cells • Short penetration of alpha emitters (2 -10 cell diameters) = highly localized tumor cell killing and minimal damage to surrounding normal tissue Perez et al. Principles and Practice of Radiation Oncology. 5 th ed. Lippincott Williams & Wilkins; 2007: 103.
 Updated Analysis of the Phase III, Double. Blind, Randomized, Multinational Study of Radium-223 Chloride in Castration. Resistant Prostate Cancer (CRPC) Patients with Bone Metastases (ALSYMPCA) Parker C et al. Proc ASCO 2012; Abstract LBA 4512.
Updated Analysis of the Phase III, Double. Blind, Randomized, Multinational Study of Radium-223 Chloride in Castration. Resistant Prostate Cancer (CRPC) Patients with Bone Metastases (ALSYMPCA) Parker C et al. Proc ASCO 2012; Abstract LBA 4512.
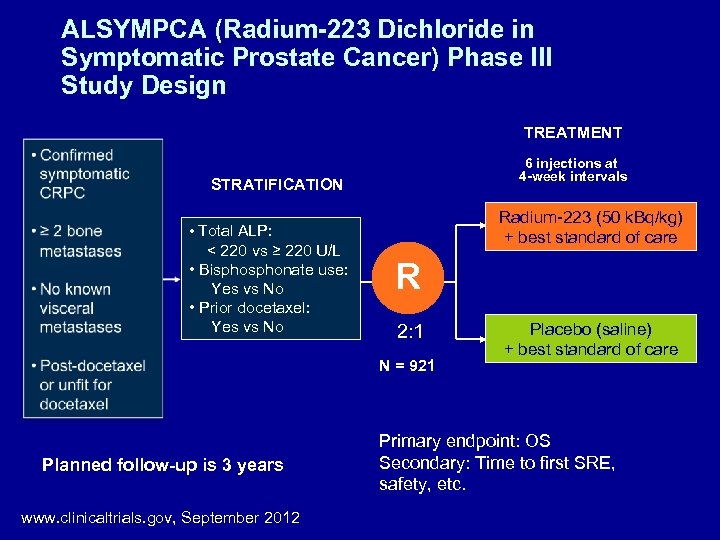 ALSYMPCA (Radium-223 Dichloride in Symptomatic Prostate Cancer) Phase III Study Design TREATMENT PATIENTS 6 injections at 4 -week intervals STRATIFICATION Radium-223 (50 k. Bq/kg) + best standard of care • Total ALP: < 220 vs ≥ 220 U/L • Bisphonate use: Yes vs No • Prior docetaxel: Yes vs No R 2: 1 N = 921 Planned follow-up is 3 years www. clinicaltrials. gov, September 2012 Placebo (saline) + best standard of care Primary endpoint: OS Secondary: Time to first SRE, safety, etc.
ALSYMPCA (Radium-223 Dichloride in Symptomatic Prostate Cancer) Phase III Study Design TREATMENT PATIENTS 6 injections at 4 -week intervals STRATIFICATION Radium-223 (50 k. Bq/kg) + best standard of care • Total ALP: < 220 vs ≥ 220 U/L • Bisphonate use: Yes vs No • Prior docetaxel: Yes vs No R 2: 1 N = 921 Planned follow-up is 3 years www. clinicaltrials. gov, September 2012 Placebo (saline) + best standard of care Primary endpoint: OS Secondary: Time to first SRE, safety, etc.
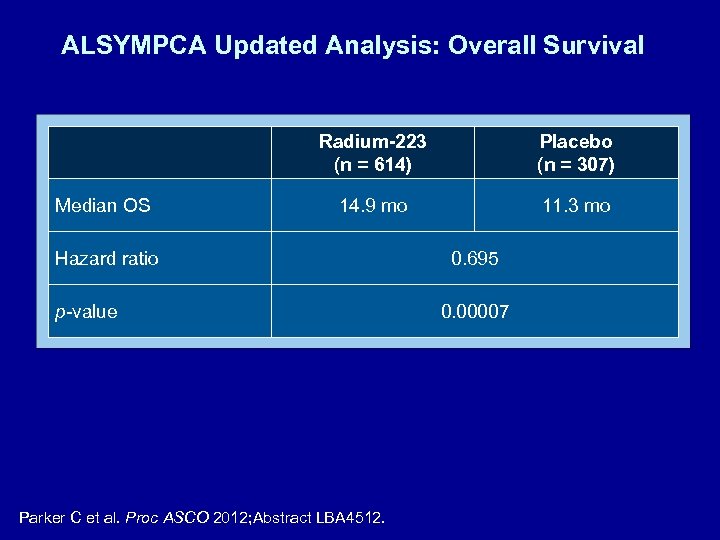 ALSYMPCA Updated Analysis: Overall Survival Radium-223 (n = 614) Median OS Placebo (n = 307) 14. 9 mo 11. 3 mo Hazard ratio p-value Parker C et al. Proc ASCO 2012; Abstract LBA 4512. 0. 695 0. 00007
ALSYMPCA Updated Analysis: Overall Survival Radium-223 (n = 614) Median OS Placebo (n = 307) 14. 9 mo 11. 3 mo Hazard ratio p-value Parker C et al. Proc ASCO 2012; Abstract LBA 4512. 0. 695 0. 00007
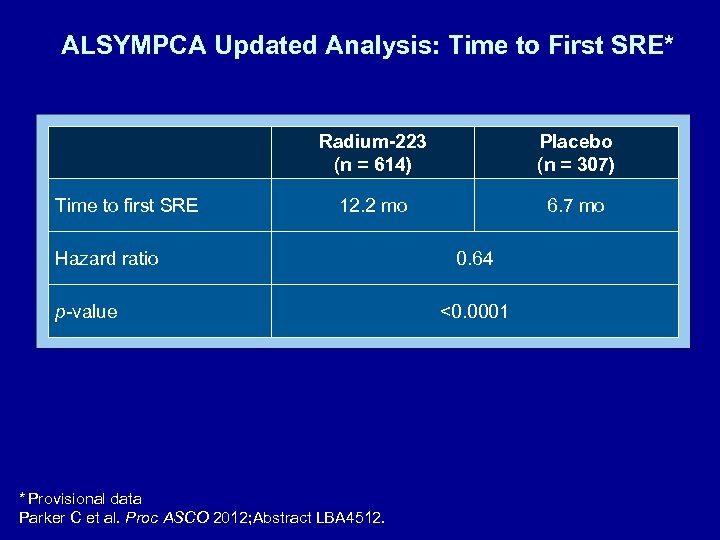 ALSYMPCA Updated Analysis: Time to First SRE* Radium-223 (n = 614) Time to first SRE Placebo (n = 307) 12. 2 mo 6. 7 mo Hazard ratio p-value * Provisional data Parker C et al. Proc ASCO 2012; Abstract LBA 4512. 0. 64 <0. 0001
ALSYMPCA Updated Analysis: Time to First SRE* Radium-223 (n = 614) Time to first SRE Placebo (n = 307) 12. 2 mo 6. 7 mo Hazard ratio p-value * Provisional data Parker C et al. Proc ASCO 2012; Abstract LBA 4512. 0. 64 <0. 0001
 Radium-223 Chloride Impact on Skeletal-Related Events and ECOG Performance Status in Patients with Castration-Resistant Prostate Cancer with Bone Metastases: Interim Results of a Phase III Trial (ALSYMPCA) Sartor AO et al. Proc ASCO 2012; Abstract 4551.
Radium-223 Chloride Impact on Skeletal-Related Events and ECOG Performance Status in Patients with Castration-Resistant Prostate Cancer with Bone Metastases: Interim Results of a Phase III Trial (ALSYMPCA) Sartor AO et al. Proc ASCO 2012; Abstract 4551.

 Faculty Case: Dr Sartor • An 85 -year-old man • Metastatic CRPC unsuitable for docetaxel due to frailty – Increasing pain – Prior treatments: LHRH/nilutamide, then abiraterone/prednisone, denosumab, low-dose dexamethasone • Expanded access protocol: Radium-223 – 4 doses with diminished pain and no side effects – PSA rising but more slowly
Faculty Case: Dr Sartor • An 85 -year-old man • Metastatic CRPC unsuitable for docetaxel due to frailty – Increasing pain – Prior treatments: LHRH/nilutamide, then abiraterone/prednisone, denosumab, low-dose dexamethasone • Expanded access protocol: Radium-223 – 4 doses with diminished pain and no side effects – PSA rising but more slowly

 Cabozantinib has resulted in bone scan improvements and pain relief in chemotherapypretreated metastatic CRPC.
Cabozantinib has resulted in bone scan improvements and pain relief in chemotherapypretreated metastatic CRPC.
 Cabozantinib (XL 184) in Chemotherapy. Pretreated Metastatic Castration Resistant Prostate Cancer (m. CRPC): Results from a Phase II Nonrandomized Expansion Cohort (NRE) Smith MR et al. Proc ASCO 2012; Abstract 4513.
Cabozantinib (XL 184) in Chemotherapy. Pretreated Metastatic Castration Resistant Prostate Cancer (m. CRPC): Results from a Phase II Nonrandomized Expansion Cohort (NRE) Smith MR et al. Proc ASCO 2012; Abstract 4513.
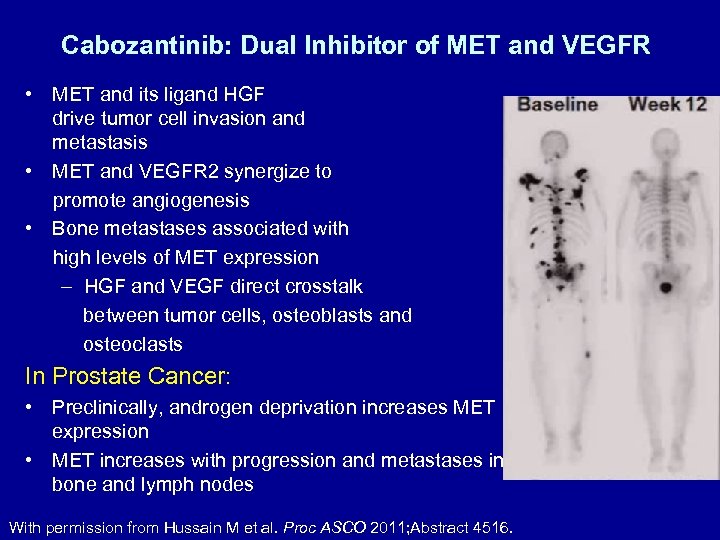 Cabozantinib: Dual Inhibitor of MET and VEGFR • MET and its ligand HGF drive tumor cell invasion and metastasis • MET and VEGFR 2 synergize to promote angiogenesis • Bone metastases associated with high levels of MET expression – HGF and VEGF direct crosstalk between tumor cells, osteoblasts and osteoclasts In Prostate Cancer: • Preclinically, androgen deprivation increases MET expression • MET increases with progression and metastases in bone and lymph nodes With permission from Hussain M et al. Proc ASCO 2011; Abstract 4516.
Cabozantinib: Dual Inhibitor of MET and VEGFR • MET and its ligand HGF drive tumor cell invasion and metastasis • MET and VEGFR 2 synergize to promote angiogenesis • Bone metastases associated with high levels of MET expression – HGF and VEGF direct crosstalk between tumor cells, osteoblasts and osteoclasts In Prostate Cancer: • Preclinically, androgen deprivation increases MET expression • MET increases with progression and metastases in bone and lymph nodes With permission from Hussain M et al. Proc ASCO 2011; Abstract 4516.
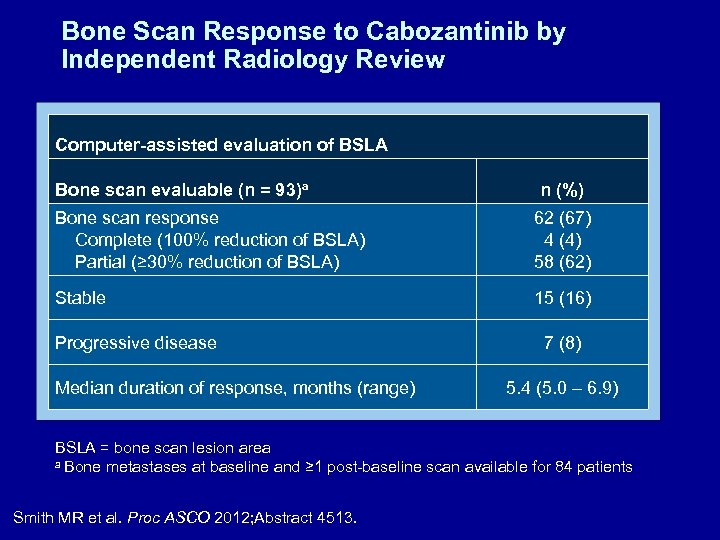 Bone Scan Response to Cabozantinib by Independent Radiology Review Computer-assisted evaluation of BSLA Bone scan evaluable (n = 93)a n (%) Bone scan response Complete (100% reduction of BSLA) Partial (≥ 30% reduction of BSLA) 62 (67) 4 (4) 58 (62) Stable 15 (16) Progressive disease Median duration of response, months (range) 7 (8) 5. 4 (5. 0 – 6. 9) BSLA = bone scan lesion area a Bone metastases at baseline and ≥ 1 post-baseline scan available for 84 patients Smith MR et al. Proc ASCO 2012; Abstract 4513.
Bone Scan Response to Cabozantinib by Independent Radiology Review Computer-assisted evaluation of BSLA Bone scan evaluable (n = 93)a n (%) Bone scan response Complete (100% reduction of BSLA) Partial (≥ 30% reduction of BSLA) 62 (67) 4 (4) 58 (62) Stable 15 (16) Progressive disease Median duration of response, months (range) 7 (8) 5. 4 (5. 0 – 6. 9) BSLA = bone scan lesion area a Bone metastases at baseline and ≥ 1 post-baseline scan available for 84 patients Smith MR et al. Proc ASCO 2012; Abstract 4513.

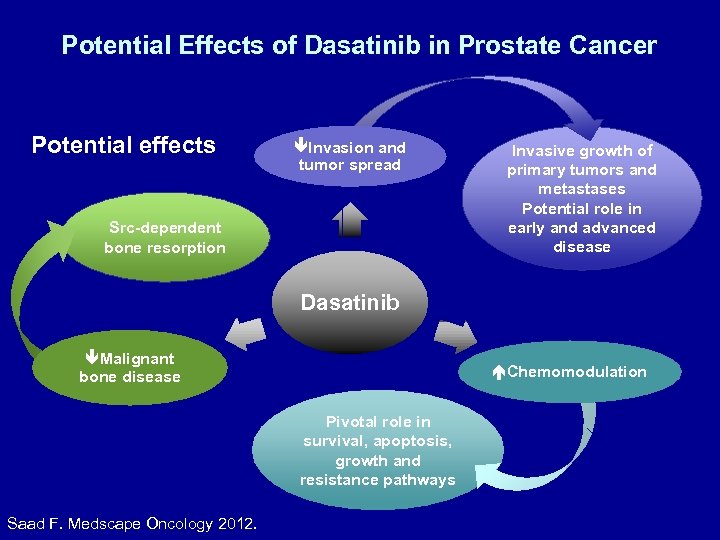 Potential Effects of Dasatinib in Prostate Cancer Potential effects Invasion and tumor spread Src-dependent bone resorption Invasive growth of primary tumors and metastases Potential role in early and advanced disease Dasatinib Malignant bone disease Chemomodulation Pivotal role in survival, apoptosis, growth and resistance pathways Saad F. Medscape Oncology 2012.
Potential Effects of Dasatinib in Prostate Cancer Potential effects Invasion and tumor spread Src-dependent bone resorption Invasive growth of primary tumors and metastases Potential role in early and advanced disease Dasatinib Malignant bone disease Chemomodulation Pivotal role in survival, apoptosis, growth and resistance pathways Saad F. Medscape Oncology 2012.
 Dasatinib Combined with Docetaxel for Castration-Resistant Prostate Cancer: Results from a Phase 1 -2 Study Araujo JC et al. Cancer 2012; 118(1): 63 -71.
Dasatinib Combined with Docetaxel for Castration-Resistant Prostate Cancer: Results from a Phase 1 -2 Study Araujo JC et al. Cancer 2012; 118(1): 63 -71.
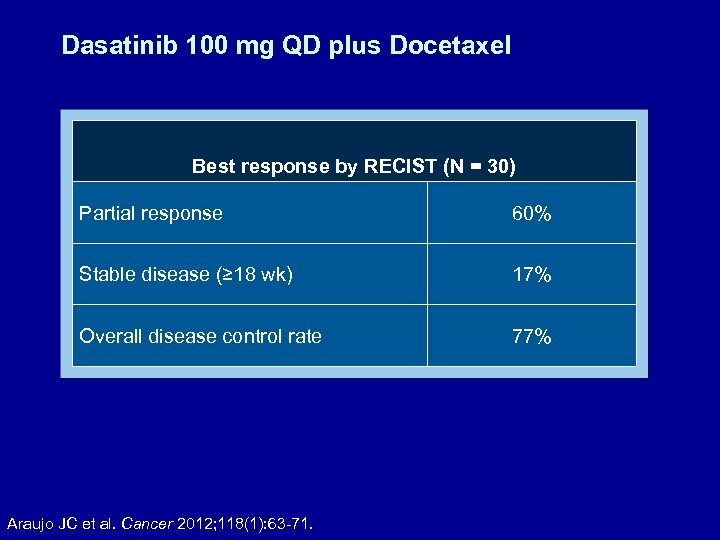
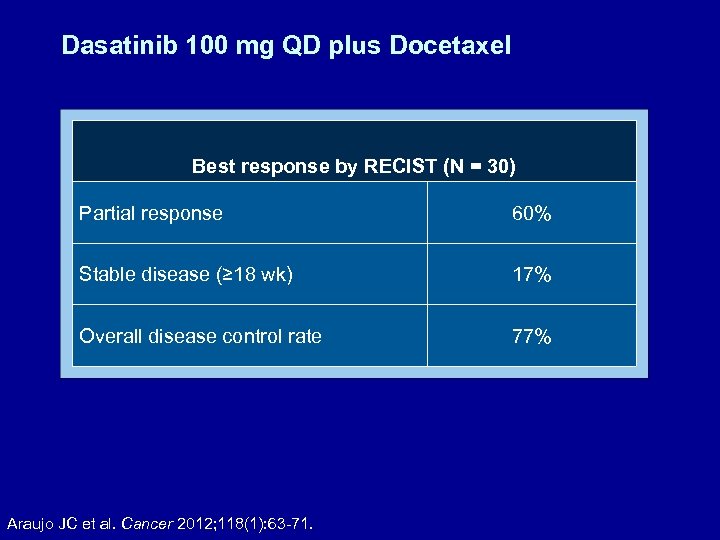 Dasatinib 100 mg QD plus Docetaxel Best response by RECIST (N = 30) Partial response 60% Stable disease (≥ 18 wk) 17% Overall disease control rate 77% Araujo JC et al. Cancer 2012; 118(1): 63 -71.
Dasatinib 100 mg QD plus Docetaxel Best response by RECIST (N = 30) Partial response 60% Stable disease (≥ 18 wk) 17% Overall disease control rate 77% Araujo JC et al. Cancer 2012; 118(1): 63 -71.
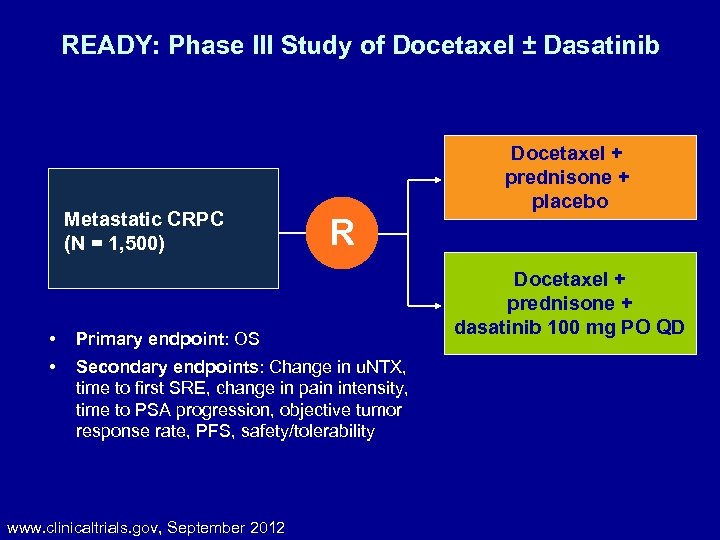 READY: Phase III Study of Docetaxel ± Dasatinib Metastatic CRPC (N = 1, 500) • • R Primary endpoint: OS Secondary endpoints: Change in u. NTX, time to first SRE, change in pain intensity, time to PSA progression, objective tumor response rate, PFS, safety/tolerability www. clinicaltrials. gov, September 2012 Docetaxel + prednisone + placebo Docetaxel + prednisone + dasatinib 100 mg PO QD
READY: Phase III Study of Docetaxel ± Dasatinib Metastatic CRPC (N = 1, 500) • • R Primary endpoint: OS Secondary endpoints: Change in u. NTX, time to first SRE, change in pain intensity, time to PSA progression, objective tumor response rate, PFS, safety/tolerability www. clinicaltrials. gov, September 2012 Docetaxel + prednisone + placebo Docetaxel + prednisone + dasatinib 100 mg PO QD
 Dasatinib 100 mg QD plus Docetaxel Best response by RECIST (N = 30) Partial response 60% Stable disease (≥ 18 wk) 17% Overall disease control rate 77% Araujo JC et al. Cancer 2012; 118(1): 63 -71.
Dasatinib 100 mg QD plus Docetaxel Best response by RECIST (N = 30) Partial response 60% Stable disease (≥ 18 wk) 17% Overall disease control rate 77% Araujo JC et al. Cancer 2012; 118(1): 63 -71.
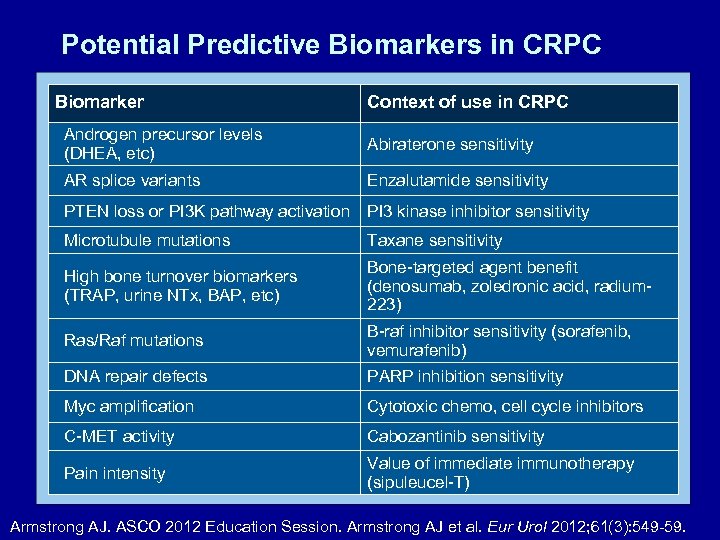 Potential Predictive Biomarkers in CRPC Biomarker Context of use in CRPC Androgen precursor levels (DHEA, etc) Abiraterone sensitivity AR splice variants Enzalutamide sensitivity PTEN loss or PI 3 K pathway activation PI 3 kinase inhibitor sensitivity Microtubule mutations Taxane sensitivity High bone turnover biomarkers (TRAP, urine NTx, BAP, etc) Bone-targeted agent benefit (denosumab, zoledronic acid, radium 223) Ras/Raf mutations B-raf inhibitor sensitivity (sorafenib, vemurafenib) DNA repair defects PARP inhibition sensitivity Myc amplification Cytotoxic chemo, cell cycle inhibitors C-MET activity Cabozantinib sensitivity Pain intensity Value of immediate immunotherapy (sipuleucel-T) Armstrong AJ. ASCO 2012 Education Session. Armstrong AJ et al. Eur Urol 2012; 61(3): 549 -59.
Potential Predictive Biomarkers in CRPC Biomarker Context of use in CRPC Androgen precursor levels (DHEA, etc) Abiraterone sensitivity AR splice variants Enzalutamide sensitivity PTEN loss or PI 3 K pathway activation PI 3 kinase inhibitor sensitivity Microtubule mutations Taxane sensitivity High bone turnover biomarkers (TRAP, urine NTx, BAP, etc) Bone-targeted agent benefit (denosumab, zoledronic acid, radium 223) Ras/Raf mutations B-raf inhibitor sensitivity (sorafenib, vemurafenib) DNA repair defects PARP inhibition sensitivity Myc amplification Cytotoxic chemo, cell cycle inhibitors C-MET activity Cabozantinib sensitivity Pain intensity Value of immediate immunotherapy (sipuleucel-T) Armstrong AJ. ASCO 2012 Education Session. Armstrong AJ et al. Eur Urol 2012; 61(3): 549 -59.



